Genome-Wide Characterization and Expression of the Hsf Gene Family in Salvia miltiorrhiza (Danshen) and the Potential Thermotolerance of SmHsf1 and SmHsf7 in Yeast
Abstract
1. Introduction
2. Results
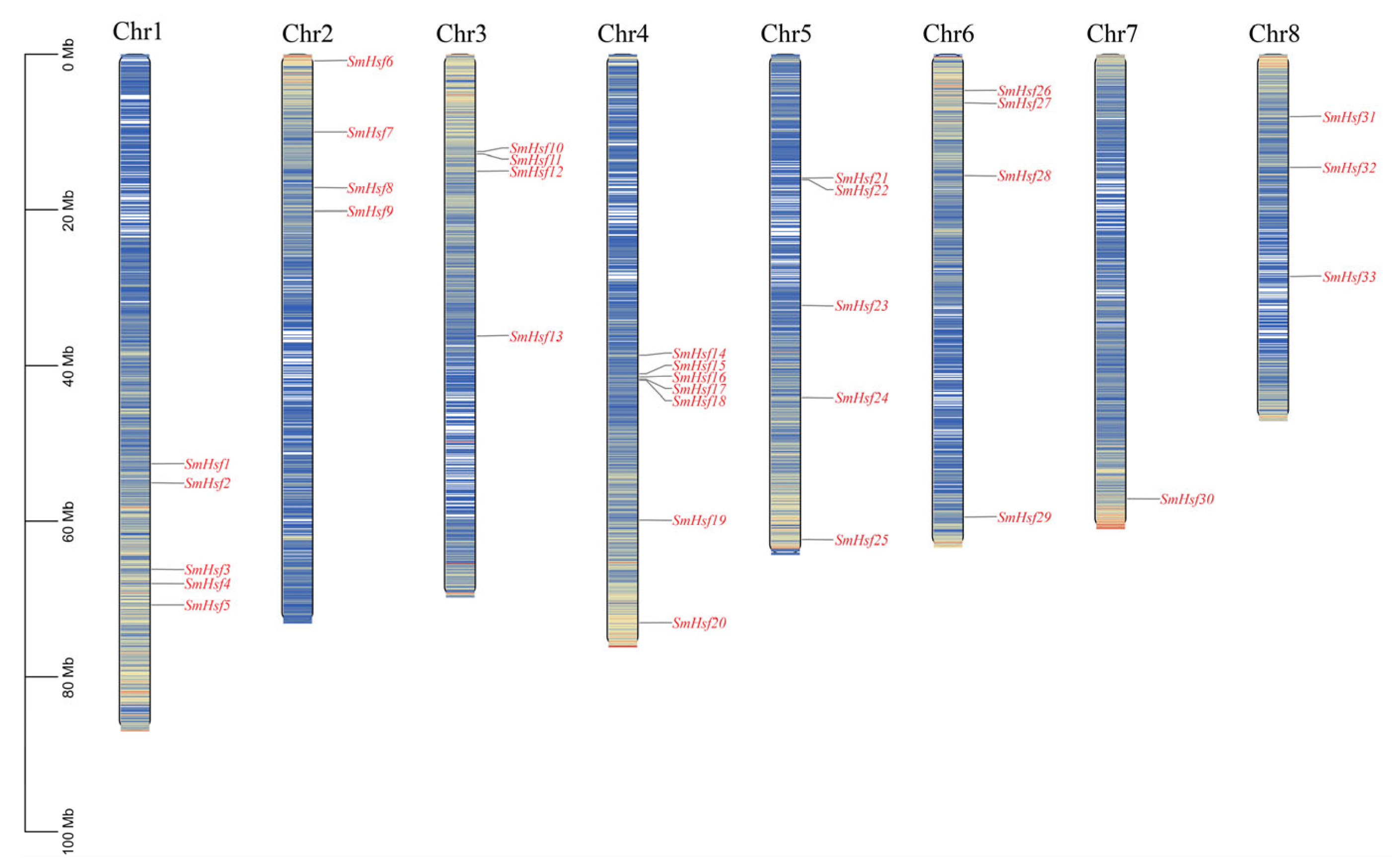
2.1. Identification and Chromosomal Distribution of Hsf Genes in S. miltiorrhiza
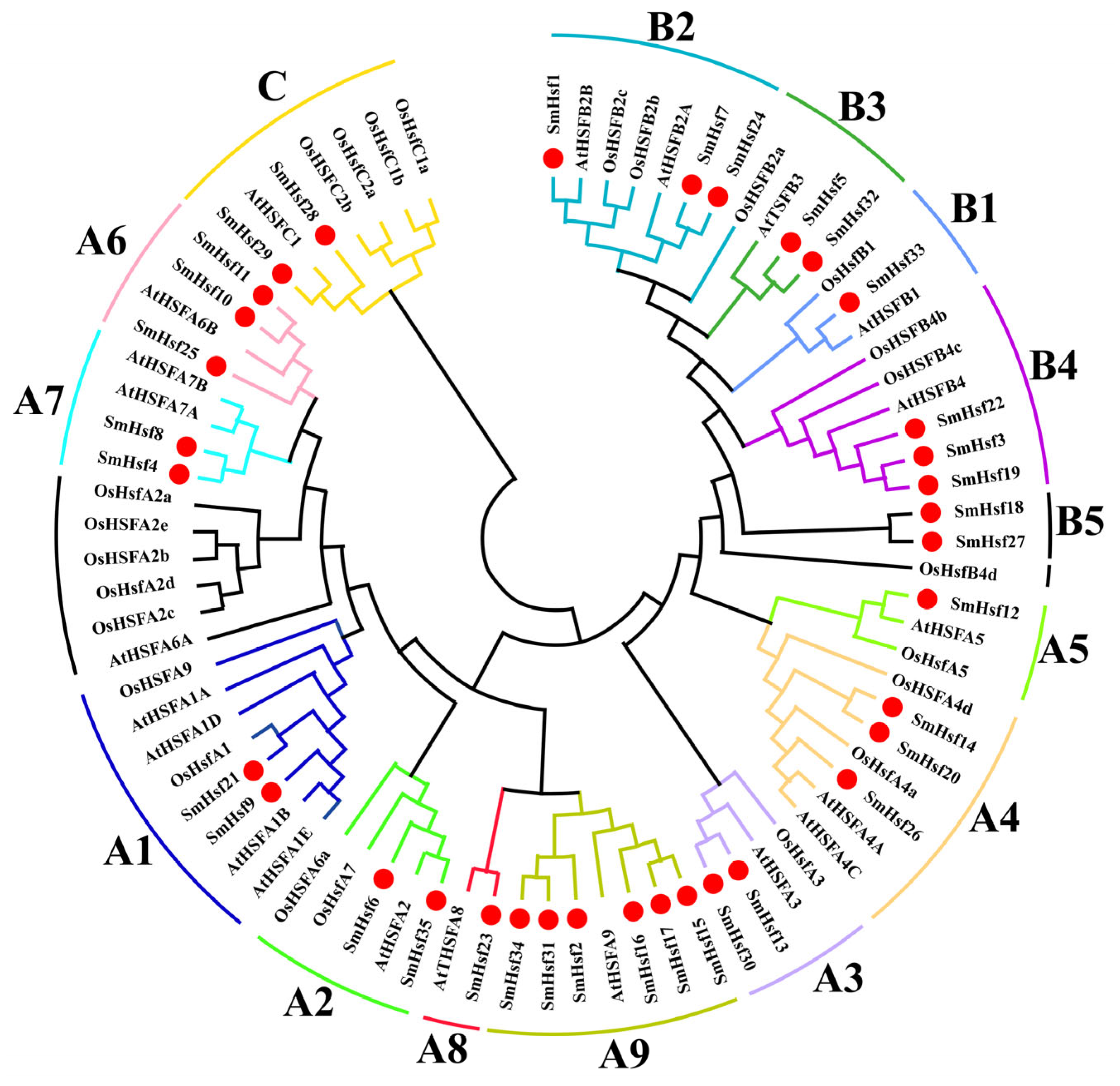
2.2. Phylogenetic and Classification Analyses of SmHsfs
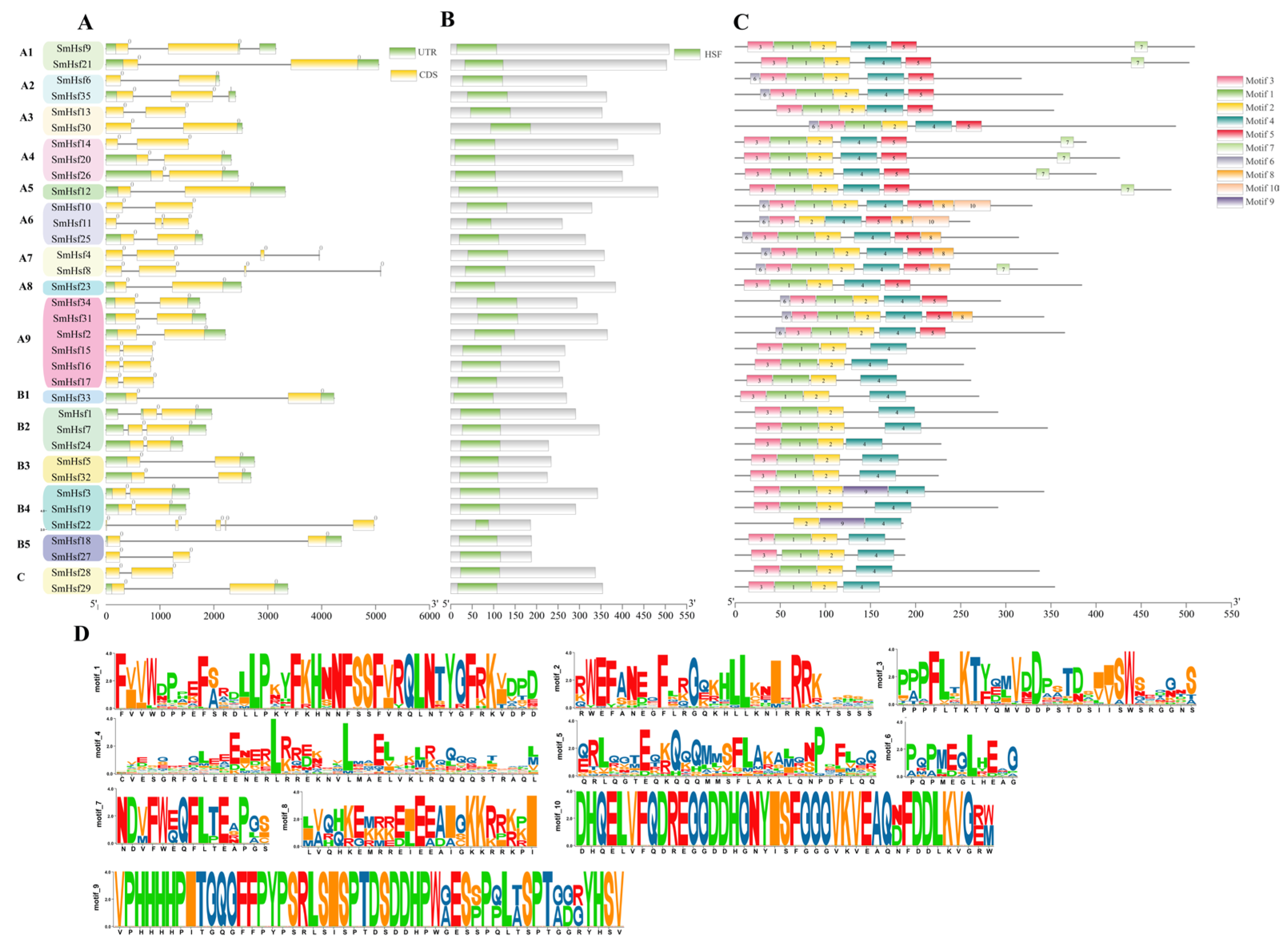
2.3. Gene Structure, Conserved Domains and Motif Analyses
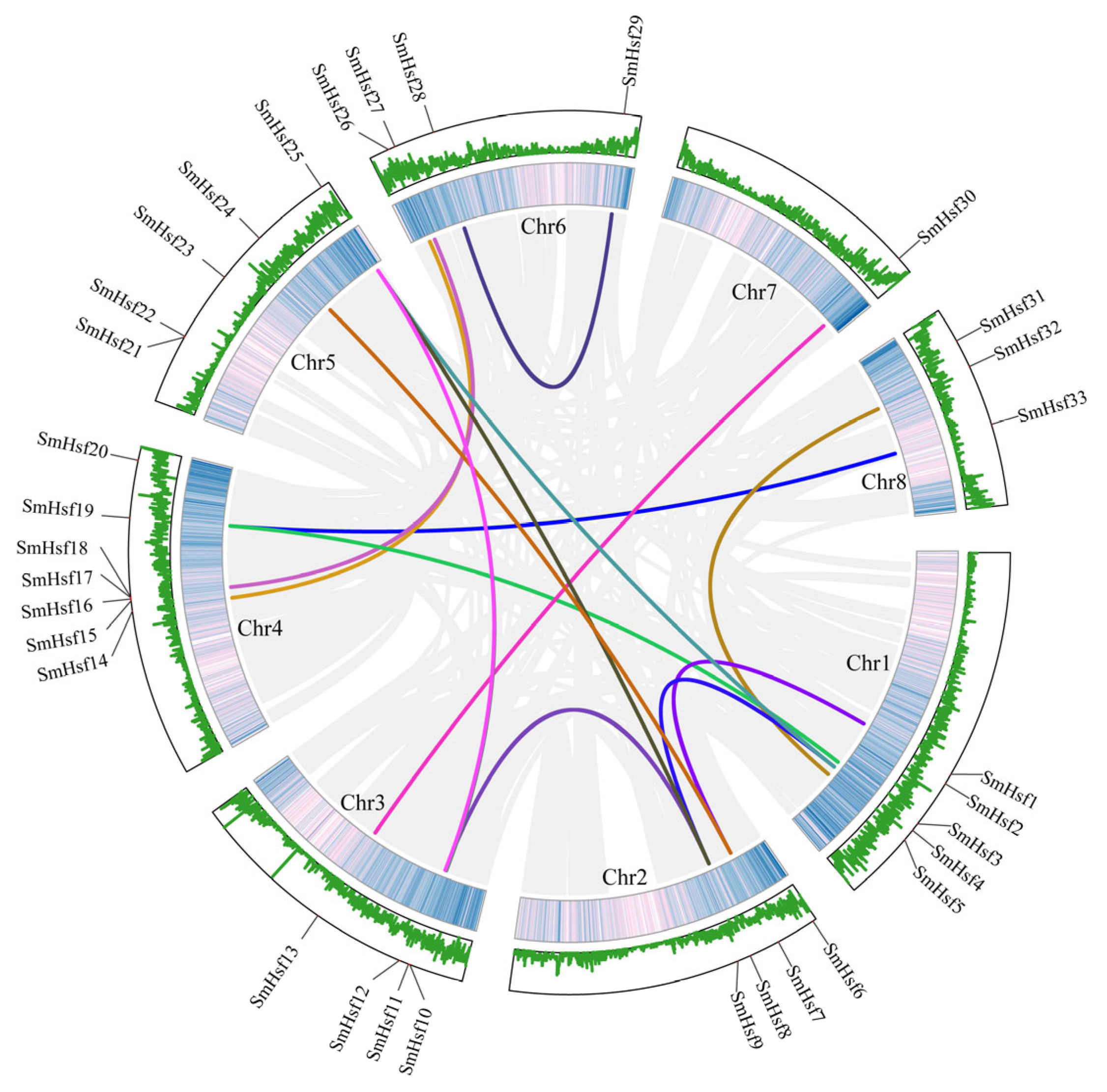
2.4. Gene Duplication and Synteny Analyses of Hsf Genes in S. miltiorrhiza
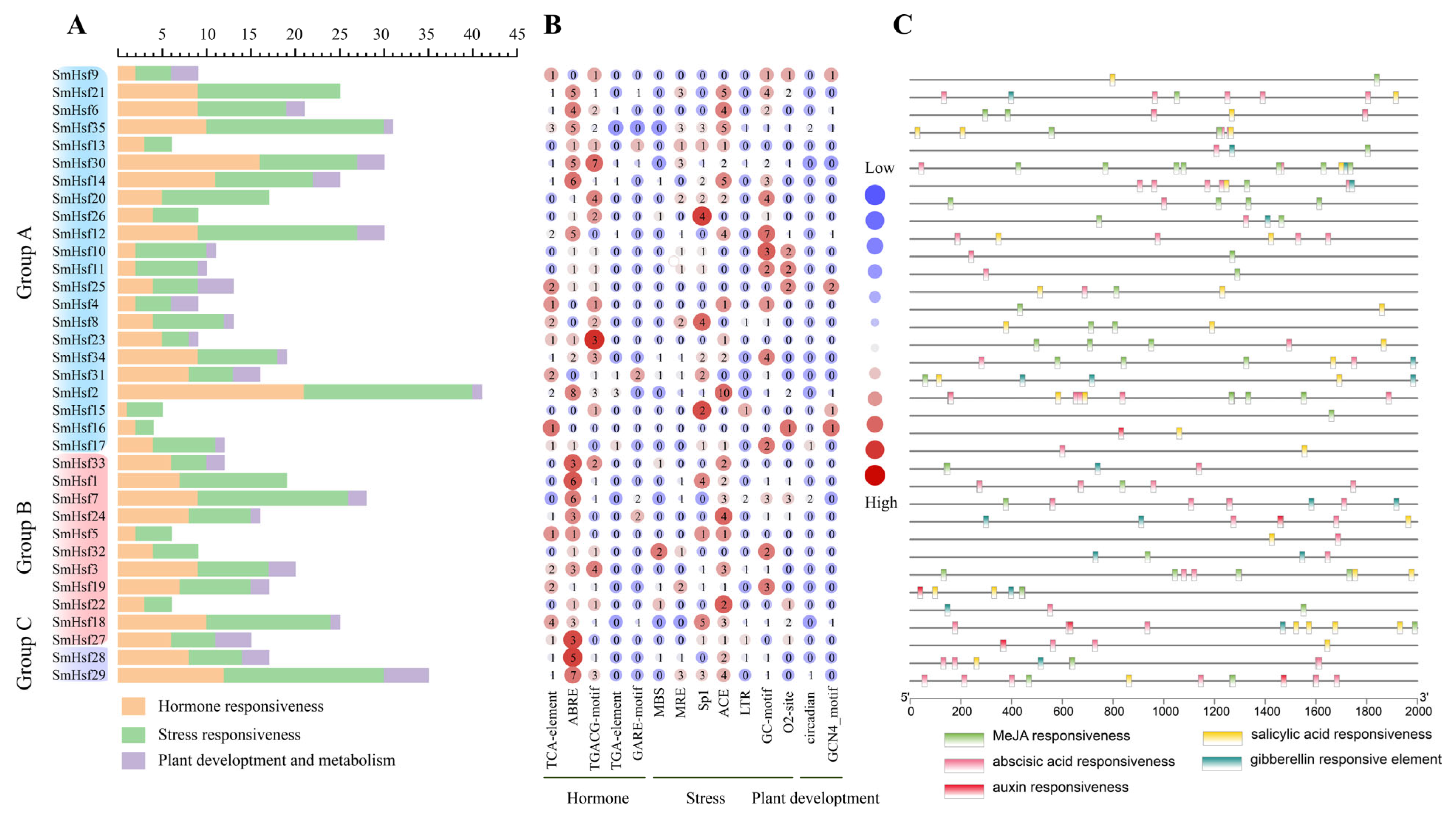
2.5. Analysis of Cis-Acting Elements and Upstream Regulators of SmHsfs
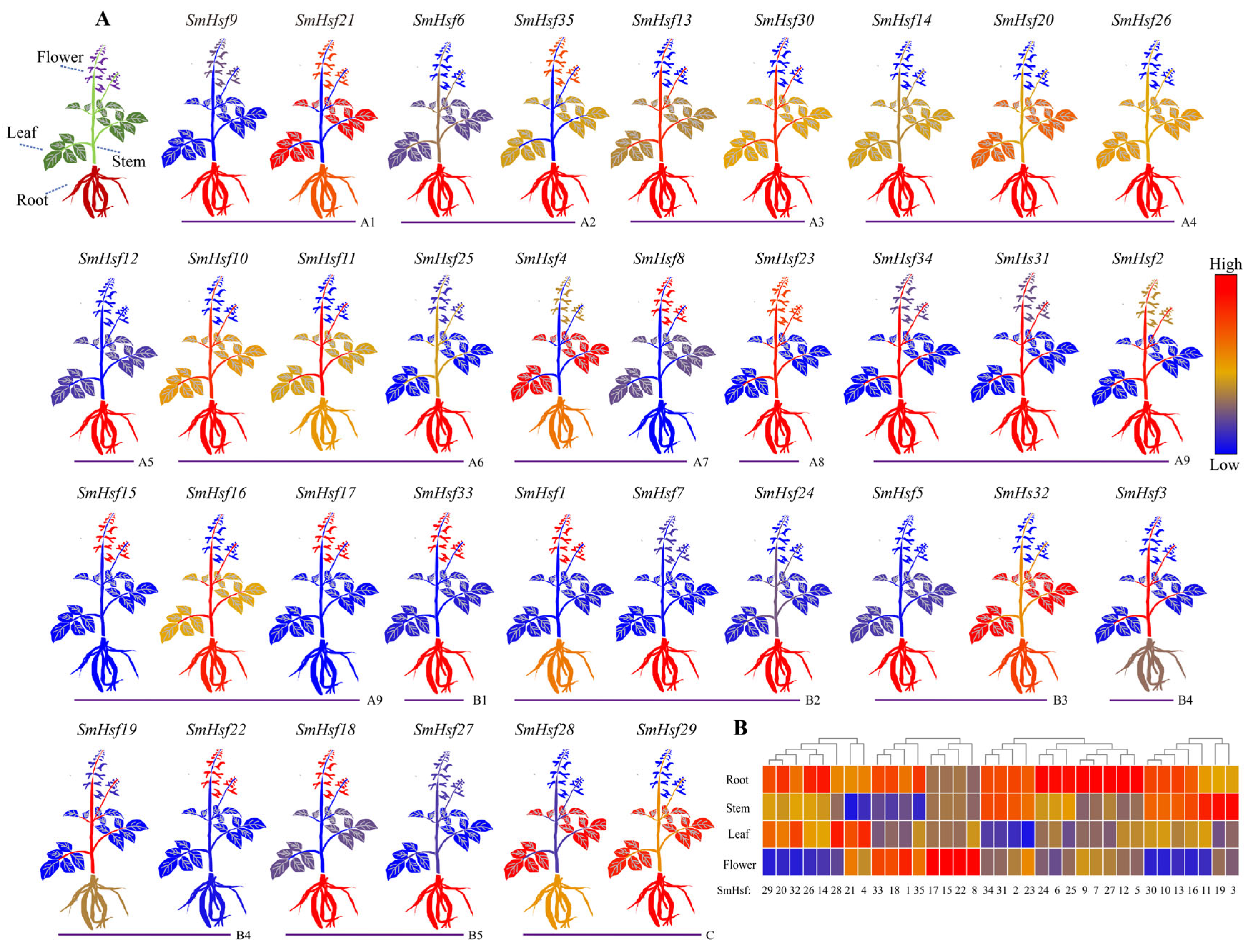
2.6. Expression Patterns of SmHsfs in S. miltiorrhiza Organs
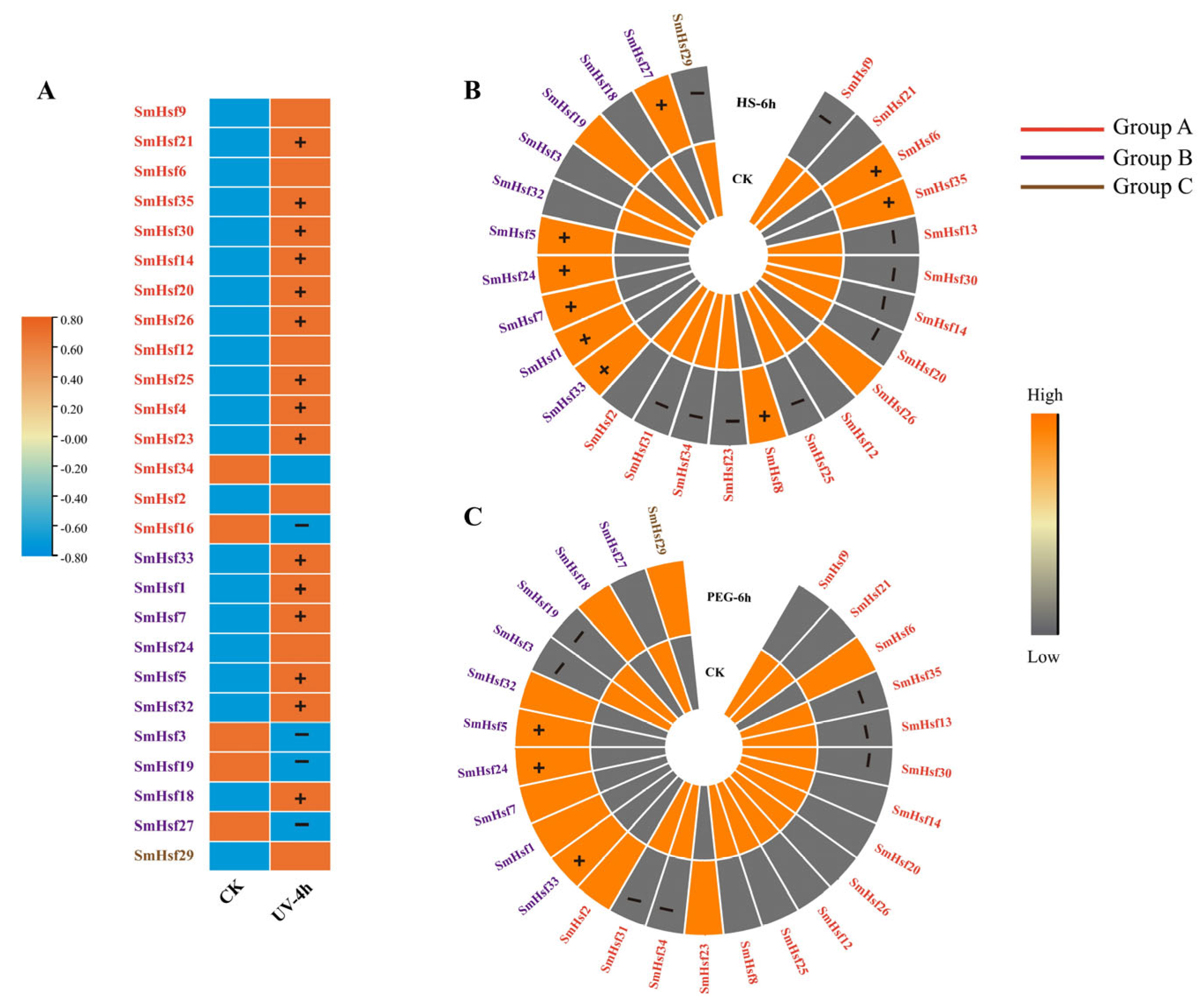
2.7. Expression Patterns of SmHsfs in Response to Hormone Treatment
2.8. Expression Patterns of SmHsfs in Response to Various Abiotic Stresses
2.9. Overexpression of SmHsf1 and SmHsf7 Enhanced Thermotolerance in Yeast Recombinant Cells
3. Materials and Methods
3.1. Identification and Sequence Analysis of Hsf Genes from S. miltiorrhiza
3.2. Duplication and Synteny Analysis of Hsf Genes
3.3. Plant Growth Conditions and Plant Stress Treatments
3.4. Transcriptome Analysis of SmHsfs Expression Patterns in Different Organs and under Abiotic Treatments
3.5. Subcellular Localization
3.6. Thermotolerance Analysis of Transgenic Yeast
