Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells
Abstract
1. Introduction
2. Results
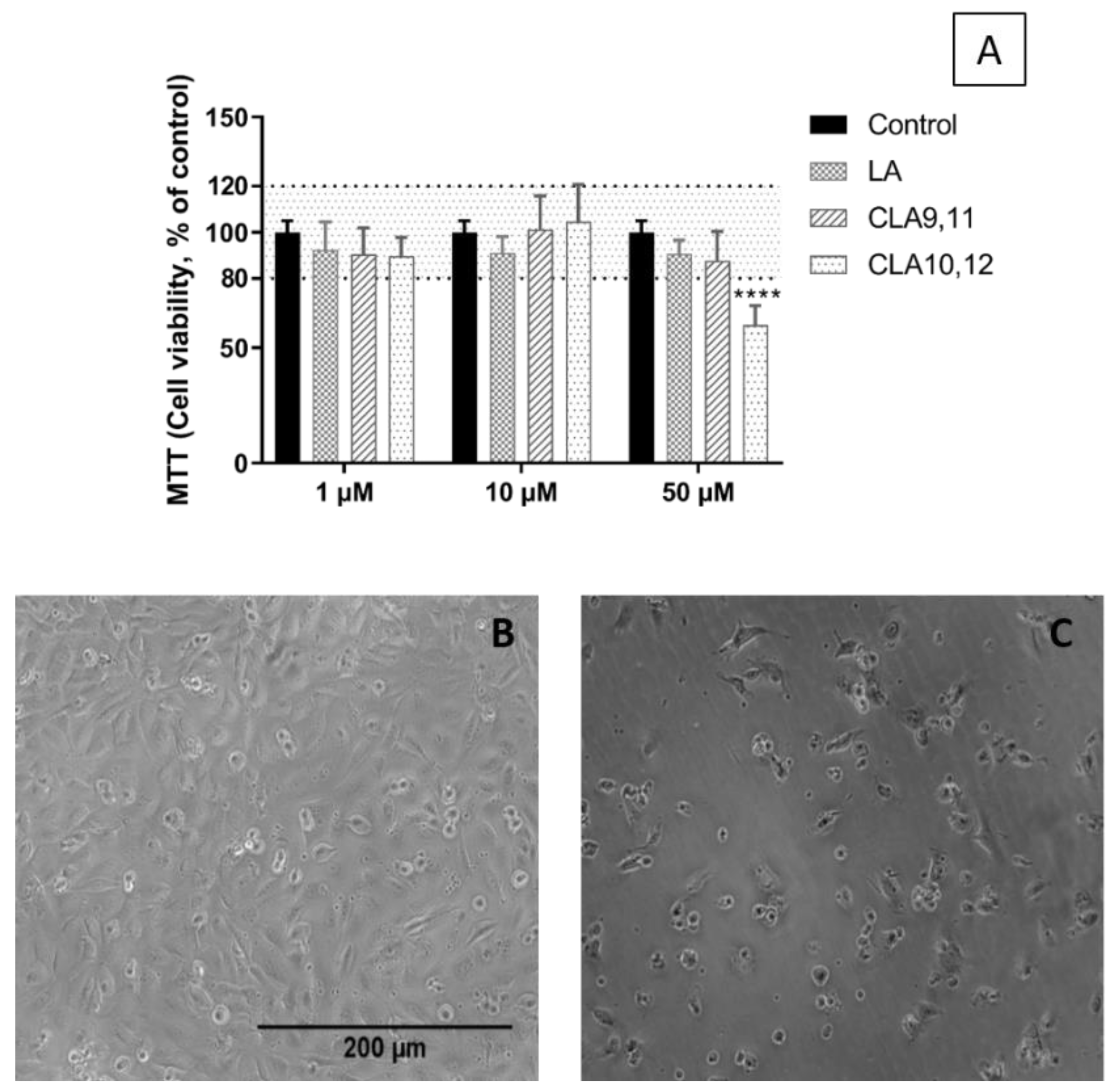
2.1. Viability of EA.hy926 Cells Incubated with FAs
2.2. FA Incorporation into EA.hy926 Cells
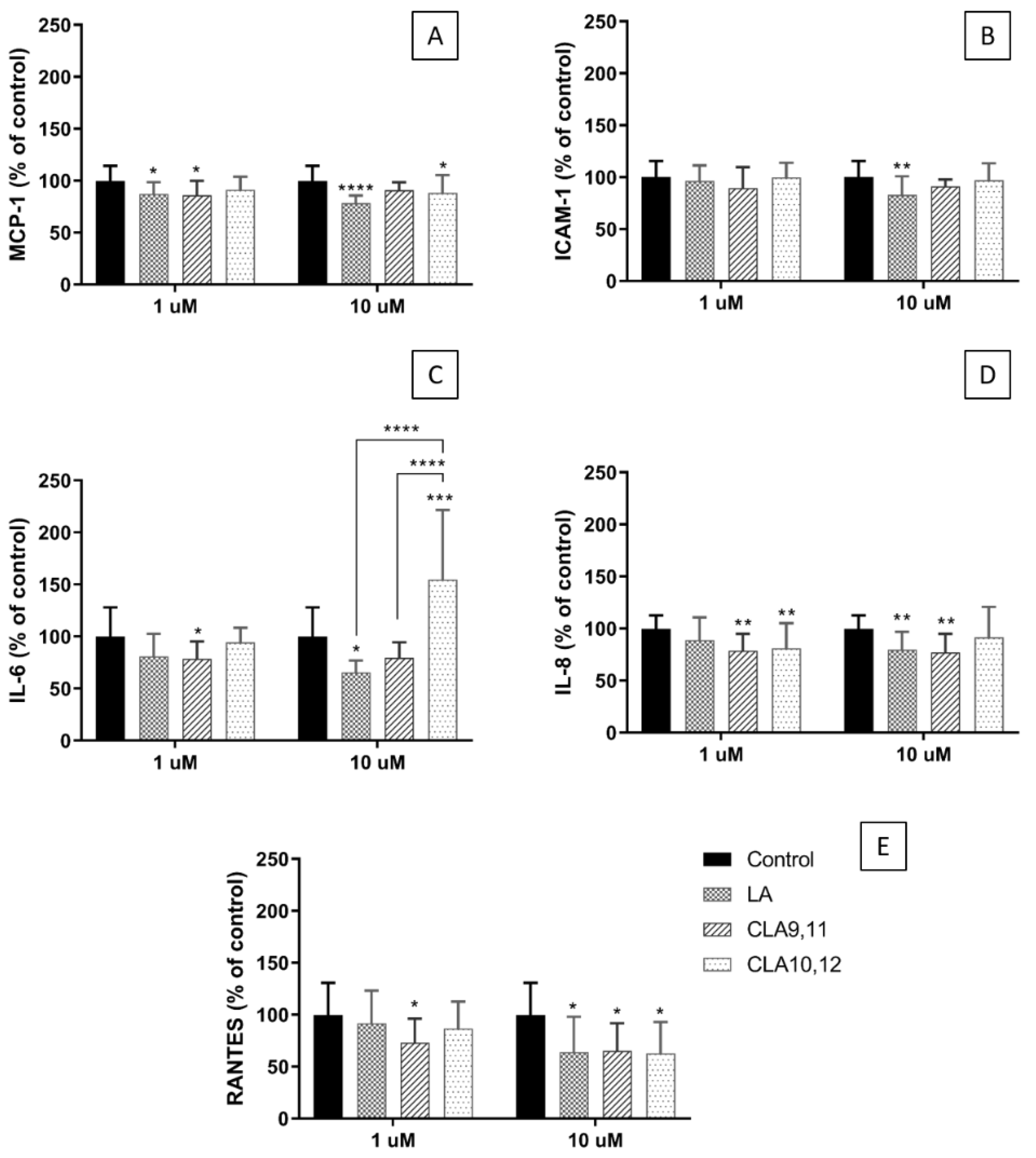
2.3. Effects of FAs on the Concentrations of Inflammatory Mediators in the Medium of Cultured ECs
2.4. Effects of FAs on the Expression of Inflammation-Related Genes
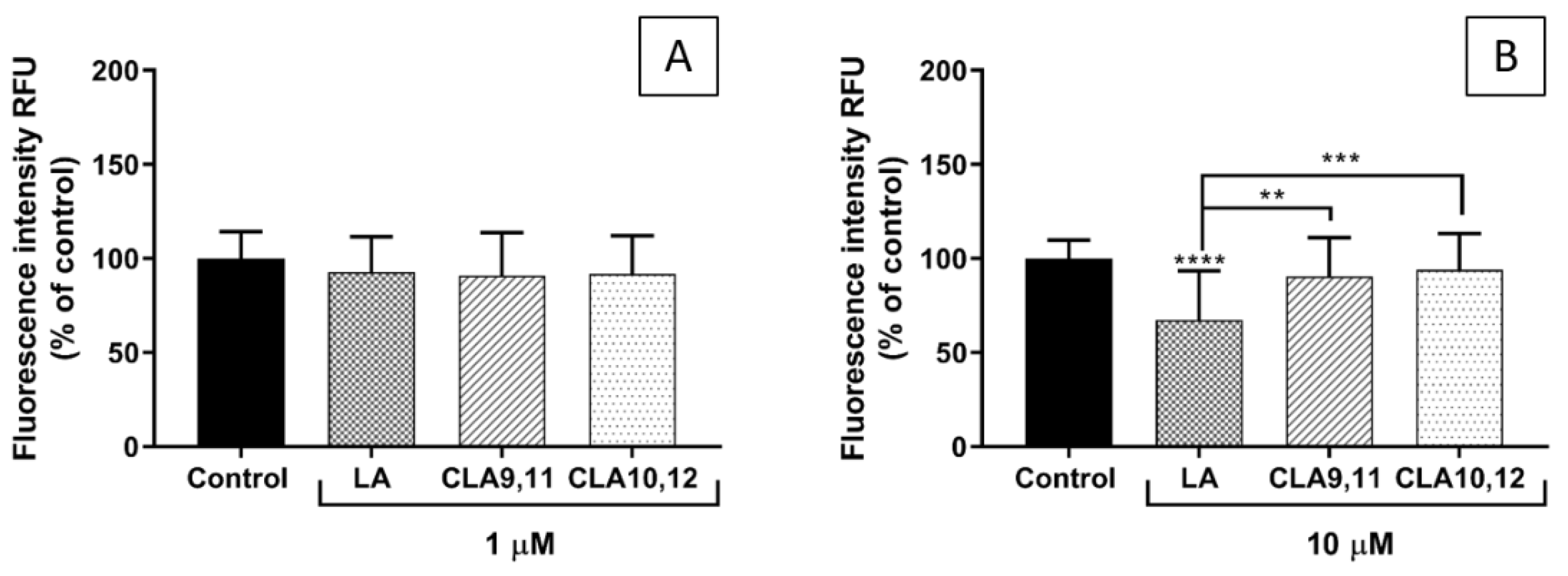
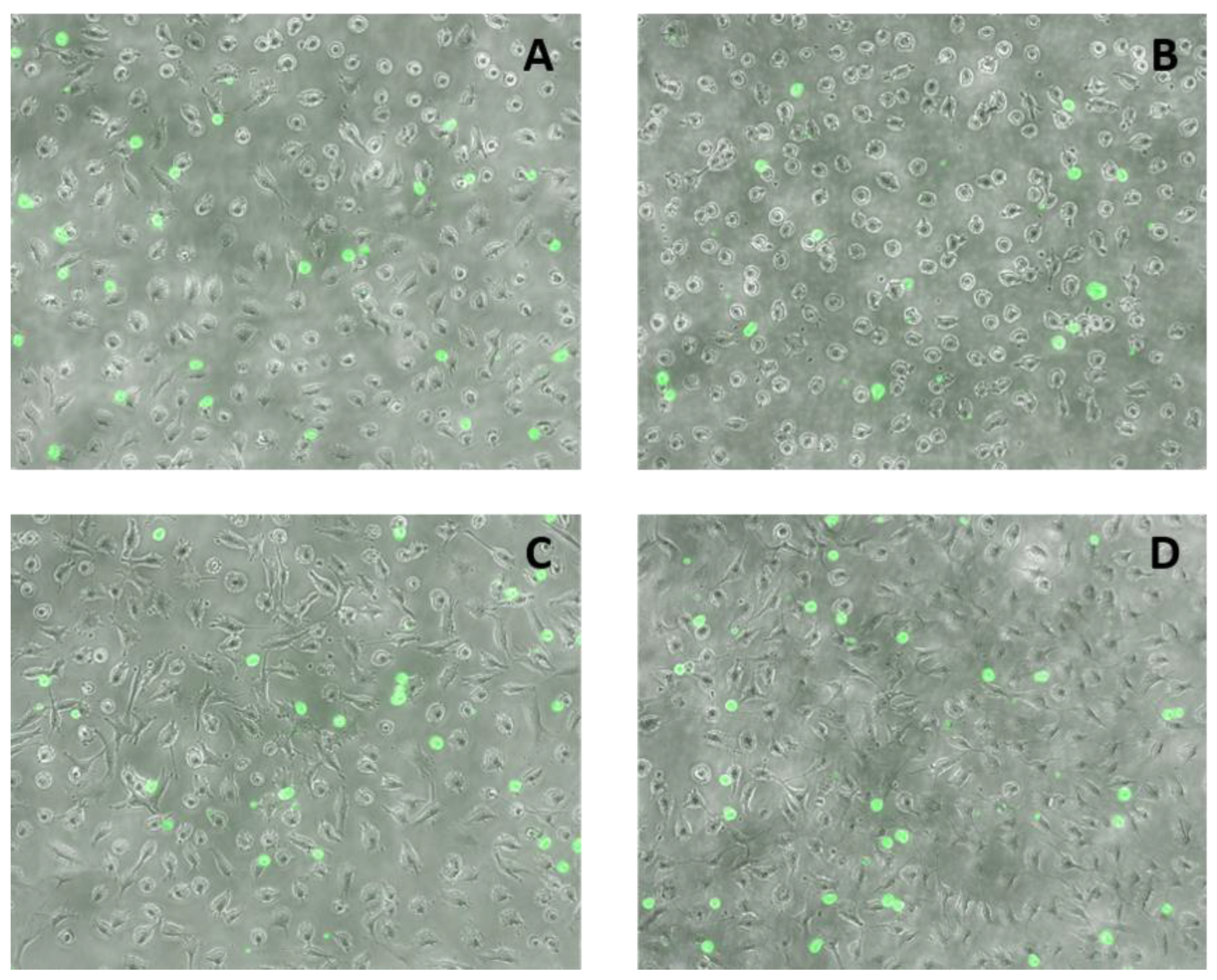
2.5. Effects of FAs on THP-1 Adhesion to EA.hy926 Cells
3. Discussion
4. Materials and Methods
4.1. Endothelial Cells
4.2. Fatty Acid Treatment
4.3. MTT Assay for Cell Viability
4.4. Fatty Acid Composition Measurement by Gas Chromatography
4.5. Measurement of Inflammatory Mediators in Cell Culture Supernatants Using Multiplex Magnetic ELISA
4.6. RNA Isolation, cDNA Synthesis, and Real-Time PCR
4.7. Adhesion of THP-1 Monocytes to ECs
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Liu, B.; Deng, Z.; Fan, Y.; Li, J.; Li, H. Lipid rafts promote trans fatty acid-induced inflammation in human umbilical vein endothelial cells. Lipids 2017, 52, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Bilodeau, J.F.; Larose, J.; Greffard, K.; Julien, P.; Barbier, O.; Rudkowska, I. Modulation of the biomarkers of inflammation and oxidative stress by ruminant trans fatty acids and dairy proteins in vascular endothelial cells (HUVEC). Prostaglandins Leukot. Essent. Fat. Acids 2017, 126, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.; Julien, P.; Bilodeau, J.F.; Barbier, O.; Rudkowska, I. Trans fatty acids suppress TNF-alpha-induced inflammatory gene expression in endothelial (HUVEC) and hepatocellular carcinoma (HepG2) cells. Lipids 2017, 52, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.A.; Baker, E.J.; De Souza, C.O.; Miles, E.A.; Calder, P.C. Differential effects of ruminant and industrial 18-carbon trans-monounsaturated fatty acids (trans vaccenic and elaidic) on the inflammatory responses of an endothelial cell line. Molecules 2021, 26, 5834. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef]
