Boosting the Separation of Adeno-Associated Virus Capsid Proteins by Liquid Chromatography and Capillary Electrophoresis Approaches
Abstract
1. Introduction
2. Results and Discussion
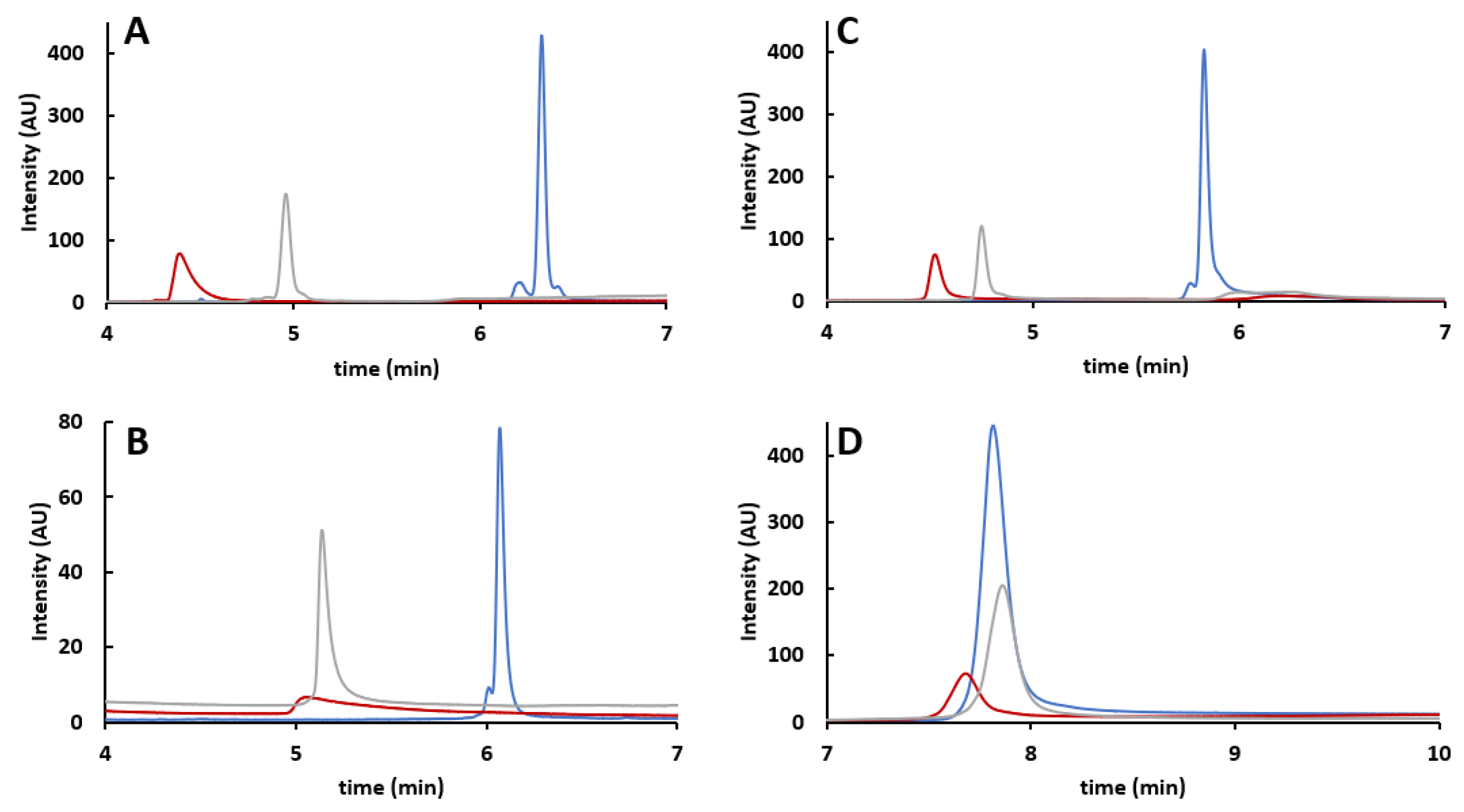
2.1. CE-SDS
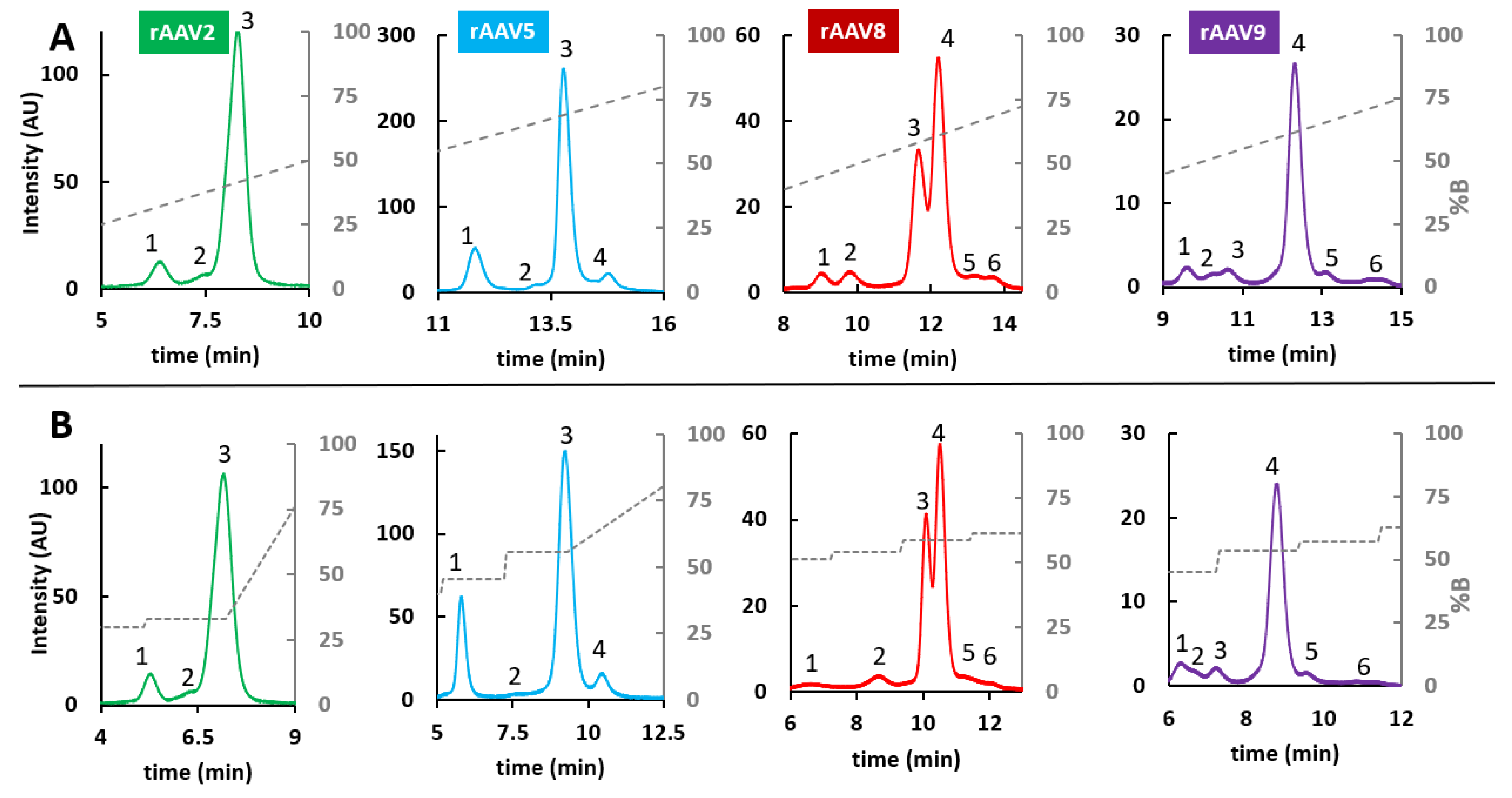
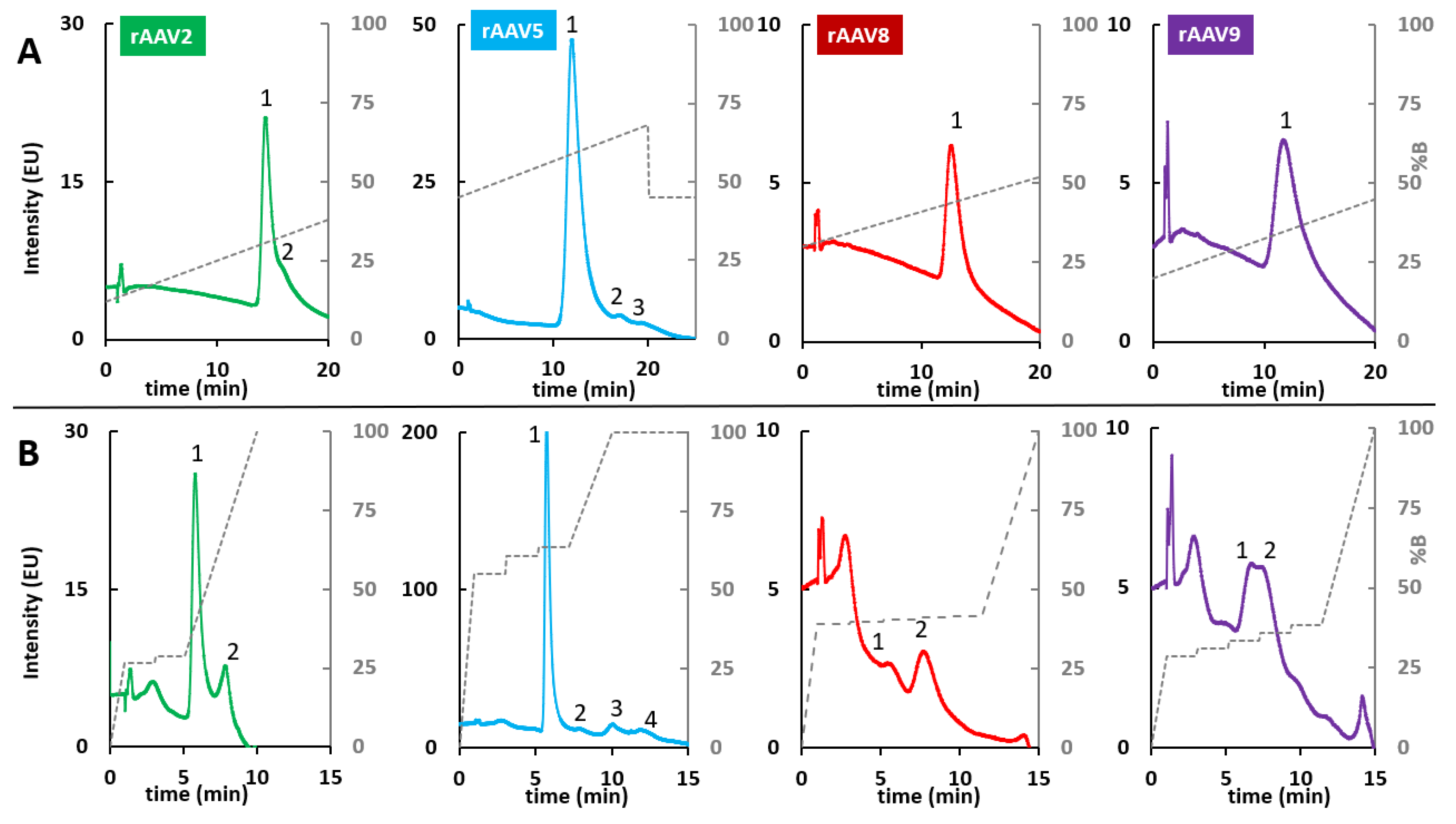
2.2. RPLC
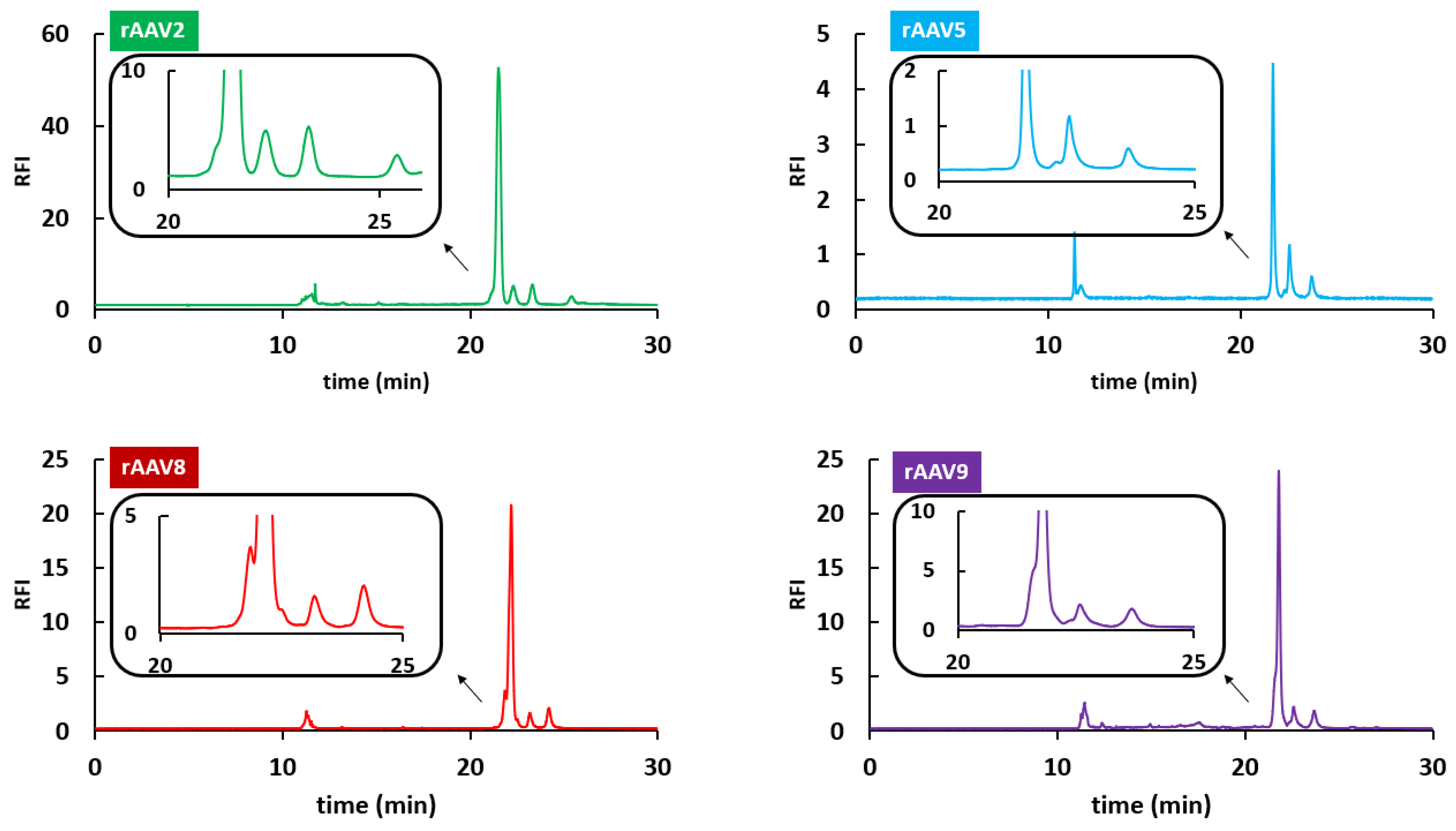
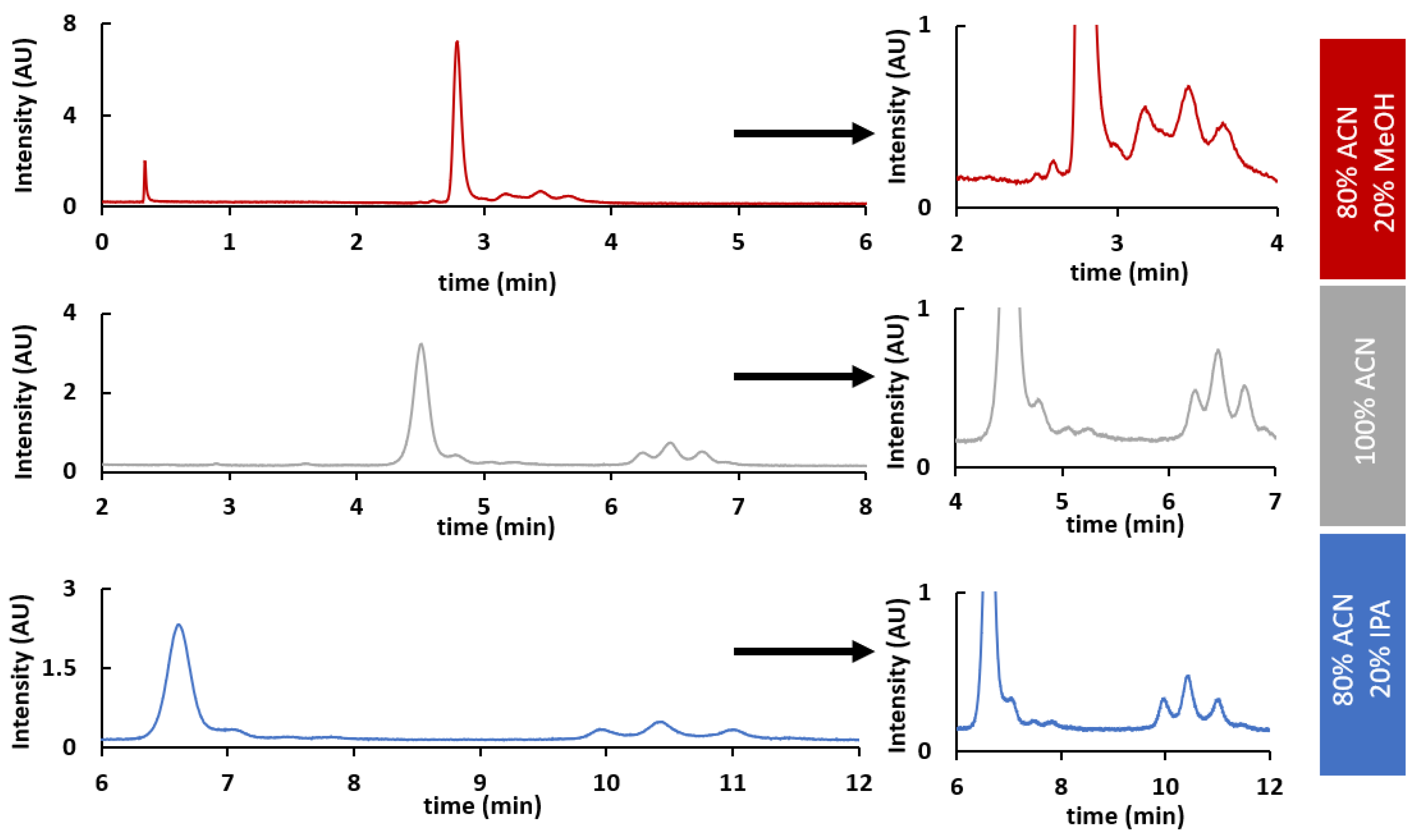
2.3. HILIC
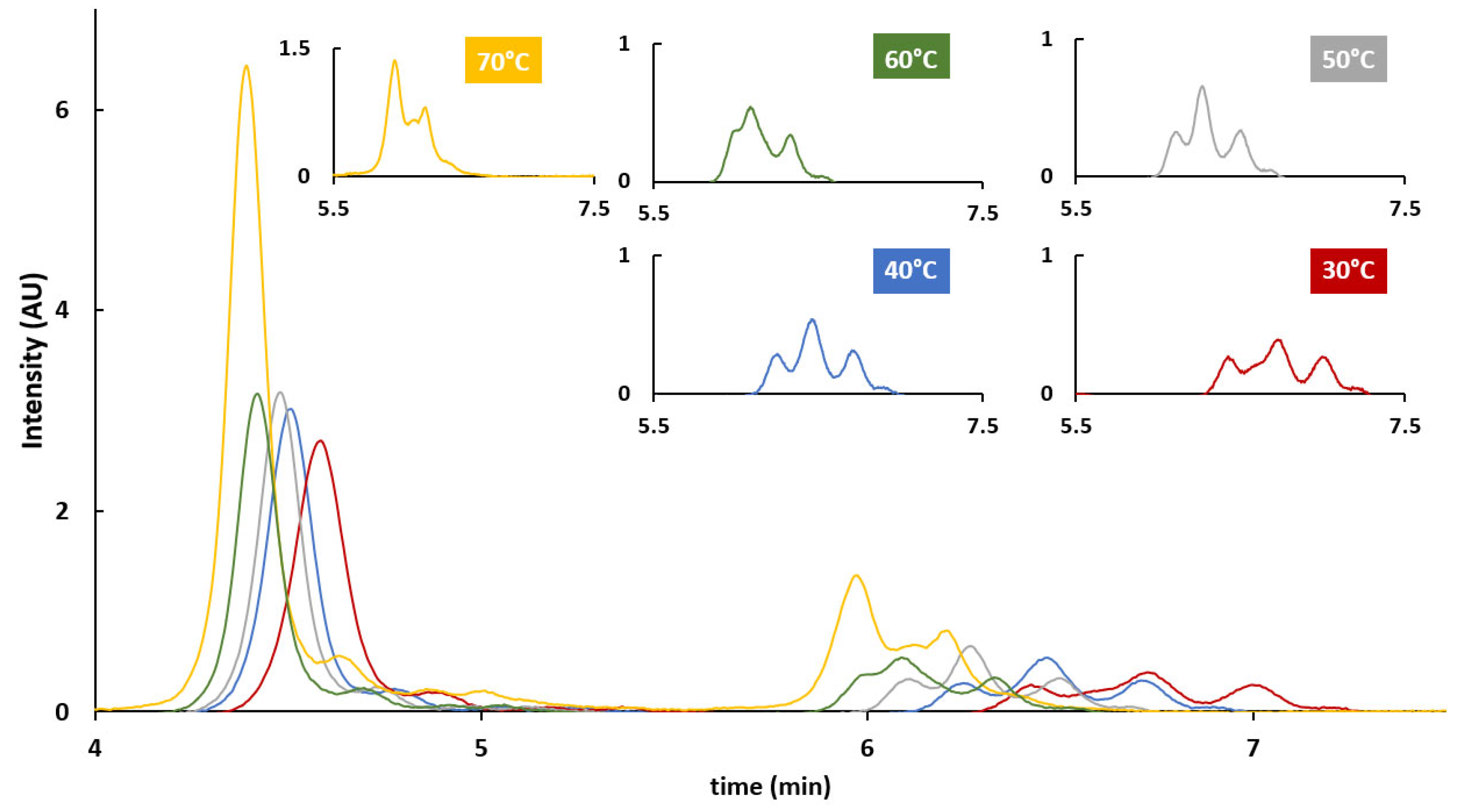
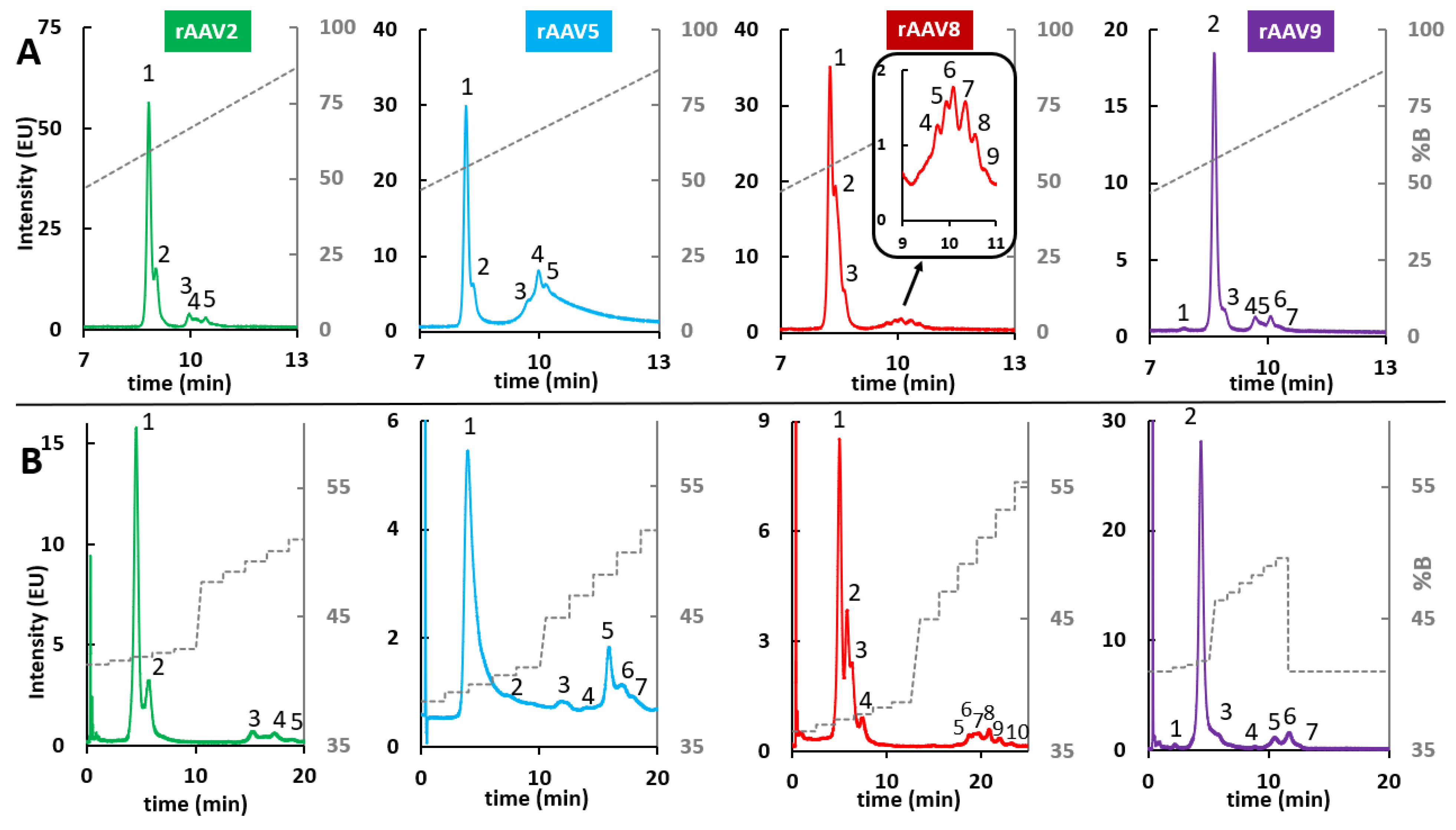
2.4. HIC
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Sample Preparation
3.3. Instrumentation and Experimental Conditions
3.3.1. CE-SDS Analysis
3.3.2. LC Analysis
RPLC Conditions
HILIC Conditions
HIC Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulaklak, K.; Gersbach, C.A. The once and future gene therapy. Nat. Commun. 