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, K.B.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress re-sponses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 211–234. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Mesihovic, A.; Ullrich, S.; Rosenkranz, R.R.; Gebhardt, P.; Bublak, D.; Eich, H.; Weber, D.; Berberich, T.; Scharf, K.-D.; Schleiff, E.; et al. HsfA7 coordinates the transition from mild to strong heat stress response by controlling the activity of the master regulator HsfA1a in tomato. Cell Rep. 2022, 38, 110224. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Wolters, H.; Jürgens, G. Survival of the Flexible: Hormonal Growth Control and Adaptation in Plant Development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Schultheiss, J.; Kunert, O.; Gase, U.; Scharf, K.-D.; Nover, L.; Rüterjans, H. Solution Structure of the DNA-Binding Domain of the Tomato Heat-Stress Transcription Factor HSF24. Eur. J. Bio-Chem. 1996, 236, 911–921. [Google Scholar] [CrossRef]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2020, 72, 1558–1575. [Google Scholar] [CrossRef]
- Peteranderl, R.; Rabenstein, M.; Shin, Y.-K.; Liu, C.W.; Wemmer, D.E.; King, D.S.; Nelson, H.C.M. Biochemical and Biophysical Characterization of the Trimerization Domain from the Heat Shock Transcription Factor. Biochemistry 1999, 38, 3559–3569. [Google Scholar] [CrossRef]
- Nover, L.; Scharf, K.-D.; Gagliardi, D.; Vergne, P.; Czarnecka-Verner, E.; Gurley, W.B. The Hsf world: Classification and properties of plant heat stress transcription factors. Cell Stress Chaperones 1996, 1, 215. [Google Scholar] [CrossRef]