- Baker, E.J.; Valenzuela, C.A.; van Dooremalen, W.T.M.; Martínez-Fernández, L.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Gamma-linolenic and pinolenic acids exert anti-inflammatory effects in cultured human endothelial cells through their elongation products. Mol. Nutr. Food Res. 2020, 64, e2000382. [Google Scholar] [CrossRef]
- Ha, Y.L.; Grimm, N.K.; Pariza, M.W. Anticarcinogens from fried ground beef: Heat-altered derivatives of linoleic acid. Carcinogenesis 1987, 8, 1881–1887. [Google Scholar] [CrossRef]
- Lee, K.N.; Kritchevsky, D.; Pariza, M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Park, Y.; Storkson, J.M.; Albright, K.J.; Liu, W.; Pariza, M.W. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 1999, 34, 235–241. [Google Scholar] [CrossRef]
- Yu, Y.; Correll, P.H.; Vanden Heuvel, J.P. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: Evidence for a PPARγ-dependent mechanism. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2002, 1581, 89–99. [Google Scholar] [CrossRef]
- Bruen, R.; Curley, S.; Kajani, S.; Lynch, G.; O’Reilly, M.E.; Dillon, E.T.; Fitzsimons, S.; Mthunzi, L.; McGillicuddy, F.C.; Belton, O. Different monocyte phenotypes result in proresolving macrophages in conjugated linoleic acid-induced attenuated progression and regression of atherosclerosis. FASEB J. 2019, 33, 11006–11020. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Jones, E.L.; Russell, J.J.; El-Khazen, S.; Moretti, E.; Hall, W.L.; Gerry, A.B.; Leake, D.S.; Grimble, R.F.; et al. Effects of dairy products naturally enriched with cis-9,trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am. J. Clin. Nutr. 2006, 83, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Grimble, R.F.; Williams, C.M.; Calder, P.C.; Yaqoob, P. Effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on