Dipeptide Nitrile CD34 with Curcumin: A New Improved Combination Strategy to Synergistically Inhibit Rhodesain of Trypanosoma brucei rhodesiense
Abstract
1. Introduction
2. Results and Discussion
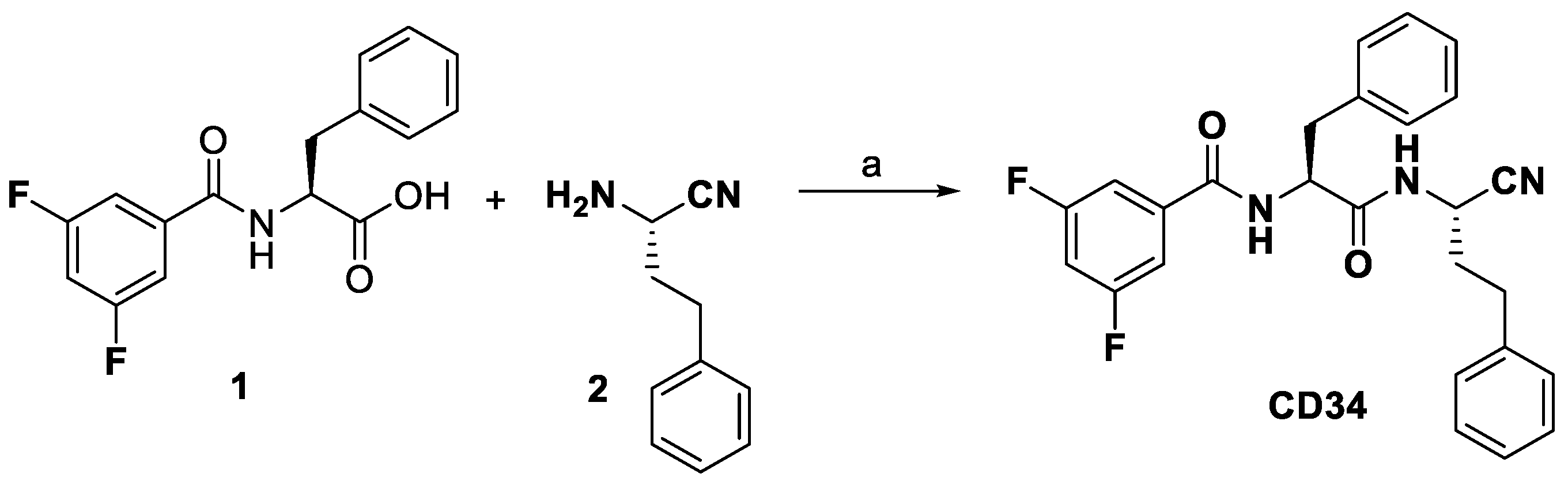
2.1. Chemistry
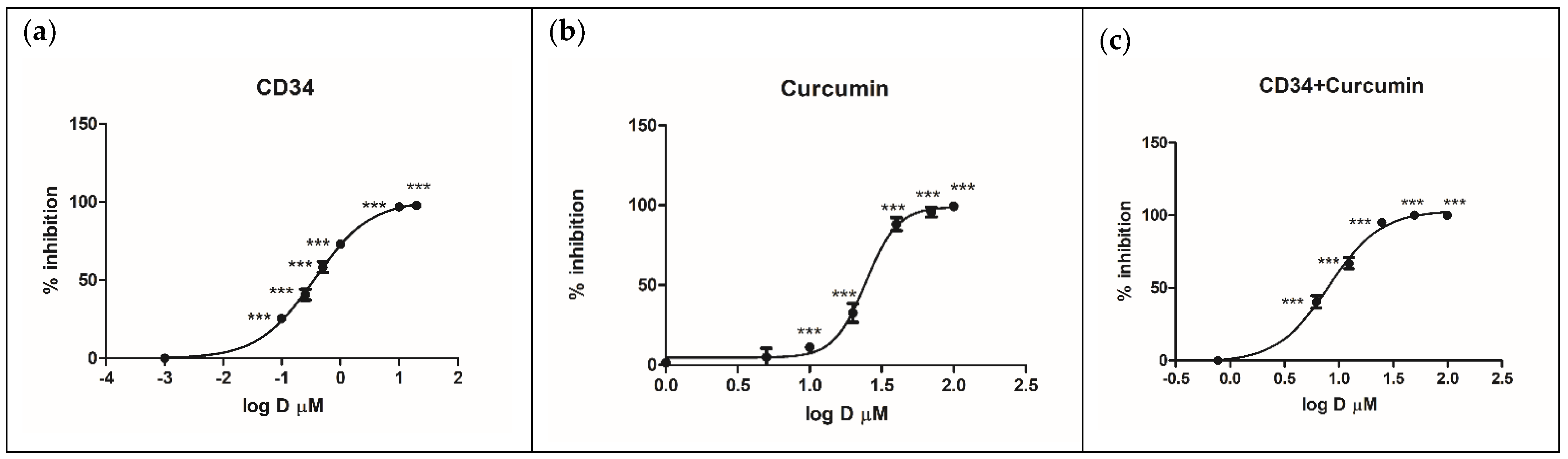
2.2. Biological Evaluation
2.2.1. Inhibition of Rhodesain and Target Selectivity
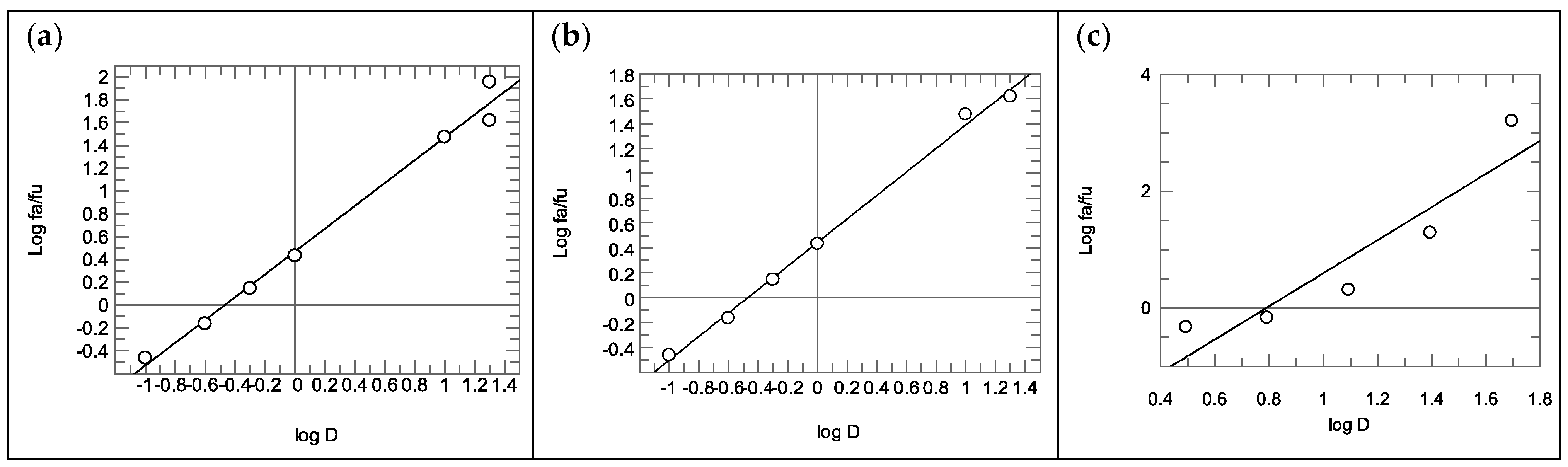
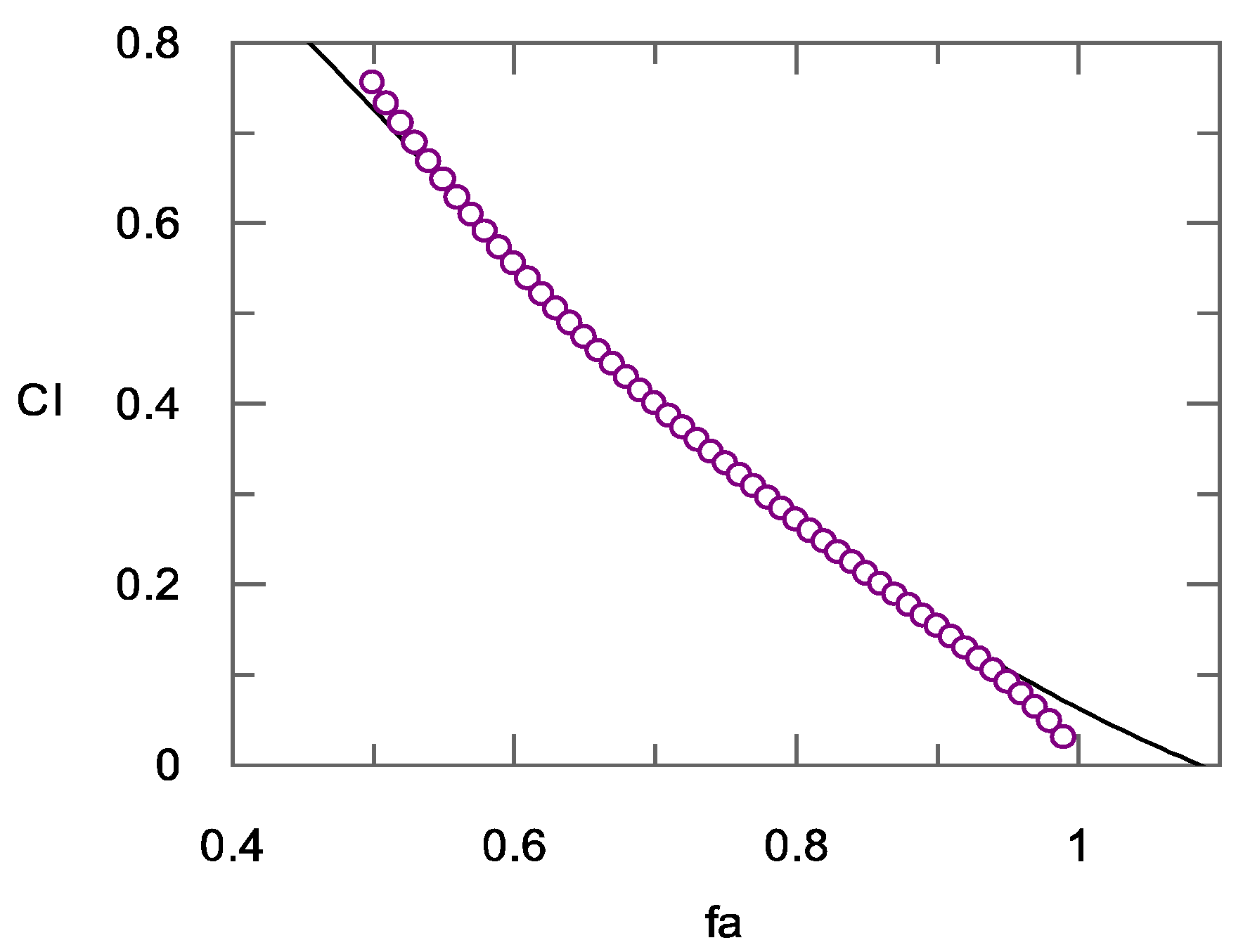
2.2.2. Calculation of the Combination Index
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of Compound CD34
3.3. Rhodesain Inhibition Assays and Calculation of the Combination Index
3.4. Combination Index
3.5. Cathepsin L Inhibition Assays
3.6. Rhodesain Expression and Purification from P. pastoris and E. coli
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/trypanosomiasis_african/en/ (accessed on 17 March 2023).
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Rodgers, J. Clinical and neuropathogenetic aspects of Human African Trypanosomiasis. Front. Immunol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Seixas, J.; Atouguia, J.; Josenando, T.; Vatunga, G.; Bilenge, C.M.M.; Lutumba, P.; Burri, C. Clinical study on the melarsoprol-related encephalopathic syndrome: Risk factors and HLA association. Trop. Med. Infect. Dis. 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Kansiime, F.; Adibaku, S.; Wamboga, C.; Idi, F.; Kato, C.D.; Yamuah, L.; Vaillant, M.; Kioy, D.; Olliaro, P.; Matovu, E. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasit. Vectors 2018, 11, 105. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- CHMP Recommends First Oral-Only Treatment for Sleeping Sickness. Available online: https://www.ema.europa.eu/en/news/chmp-recommends-first-oral-only-treatment-sleeping-sickness (accessed on 17 March 2023).