- Kotak, S.; Port, M.; Ganguli, A.; Bicker, F.; von Koskull-Döring, P. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature com-bination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular lo-calization. Plant J. 2004, 39, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Verner, E.; Pan, S.; Salem, T.; Gurley, W.B. Plant class B HSFs inhibit transcription and exhibit affinity for TFIIB and TBP. Plant Mol. Biol. 2004, 56, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Heerklotz, D.; Döring, P.; Bonzelius, F.; Winkelhaus, S.; Nover, L. The Balance of Nuclear Import and Export Determines the Intracellular Distribution and Function of Tomato Heat Stress Transcription Factor HsfA2. Mol. Cell. Biol. 2001, 21, 1759–1768. [Google Scholar] [CrossRef]
- Lyck, R.; Harmening, U.; Höhfeld, I.; Treuter, E.; Scharf, K.-D.; Nover, L. Intracellular distribution and identification of the nuclear localization signals of two plant heat-stress transcription factors. Planta 1997, 202, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.-D. In the com-plex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotol-erance in tomato. Genes Dev. 2002, 16, 1555–1567. [Google Scholar] [CrossRef]
- Busch, W.; Wunderlich, M.; Schöffl, F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 2005, 41, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shigeoka, S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006, 48, 535–547. [Google Scholar] [CrossRef]