immune cell function in healthy humans. Am. J. Clin. Nutr. 2004, 80, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Tricon, S.; Burdge, G.C.; Williams, C.M.; Calder, P.C.; Yaqoob, P. The effects of conjugated linoleic acid on human health-related outcomes. Proc. Nutr. Soc. 2005, 64, 171–182. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.M.; Eisenberg, B.L.; Lewis, L.D.; Froehlich, H.M.; Wells, W.A.; Eastman, A.; Kuemmerle, N.B.; Rosenkrantz, K.M.; Barth, R.J., Jr.; Schwartz, G.N.; et al. A proof of principle clinical trial to determine whether conjugated linoleic acid modulates the lipogenic pathway in human breast cancer tissue. Breast Cancer Res. Treat. 2013, 138, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mądry, E.; Malesza, I.J.; Subramaniapillai, M.; Czochralska-Duszyńska, A.; Walkowiak, M.; Miśkiewicz-Chotnicka, A.; Walkowiak, J.; Lisowska, A. Body fat changes and liver safety in obese and overweight women supplemented with conjugated linoleic acid: A 12-week randomised, double-blind, placebo-controlled trial. Nutrients 2020, 12, 1811. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Tso, P.; Czarnecki, S.K. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J. Am. Coll. Nutr. 2000, 19, 472s–477s. [Google Scholar] [CrossRef]
- Navarro, V.; Macarulla, M.T.; Fernández-Quintela, A.; Rodríguez, V.M.; Simón, E.; Portillo, M.P. Effects of trans-10,cis-12 conjugated linoleic acid on cholesterol metabolism in hypercholesterolaemic hamsters. Eur. J. Nutr. 2007, 46, 213–219. [Google Scholar] [CrossRef]
- Franczyk-Zarów, M.; Kostogrys, R.B.; Szymczyk, B.; Jawień, J.; Gajda, M.; Cichocki, T.; Wojnar, L.; Chlopicki, S.; Pisulewski, P.M. Functional effects of eggs, naturally enriched with conjugated linoleic acid, on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice (apoE/LDLR-/-). Br. J. Nutr. 2008, 99, 49–58. [Google Scholar] [CrossRef]