- Ettari, R.; Previti, S.; Tamborini, L.; Cullia, G.; Grasso, S.; Zappalà, M. The inhibition of cysteine proteases rhodesain and TbCatB: A valuable approach to treat Human African Trypanosomiasis. Mini Rev. Med. Chem. 2016, 16, 1374–1391. [Google Scholar] [CrossRef]
- Ettari, R.; Tamborini, L.; Angelo, I.C.; Micale, N.; Pinto, A.; De Micheli, C.; Conti, P. Inhibition of rhodesain as a novel therapeutic modality for human African trypanosomiasis. J. Med. Chem. 2013, 56, 5637–5658. [Google Scholar] [CrossRef]
- Previti, S.; Di Chio, C.; Ettari, R.; Zappalà, M. Dual inhibition of parasitic targets: A valuable strategy to treat malaria and neglected tropical diseases. Curr. Med. Chem. 2022, 29, 2952–2978. [Google Scholar] [CrossRef]
- Nikolskaia, O.V.; de Lima, A.A.P.; Kim, Y.V.; Lonsdale-Eccles, J.D.; Fukuma, T.; Scharfstein, J.; Grab, D.J. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J. Clin. Investig. 2006, 116, 2739–2747. [Google Scholar] [CrossRef]
- Barry, J.D.; McCulloch, R. Antigenic variation in trypanosomes: Enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 2001, 49, 1–70. [Google Scholar] [CrossRef] [PubMed]
- Lalmanach, G.; Boulange, A.; Serveau, C.; Lecaille, F.; Scharfstein, J.; Gauthier, F.; Authie, E. Congopain from Trypanosoma congolense: Drug target and vaccine candidate. Biol. Chem. 2002, 383, 739–749. [Google Scholar] [CrossRef]
- Caffrey, C.R.; Hansell, E.; Lucas, K.D.; Brinen, L.S.; Alvarez Hernandez, A.; Cheng, J.; Gwaltney, S.L., II; Roush, W.R.; Stierhof, Y.D.; Bogyo, M.; et al. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 2001, 118, 61–73. [Google Scholar] [CrossRef]
- Previti, S.; Ettari, R.; Calcaterra, E.; Di Chio, C.; Ravichandran, R.; Zimmer, C.; Hammerschmidt, S.; Wagner, A.; Cosconati, S.; Schirmeister, T.; et al. Development of urea bond-containing Michael acceptors as antitrypanosomal agents targeting rhodesain. ACS Med. Chem. Lett. 2022, 13, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Previti, S.; Ettari, R.; Di Chio, C.; Ravichandran, R.; Bogacz, M.; Hellmich, U.A.; Schirmeister, T.; Cosconati, S.; Zappalà, M. Development of reduced peptide bond pseudopeptide Michael acceptors for the treatment of Human African Trypanosomiasis. Molecules 2022, 27, 3765. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, S.; Ettari, R.; Previti, S.; Amendola, G.; Wagner, A.; Cosconati, S.; Hellmich, U.A.; Schirmeister, T.; Zappalà, M. Peptidyl vinyl ketone irreversible inhibitors of rhodesain: Modifications of the P2 fragment. ChemMedChem 2020, 15, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Previti, S.; Maiorana, S.; Amendola, G.; Wagner, A.; Cosconati, S.; Schirmeister, T.; Hellmich, U.A.; Zappalà, M. Optimization strategy of novel peptide-based Michael acceptors for the treatment of Human African Trypanosomiasis. J. Med. Chem. 2019, 62, 10617–10629. [Google Scholar] [CrossRef] [PubMed]
- Previti, S.; Ettari, R.; Cosconati, S.; Amendola, G.; Chouchene, K.; Wagner, A.; Hellmich, U.A.; Ulrich, K.; Krauth-Siegel, R.L.; Wich, P.R.; et al. Development of novel peptide-based Michael acceptors targeting rhodesain and falcipain-2 for the treatment of Neglected Tropical Diseases (NTDs). J. Med. Chem. 2017, 60, 6911–6923. [Google Scholar] [CrossRef]
- Ettari, R.; Previti, S.; Cosconati, S.; Maiorana, S.; Schirmeister, T.; Grasso, S.; Zappalà, M. Development of novel 1,4-benzodiazepine-based Michael acceptors as antitrypanosomal agents. Bioorg. Med. Chem. Lett. 2016, 26, 3453–3456. [Google Scholar] [CrossRef]
- Ettari, R.; Previti, S.; Cosconati, S.; Kesselring, J.; Schirmeister, T.; Grasso, S.; Zappalà, M. Synthesis and biological evaluation of novel peptidomimetics as rhodesain inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1184–1191. [Google Scholar] [CrossRef]