- Nishizawa-Yokoi, A.; Nosaka, R.; Hayashi, H.; Tainaka, H.; Maruta, T.; Tamoi, M.; Ikeda, M.; Ohme-Takagi, M.; Yoshimura, K.; Yabuta, Y.; et al. HsfA1d and HsfA1e Involved in the Transcriptional Regulation of HsfA2 Function as Key Regulators for the Hsf Signaling Network in Response to Environmental Stress. Plant Cell Physiol. 2011, 52, 933–945. [Google Scholar] [CrossRef]
- Chan-Schaminet, K.Y.; Baniwal, S.K.; Bublak, D.; Nover, L.; Scharf, K.-D. Specific Interaction between Tomato HsfA1 and HsfA2 Creates Hetero-oligomeric Superactivator Complexes for Synergistic Activation of Heat Stress Gene Expression. J. Biol. Chem. 2009, 284, 20848–20857. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Carranco, R.; Hiratsu, K.; Ohme-Takagi, M.; Jordano, J. Loss of function of the HSFA9 seed longevity program. Plant Cell Environ. 2010, 33, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; Liao, H.-T.; Charng, Y.-Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.; Groß-Hardt, R.; Schöffl, F. Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol. Biol. 2014, 85, 541–550. [Google Scholar] [CrossRef]
- Yin, X.; Fan, H.; Chen, Y.; Li, L.; Song, W.; Fan, Y.; Zhou, W.; Ma, G.; Alolga, R.N.; Li, W.; et al. Integrative omic and transgenic analyses reveal the positive effect of ultraviolet-B irradiation on salvianolic acid biosynthesis through upregulation of SmNAC1. Plant J. 2020, 104, 781–799. [Google Scholar] [CrossRef]
- Sui, C. Salvia miltiorrhiza Resources, Cultivation, and Breeding. In The Salvia Miltiorrhiza Genome; Lu, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 17–32. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Wang, Y.-P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef]