- Taylor, J.S.; Williams, S.R.; Rhys, R.; James, P.; Frenneaux, M.P. Conjugated linoleic acid impairs endothelial function. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 307–312. [Google Scholar] [CrossRef]
- Blankson, H.; Stakkestad, J.A.; Fagertun, H.; Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J. Nutr. 2000, 130, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Fielitz, K.; Laue, C.; Winkler, P.; Rubin, D.; Helwig, U.; Giller, K.; Kammann, J.; Schwedhelm, E.; Böger, R.H.; et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J. Am. Coll. Nutr. 2011, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Raff, M.; Straarup, E.M.; Lund, P.; Basu, S.; Bruun, J.M. An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in postmenopausal women. J. Nutr. 2008, 138, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Plantinga, Y.; de Roos, B.; Mennen, L.I.; Bots, M.L. Dietary supplementation with cis-9,trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am. J. Clin. Nutr. 2010, 91, 175–183. [Google Scholar] [CrossRef]
- Naumann, E.; Carpentier, Y.A.; Saebo, A.; Lassel, T.S.; Chardigny, J.M.; Sébédio, J.L.; Mensink, R.P. Cis-9, trans- 11 and trans-10, cis-12 conjugated linoleic acid (CLA) do not affect the plasma lipoprotein profile in moderately overweight subjects with LDL phenotype B. Atherosclerosis 2006, 188, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Moloney, F.; Nugent, A.P.; Doyle, L.; Cashman, K.D.; Roche, H.M. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. J. Nutr. Biochem. 2007, 18, 658–666. [Google Scholar] [CrossRef]
- Noone, E.J.; Roche, H.M.; Nugent, A.P.; Gibney, M.J. The effect of dietary supplementation using isomeric blends of conjugated linoleic acid on lipid metabolism in healthy human subjects. Br. J. Nutr. 2002, 88, 243–251. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Jones, E.L.; Grimble, R.F.; Williams, C.M.; Yaqoob, P.; Calder, P.C. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am. J. Clin. Nutr. 2004, 80, 614–620. [Google Scholar] [CrossRef]