2020, 11, 5820. [Google Scholar] [CrossRef]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- FDA Approaved Cellular and Gene Therapy Products—LUXTURNA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna (accessed on 2 May 2023).
- FDA Approaved Cellular and Gene Therapy Products—ZOLGENSMA. Available online: https://www.fda.gov/vaccines-blood-biologics/zolgensma (accessed on 2 May 2023).
- FDA Approaved Cellular and Gene Therapy Products—HEMGENIX. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/hemgenix (accessed on 2 May 2023).
- The Journal of Gene Medicine Gene Therapy Clinical Trials Worldwide. Available online: https://a873679.fmphost.com/fmi/webd/GTCT (accessed on 5 April 2023).
- Dismuke, D.; Tenenbaum, L.; Samulski, R. Biosafety of Recombinant Adeno-associated Virus Vectors. Curr. Gene Ther. 2014, 13, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Leone, P.; Samulski, R.J.; Xiao, X.; Pfaff, D.W.; O’Malley, K.L.; During, M.J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994, 8, 148–154. [Google Scholar] [CrossRef]
- Aschauer, D.F.; Kreuz, S.; Rumpel, S. Analysis of Transduction Efficiency, Tropism and Axonal Transport of AAV Serotypes 1, 2, 5, 6, 8 and 9 in the Mouse Brain. PLoS ONE 2013, 8, e76310. [Google Scholar] [CrossRef]
- Burger, C.; Gorbatyuk, O.S.; Velardo, M.J.; Peden, C.S.; Williams, P.; Zolotukhin, S.; Reier, P.J.; Mandel, R.J.; Muzyczka, N. Recombinant AAV Viral Vectors Pseudotyped with Viral Capsids from Serotypes 1, 2, and 5 Display Differential Efficiency and Cell Tropism after Delivery to Different Regions of the Central Nervous System. Mol. Ther. 2004, 10, 302–317. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Nicklin, S.A.; Büning, H.; Brosnan, M.J.; Leike, K.; Papadakis, E.D.; Hallek, M.; Baker, A.H. Targeted Gene Delivery to Vascular Tissue In Vivo by Tropism-Modified Adeno-Associated Virus Vectors. Circulation 2004, 109, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tse, L.V.; Klinc, K.A.; Madigan, V.J.; Castellanos Rivera, R.M.; Wells, L.F.; Havlik, L.P.; Smith, J.K.; Agbandje-McKenna, M.; Asokan, A. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl. Acad. Sci. USA 2017, 114, E4812–E4821. [Google Scholar] [CrossRef] [PubMed]
- Leborgne, C.; Barbon, E.; Alexander, J.M.; Hanby, H.; Delignat, S.; Cohen, D.M.; Collaud, F.; Muraleetharan, S.; Lupo, D.; Silverberg, J.; et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020, 26, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, L.; Nass, S.; O’Riordan, C.; Pastor, E.; Zhang, X.K. Direct Liquid Chromatography/Mass Spectrometry Analysis for Complete Characterization of Recombinant Adeno-Associated Virus Capsid Proteins. Hum. Gene Ther. Methods 2017, 28, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Kuck, D.; Kern, A.; Kleinschmidt, J.A. Development of AAV serotype-specific ELISAs using novel monoclonal antibodies. J. Virol. Methods 2007, 140, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Sena-Esteves, M.; Gao, G. Analysis of Recombinant Adeno-Associated Virus (rAAV) Purity Using Silver-Stained SDS-PAGE. Cold Spring Harb. Protoc. 2020, 2020, 095679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Meagher, M.M. Highly Sensitive SDS Capillary Gel Electrophoresis with Sample Stacking Requiring Only Nanograms of Adeno-Associated Virus Capsid Proteins. Methods Mol. Biol. 2019, 1972, 263–270. [Google Scholar] [PubMed]
- Liu, A.P.; Patel, S.K.; Xing, T.; Yan, Y.; Wang, S.; Li, N. Characterization of Adeno-Associated Virus Capsid Proteins Using Hydrophilic Interaction Chromatography Coupled with Mass Spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Park, J.; Barrett, H.; Dooley, S.; Davies, C.; Verhagen, M.F. Capillary Electrophoresis-Sodium Dodecyl