- Song, Z.; Lin, C.; Xing, P.; Fen, Y.; Jin, H.; Zhou, C.; Gu, Y.Q.; Wang, J.; Li, X. A high-quality reference genome sequence of Salvia miltiorrhiza provides insights into tanshinone synthesis in its red rhizomes. Plant Genome 2020, 13, e20041. [Google Scholar] [CrossRef]
- Maher, C.; Stein, L.; Ware, D. Evolution of Arabidopsis microRNA families through duplication events. Genome Res. 2006, 16, 510–519. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246–247, 153135. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Huang, Y.; Li, B.; Ma, W.; Wang, D.; Cao, X.; Wang, Z. Genome-Wide Identification of the TIFY Family in Salvia miltiorrhiza Reveals That SmJAZ3 Interacts With SmWD40-170, a Relevant Protein That Modulates Secondary Metabolism and Development. Front. Plant Sci. 2021, 12, 630424. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Ji, Q.; Wang, C.; Luo, L.; Yuan, Y.; Wang, Y.; Wang, J. Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J. Genet. Genom. 2008, 35, 105–118. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Jiang, H.-Y.; Chu, Z.-X.; Tang, X.-L.; Zhu, S.-W.; Cheng, B.-J. Genome-wide identification, classification and analysis of heat shock transcription factor family in maize. BMC Genom. 2011, 12, 76. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Wasternack, C.; Xie, D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014, 21, 112–119. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Rose, A.B. Intron-Mediated Regulation of Gene Expression. In Nuclear Pre-MRNA Processing in Plants; Reddy, A.S.N., Golovkin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 277–290. [Google Scholar] [CrossRef]