- Ettari, R.; Pinto, A.; Previti, S.; Tamborini, L.; Angelo, I.C.; La Pietra, V.; Marinelli, L.; Novellino, E.; Schirmeister, T.; Zappalà, M.; et al. Development of novel dipeptide-like rhodesain inhibitors containing the 3-bromoisoxazoline warhead in a constrained conformation. Bioorg. Med. Chem. 2015, 23, 7053–7060. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Pinto, A.; Tamborini, L.; Angelo, I.C.; Grasso, S.; Zappalà, M.; Capodicasa, N.; Yzeiraj, L.; Gruber, E.; Aminake, M.N.; et al. Synthesis and biological evaluation of papain-family cathepsin L-like cysteine protease inhibitors containing a 1,4-benzodiazepine scaffold as antiprotozoal agents. ChemMedChem 2014, 9, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Tamborini, L.; Angelo, I.C.; Grasso, S.; Schirmeister, T.; Lo Presti, L.; De Micheli, C.; Pinto, A.; Conti, P. Development of rhodesain inhibitors with a 3-bromoisoxazoline warhead. ChemMedChem 2013, 8, 2070–2076. [Google Scholar] [CrossRef]
- Ettari, R.; Micale, N.; Grazioso, G.; Bova, F.; Schirmeister, T.; Grasso, S.; Zappalà, M. Synthesis and molecular modeling studies of derivatives of a highly potent peptidomimetic vinyl ester as falcipain-2 inhibitors. ChemMedChem 2012, 7, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Zappalà, M.; Micale, N.; Schirmeister, T.; Gelhaus, C.; Leippe, M.; Evers, A.; Grasso, S. Synthesis of novel peptidomimetics as inhibitors of protozoan cysteine proteases falcipain-2 and rhodesain. Eur. J. Med. Chem. 2010, 45, 3228–3233. [Google Scholar] [CrossRef]
- Bova, F.; Ettari, R.; Micale, N.; Carnovale, C.; Schirmeister, T.; Gelhaus, C.; Leippe, M.; Grasso, S.; Zappalà, M. Constrained peptidomimetics as antiplasmodial falcipain-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 4928–4938. [Google Scholar] [CrossRef]
- Di Chio, C.; Previti, S.; Amendola, G.; Ravichandran, R.; Wagner, A.; Cosconati, S.; Hellmich, U.A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Development of novel dipeptide nitriles as inhibitors of rhodesain of Trypanosoma brucei rhodesiense. Eur. J. Med. Chem. 2022, 236, 114328. [Google Scholar] [CrossRef]
- Di Chio, C.; Previti, S.; De Luca, F.; Allegra, A.; Zappalà, M.; Ettari, R. Drug combination studies of PS-1 and quercetin against rhodesain of Trypanosoma brucei rhodesiense. Nat. Prod. Res. 2021, 36, 4282–4286. [Google Scholar] [CrossRef]
- Ettari, R.; Previti, S.; Di Chio, C.; Maiorana, S.; Allegra, A.; Schirmeister, T.; Zappalà, M. Drug synergism: Studies of combination of RK-52 and curcumin against rhodesain of Trypanosoma brucei rhodesiense. ACS Med. Chem. Lett. 2020, 11, 806–810. [Google Scholar] [CrossRef]
- Ettari, R.; Previti, S.; Maiorana, S.; Allegra, A.; Schirmeister, T.; Grasso, S.; Zappalà, M. Drug combination studies of curcumin and genistein against rhodesain of Trypanosoma brucei rhodesiense. Nat. Prod. Res. 2019, 33, 3577–3581. [Google Scholar] [CrossRef]
- Di Chio, C.; Previti, S.; De Luca, F.; Bogacz, M.; Zimmer, C.; Wagner, A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Drug combination studies of the dipeptide nitrile CD24 with curcumin: A new strategy to synergistically inhibit rhodesain of Trypanosoma brucei rhodesiense. Int. J. Mol. Sci. 2022, 23, 14470. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Kerr, I.D.; Wu, P.; Marion-Tsukamaki, R.; Mackey, Z.B.; Brinen, L.S. Crystal Structures of TbCatB and rhodesain, potential chemotherapeutic targets and major cysteine proteases of Trypanosoma brucei. PLoS Negl. Trop. Dis. 2010, 4, e701. [Google Scholar] [CrossRef] [PubMed]