Sulfate with Laser-Induced Fluorescence Detection as a Highly Sensitive and Quality Control-Friendly Method for Monitoring Adeno-Associated Virus Capsid Protein Purity. Hum. Gene Ther. 2021, 32, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Meagher, M.M. Sample Stacking Provides Three Orders of Magnitude Sensitivity Enhancement in SDS Capillary Gel Electrophoresis of Adeno-Associated Virus Capsid Proteins. Anal. Chem. 2017, 89, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yowanto, H.; Mollah, S. Sciex Application Note RUO-MKT-02-10086-A: Purity Analysis of Adeno-Associated Virus (AAV) Capsid Proteins Using CE-LIF Technology. Available online: https://sciex.com/content/dam/SCIEX/pdf/tech-notes/all/AAV-Purity-CE-LIF.pdf (accessed on 5 April 2023).
- Römer, J.; Montealegre, C.; Schlecht, J.; Kiessig, S.; Moritz, B.; Neusüß, C. Online mass spectrometry of CE (SDS)-separated proteins by two-dimensional capillary electrophoresis. Anal. Bioanal. Chem. 2019, 411, 7197–7206. [Google Scholar] [CrossRef]
- Römer, J.; Stolz, A.; Kiessig, S.; Moritz, B.; Neusüß, C. Online top-down mass spectrometric identification of CE(SDS)-separated antibody fragments by two-dimensional capillary electrophoresis. J. Pharm. Biomed. Anal. 2021, 201, 114089. [Google Scholar] [CrossRef]
- Murisier, A.; D’Atri, V.; Larraillet, V.; Pirner, S.; Guillarme, D. Boosting the Liquid Chromatography Separation of Complex Bispecific Antibody Products by Using the Multi-Isocratic Elution Mode. Separations 2022, 9, 243. [Google Scholar] [CrossRef]
- Zhang, X.; Koza, S.; Yu, Y.Q.; Chen, W. Waters Application Note 720006825—Optimizing Adeno-Associated Virus (AAV) Capsid Protein Analysis Using UPLC and UPLC-MS. Available online: https://www.waters.com/content/dam/waters/en/app-notes/2020/720006869/720006869-en.pdf (accessed on 5 April 2023).