- Todd, A.E.; Orengo, C.A.; Thornton, J.M. Evolution of function in protein superfamilies, from a struc-tural perspective. J. Mol. Biol. 2001, 307, 1113–1143. [Google Scholar] [CrossRef]
- Demuth, J.P.; Hahn, M.W. The life and death of gene families. Bioessays 2009, 31, 29–39. [Google Scholar] [CrossRef]
- Achaz, G.; Coissac, E.; Viari, A.; Netter, P. Analysis of Intrachromosomal Duplications in Yeast Saccharomyces cerevisiae: A Possible Model for Their Origin. Mol. Biol. Evol. 2000, 17, 1268–1275. [Google Scholar] [CrossRef]
- Blanc, G.; Hokamp, K.; Wolfe, K.H. A recent polyploidy superimposed on older large-scale duplica-tions in the Arabidopsis genome. Genome Res. 2003, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Friedman, R.; Ekollu, V.; Rose, J.R. Non-random association of transposable elements with duplicated genomic blocks in Arabidopsis thaliana. Mol. Phylogenet. Evol. 2003, 29, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; De Bodt, S.; Raes, J.; Casneuf, T.; Van Montagu, M.; Kuiper, M.; Van de Peer, Y. Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 5454–5459. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, A.; Piconese, S.; Tassone, P.; Ferrari, S.; Migliaccio, F. A new mutant of Arabidopsis disturbed in its roots, right-handed slanting, and gravitropism defines a gene that encodes a heat-shock factor. J. Exp. Bot. 2008, 59, 1363–1374. [Google Scholar] [CrossRef]