- Raff, M.; Tholstrup, T.; Basu, S.; Nonboe, P.; Sorensen, M.T.; Straarup, E.M. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J. Nutr. 2008, 138, 509–514. [Google Scholar] [CrossRef]
- Sofi, F.; Buccioni, A.; Cesari, F.; Gori, A.M.; Minieri, S.; Mannini, L.; Casini, A.; Gensini, G.F.; Abbate, R.; Antongiovanni, M. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: A dietary intervention study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 117–124. [Google Scholar] [CrossRef]
- Penedo, L.A.; Nunes, J.C.; Gama, M.A.; Leite, P.E.; Quirico-Santos, T.F.; Torres, A.G. Intake of butter naturally enriched with cis9,trans11 conjugated linoleic acid reduces systemic inflammatory mediators in healthy young adults. J. Nutr. Biochem. 2013, 24, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.K.; Bůžková, P.; Matthan, N.R.; Djoussé, L.; Kizer, J.R.; Mukamal, K.J.; Polak, J.F.; Lichtenstein, A.H. Serum non-esterified fatty acids, carotid artery intima-media thickness and flow-mediated dilation in older adults: The Cardiovascular Health Study (CHS). Nutrients 2021, 13, 3052. [Google Scholar] [CrossRef] [PubMed]
- Goua, M.; Mulgrew, S.; Frank, J.; Rees, D.; Sneddon, A.A.; Wahle, K.W. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: Involvement of the transcription factor NF-kappaB? Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 33–43. [Google Scholar] [CrossRef]
- Stachowska, E.; Siennicka, A.; Baśkiewcz-Hałasa, M.; Bober, J.; Machalinski, B.; Chlubek, D. Conjugated linoleic acid isomers may diminish human macrophages adhesion to endothelial surface. Int. J. Food Sci. Nutr. 2012, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, A.A.; McLeod, E.; Wahle, K.W.; Arthur, J.R. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: Suppression by conjugated linoleic acid. Biochim. Biophys. Acta 2006, 1761, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Differential inflammatory responses in cultured endothelial cells exposed to two conjugated linoleic acids (CLAs) under a pro-inflammatory condition. Int. J. Mol. Sci. 2022, 23, 6101. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Chen, X. Interactions between nuclear receptors glucocorticoid receptor α and peroxisome proliferator-activated receptor α form a negative feedback loop. Rev. Endocr. Metab. Disord. 2022, 23, 893–903. [Google Scholar] [CrossRef]
- Forcina, L.; Franceschi, C.; Musarò, A. The hormetic and hermetic role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef]
- Asbaghi, O.; Shimi, G.; Naseri, K.; Saadati, S.; Kelishadi, M.R.; Doaei, S.; Haghighat, N. The effects of conjugated linoleic acid supplementation on blood pressure and endothelial function in adults: A systematic review and dose-response meta-analysis. Eur. J. Pharmacol. 2022, 931, 175162. [Google Scholar] [CrossRef]
- Soto-Vaca, A.; Losso, J.N.; McDonough, K.; Finley, J.W. Differential effect of 14 free fatty acids in the expression of inflammation markers on human arterial coronary cells. J. Agric. Food Chem. 2013, 61, 10074–10079. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 874. https://doi.org/10.3390/ijms24010874
Valenzuela CA, Baker EJ, Miles EA, Calder PC. Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(1):874. https://doi.org/10.3390/ijms24010874
Chicago/Turabian StyleValenzuela, Carina A., Ella J. Baker, Elizabeth A. Miles, and Philip C. Calder. 2023. "Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells" International Journal of Molecular Sciences 24, no. 1: 874. https://doi.org/10.3390/ijms24010874
APA StyleValenzuela, C. A., Baker, E. J., Miles, E. A., & Calder, P. C. (2023). Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells. International Journal of Molecular Sciences, 24(1), 874. https://doi.org/10.3390/ijms24010874