- Bobály, B.; D’Atri, V.; Lauber, M.; Beck, A.; Guillarme, D.; Fekete, S. Characterizing various monoclonal antibodies with milder reversed phase chromatography conditions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1096, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Dumont, E.; Vandenheede, I.; Guillarme, D.; Sandra, P.; Sandra, K. Hydrophilic interaction chromatography for the characterization of therapeutic monoclonal antibodies at protein, peptide, and glycan levels. LCGC Eur. 2017, 30, 424–434. [Google Scholar]
- D’Atri, V.; Goyon, A.; Bobaly, B.; Beck, A.; Fekete, S.; Guillarme, D. Protocols for the analytical characterization of therapeutic monoclonal antibodies. III—Denaturing chromatographic techniques hyphenated to mass spectrometry. J. Chromatogr. B 2018, 1096, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bobály, B.; D’Atri, V.; Beck, A.; Guillarme, D.; Fekete, S. Analysis of recombinant monoclonal antibodies in hydrophilic interaction chromatography: A generic method development approach. J. Pharm. Biomed. Anal. 2017, 145, 24–32. [Google Scholar] [CrossRef]
- Periat, A.; Fekete, S.; Cusumano, A.; Veuthey, J.-L.; Beck, A.; Lauber, M.; Guillarme, D. Potential of hydrophilic interaction chromatography for the analytical characterization of protein biopharmaceuticals. J. Chromatogr. A 2016, 1448, 81–92. [Google Scholar] [CrossRef]
- D’Atri, V.; Fekete, S.; Beck, A.; Lauber, M.; Guillarme, D. Hydrophilic Interaction Chromatography Hyphenated with Mass Spectrometry: A Powerful Analytical Tool for the Comparison of Originator and Biosimilar Therapeutic Monoclonal Antibodies at the Middle-up Level of Analysis. Anal. Chem. 2017, 89, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Nováková, L.; Fekete, S.; Stoll, D.; Lauber, M.; Beck, A.; Guillarme, D. Orthogonal Middle-up Approaches for Characterization of the Glycan Heterogeneity of Etanercept by Hydrophilic Interaction Chromatography Coupled to High-Resolution Mass Spectrometry. Anal. Chem. 2019, 91, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Veuthey, J.-L.; Beck, A.; Guillarme, D. Hydrophobic interaction chromatography for the characterization of monoclonal antibodies and related products. J. Pharm. Biomed. Anal. 2016, 130, 3–18. [Google Scholar] [CrossRef]
- Fekete, S.; Murisier, A.; Guillarme, D. Hydrophobic Interaction Chromatography (HIC) for the Characterization of Therapeutic Monoclonal Antibodies and Related Products, Part 1: Theoretical Aspects. LCGC Eur. 2021, 34, 101–105. [Google Scholar]
- Fekete, S.; Murisier, A.; Verscheure, L.; Sandra, K.; Guillarme, D. Hydrophobic Interaction Chromatography (HIC) for the Characterization of Therapeutic Monoclonal Antibodies and Related Products, Part 2: Practical Considerations. LCGC Eur. 2021, 34, 139–148. [Google Scholar]
- Cusumano, A.; Guillarme, D.; Beck, A.; Fekete, S. Practical method development for the separation of monoclonal antibodies and antibody-drug-conjugate species in hydrophobic interaction chromatoraphy, part 2: Optimization of the phase system. J. Pharm. Biomed. Anal. 2016, 121, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Jose, A.; Chipman, P.; Bhattacharya, N.; Daneshparvar, N.; McKenna, R.; Agbandje-McKenna, M. Completion of the AAV Structural Atlas: Serotype Capsid Structures Reveals Clade-Specific Features. Viruses 2021, 13, 101. [Google Scholar] [CrossRef]
- Bouvarel, T.; Duivelshof, B.L.; Camperi, J.; Schlothauer, T.; Knaupp, A.; Stella, C.; Guillarme, D. Extending the performance of FcRn and FcγRIIIa affinity liquid chromatography for protein biopharmaceuticals. J. Chromatogr. A 2022, 1682, 463518. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; D’Atri, V.; Bobaly, B.; Wagner-Rousset, E.; Beck, A.; Fekete, S.; Guillarme, D. Protocols for the analytical characterization of therapeutic monoclonal antibodies. I—Non-denaturing chromatographic techniques. J. Chromatogr. B 2017, 1058, 73–84. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebischer, M.K.; Bouvarel, T.; Barrozo, E.; Kochardt, D.; Elger, C.; Haindl, M.; Ruppert, R.; Guillarme, D.; D’Atri, V. Boosting the Separation of Adeno-Associated Virus Capsid Proteins by Liquid Chromatography and Capillary Electrophoresis Approaches. Int. J. Mol. Sci. 2023, 24, 8503. https://doi.org/10.3390/ijms24108503
Aebischer MK, Bouvarel T, Barrozo E, Kochardt D, Elger C, Haindl M, Ruppert R, Guillarme D, D’Atri V. Boosting the Separation of Adeno-Associated Virus Capsid Proteins by Liquid Chromatography and Capillary Electrophoresis Approaches. International Journal of Molecular Sciences. 2023; 24(10):8503. https://doi.org/10.3390/ijms24108503
Chicago/Turabian StyleAebischer, Megane K., Thomas Bouvarel, Emmalyn Barrozo, Dominik Kochardt, Carsten Elger, Markus Haindl, Raphael Ruppert, Davy Guillarme, and Valentina D’Atri. 2023. "Boosting the Separation of Adeno-Associated Virus Capsid Proteins by Liquid Chromatography and Capillary Electrophoresis Approaches" International Journal of Molecular Sciences 24, no. 10: 8503. https://doi.org/10.3390/ijms24108503
APA StyleAebischer, M. K., Bouvarel, T., Barrozo, E., Kochardt, D., Elger, C., Haindl, M., Ruppert, R., Guillarme, D., & D’Atri, V. (2023). Boosting the Separation of Adeno-Associated Virus Capsid Proteins by Liquid Chromatography and Capillary Electrophoresis Approaches. International Journal of Molecular Sciences, 24(10), 8503. https://doi.org/10.3390/ijms24108503