- Tan, B.; Yan, L.; Li, H.; Lian, X.; Cheng, J.; Wang, W.; Zheng, X.; Wang, X.; Li, J.; Ye, X.; et al. Ge-nome-wide identification of HSF family in peach and functional analysis of PpHSF5 involvement in root and aerial organ development. PeerJ 2021, 9, e1096. [Google Scholar] [CrossRef]
- Li, W.; Wan, X.-L.; Yu, J.-Y.; Wang, K.-L.; Zhang, J. Genome-Wide Identification, Classification, and Expression Analysis of the Hsf Gene Family in Carnation (Dianthus caryophyllus). Int. J. Mol. Sci. 2019, 20, 5233. [Google Scholar] [CrossRef]
- Ogawa, D.; Yamaguchi, K.; Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007, 58, 3373–3383. [Google Scholar] [CrossRef]
- Yokotani, N.; Ichikawa, T.; Kondou, Y.; Matsui, M.; Hirochika, H.; Iwabuchi, M.; Oda, K. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta 2008, 227, 957–967. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis HsfB1 and HsfB2b Act as Repressors of the Expression of Heat-Inducible Hsfs but Positively Regulate the Acquired Thermotolerance. Plant Physiol. 2011, 157, 1243–1254. [Google Scholar] [CrossRef]
- Bharti, K.; von Koskull-Döring, P.; Bharti, S.; Kumar, P.; Tintschl-Körbitzer, A.; Treuter, E.; Nover, L. Tomato Heat Stress Transcription Factor HsfB1 Represents a Novel Type of General Transcription Coactivator with a Histone-Like Motif Interacting with the Plant CREB Binding Protein Ortholog HAC1. Plant Cell 2004, 16, 1521–1535. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, C.; Zhang, L.; Liu, H.; Yu, J.; Hu, Z.; Hua, W. Systematic Analysis of Hsf Family Genes in the Brassica napus Genome Reveals Novel Responses to Heat, Drought and High CO2 Stresses. Front. Plant Sci. 2017, 8, 1174. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, R.; Wang, S.; Wang, X.; Peng, J.; Guo, J.; Cui, G.; Chen, M.; Mu, J.; Lai, C.; Huang, L.; et al. Genome-Wide Characterization and Expression of the Hsf Gene Family in Salvia miltiorrhiza (Danshen) and the Potential Thermotolerance of SmHsf1 and SmHsf7 in Yeast. Int. J. Mol. Sci. 2023, 24, 8461. https://doi.org/10.3390/ijms24108461
Qu R, Wang S, Wang X, Peng J, Guo J, Cui G, Chen M, Mu J, Lai C, Huang L, et al. Genome-Wide Characterization and Expression of the Hsf Gene Family in Salvia miltiorrhiza (Danshen) and the Potential Thermotolerance of SmHsf1 and SmHsf7 in Yeast. International Journal of Molecular Sciences. 2023; 24(10):8461. https://doi.org/10.3390/ijms24108461
Chicago/Turabian StyleQu, Renjun, Shiwei Wang, Xinxin Wang, Jiaming Peng, Juan Guo, Guanghong Cui, Meilan Chen, Jing Mu, Changjiangsheng Lai, Luqi Huang, and et al. 2023. "Genome-Wide Characterization and Expression of the Hsf Gene Family in Salvia miltiorrhiza (Danshen) and the Potential Thermotolerance of SmHsf1 and SmHsf7 in Yeast" International Journal of Molecular Sciences 24, no. 10: 8461. https://doi.org/10.3390/ijms24108461
APA StyleQu, R., Wang, S., Wang, X., Peng, J., Guo, J., Cui, G., Chen, M., Mu, J., Lai, C., Huang, L., Wang, S., & Shen, Y. (2023). Genome-Wide Characterization and Expression of the Hsf Gene Family in Salvia miltiorrhiza (Danshen) and the Potential Thermotolerance of SmHsf1 and SmHsf7 in Yeast. International Journal of Molecular Sciences, 24(10), 8461. https://doi.org/10.3390/ijms24108461