- Pourkavoos, N. Unique risks, benefits, and challenges of developing drug-drug combination products in a pharmaceutical industrial setting. Comb. Prod. Ther. 2012, 2, 2. [Google Scholar] [CrossRef]
- Sisi, W.; Hongyong, Z.; Liang, C.; Christopher, E.; Chong-Xian, P.A.N. Analysis of the cytotoxic activity of carboplatin and gemcitabine combination. Anticancer Res. 2010, 30, 4573. [Google Scholar]
- Previti, S.; Ettari, R.; Calcaterra, E.; Di Maro, S.; Hammerschmidt, S.J.; Muller, C.; Ziebuhr, J.; Schirmeister, T.; Cosconati, S.; Zappalà, M. Structure-based lead optimization of peptide-based vinyl methyl ketones as SARS-CoV-2 main protease inhibitors. Eur. J. Med. Chem. 2023, 247, 115021. [Google Scholar] [CrossRef]
- Johe, P.; Jaenicke, E.; Neuweiler, H.; Schirmeister, T.; Kersten, C.; Hellmich, U.A. Structure, interdomain dynamics, and pH-dependent autoactivation of pro-rhodesain, the main lysosomal cysteine protease from African trypanosomes. J. Biol. Chem. 2021, 296, 100565. [Google Scholar] [CrossRef]

| Ki (nM) Rhodesain | Ki (nM) Cathepsin L | Selectivity Index (SI) | |
|---|---|---|---|
| CD24 | 16 ± 1.8 [29] | 34 ± 0.1 | 2.12 |
| CD34 | 27 ± 2.7 | 196 ± 22 | 7.26 |
| E-64 | 13 ± 0.2 | 30 ± 3 |
| Cmps | 1/32 × IC50 | 1/4 × IC50 | 1/2 × IC50 | IC50 | 2 × IC50 | 4 × IC50 |
|---|---|---|---|---|---|---|
| CD34 | 0.011 µM | 0.0875 µM | 0.175 µM | 0.35 µM | 0.7 µM | 1.4 µM |
| Curcumin | 0.76 µM | 6.125 µM | 12.25 µM | 24.5 µM | 49 µM | 98 µM |
| CD34 + Curcumin | 0.011 + 0.76 µM | 0.0875 + 6.125 µM | 0.175 + 12.25 µM | 0.35 + 24.5 µM | 0.7 + 49 µM | 1.4 + 98 µM |
| Fraction Affected (fa) | % of Rhodesain Inhibition | CI CD24 + Curcumin | Diagnosis of Combined Effect CD24+ Curcumin [33] | CI CD34 + Curcumin | Diagnosis of Combined Effect CD34+ Curcumin |
|---|---|---|---|---|---|
| 0.50 | 50% | 1.08 | Additive | 0.75 | Moderate Synergism |
| 0.60 | 60% | 0.93 | Slight Synergism | 0.55 | Synergism |
| 0.70 | 70% | 0.81 | Moderate Synergism | 0.40 | Synergism |
| 0.80 | 80% | 0.70 | Synergism | 0.27 | Strong Synergism |
| 0.90 | 90% | 0.59 | Synergism | 0.15 | Strong Synergism |
| 1 | 100% | 0.45 | Synergism | 0.03 | Very strong Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Chio, C.; Previti, S.; Totaro, N.; De Luca, F.; Allegra, A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Dipeptide Nitrile CD34 with Curcumin: A New Improved Combination Strategy to Synergistically Inhibit Rhodesain of Trypanosoma brucei rhodesiense. Int. J. Mol. Sci. 2023, 24, 8477. https://doi.org/10.3390/ijms24108477
Di Chio C, Previti S, Totaro N, De Luca F, Allegra A, Schirmeister T, Zappalà M, Ettari R. Dipeptide Nitrile CD34 with Curcumin: A New Improved Combination Strategy to Synergistically Inhibit Rhodesain of Trypanosoma brucei rhodesiense. International Journal of Molecular Sciences. 2023; 24(10):8477. https://doi.org/10.3390/ijms24108477
Chicago/Turabian StyleDi Chio, Carla, Santo Previti, Noemi Totaro, Fabiola De Luca, Alessandro Allegra, Tanja Schirmeister, Maria Zappalà, and Roberta Ettari. 2023. "Dipeptide Nitrile CD34 with Curcumin: A New Improved Combination Strategy to Synergistically Inhibit Rhodesain of Trypanosoma brucei rhodesiense" International Journal of Molecular Sciences 24, no. 10: 8477. https://doi.org/10.3390/ijms24108477
APA StyleDi Chio, C., Previti, S., Totaro, N., De Luca, F., Allegra, A., Schirmeister, T., Zappalà, M., & Ettari, R. (2023). Dipeptide Nitrile CD34 with Curcumin: A New Improved Combination Strategy to Synergistically Inhibit Rhodesain of Trypanosoma brucei rhodesiense. International Journal of Molecular Sciences, 24(10), 8477. https://doi.org/10.3390/ijms24108477







