Microglia in Cultured Porcine Retina: Qualitative Immunohistochemical Analyses of Reactive Microglia in the Outer Retina
Abstract
1. Introduction
2. Results
2.1. Overview of Microglia Associated with the OPL in Healthy and Degenerating Retina
2.2. Reactive Microglia in Degenerating Retina
2.2.1. IB4
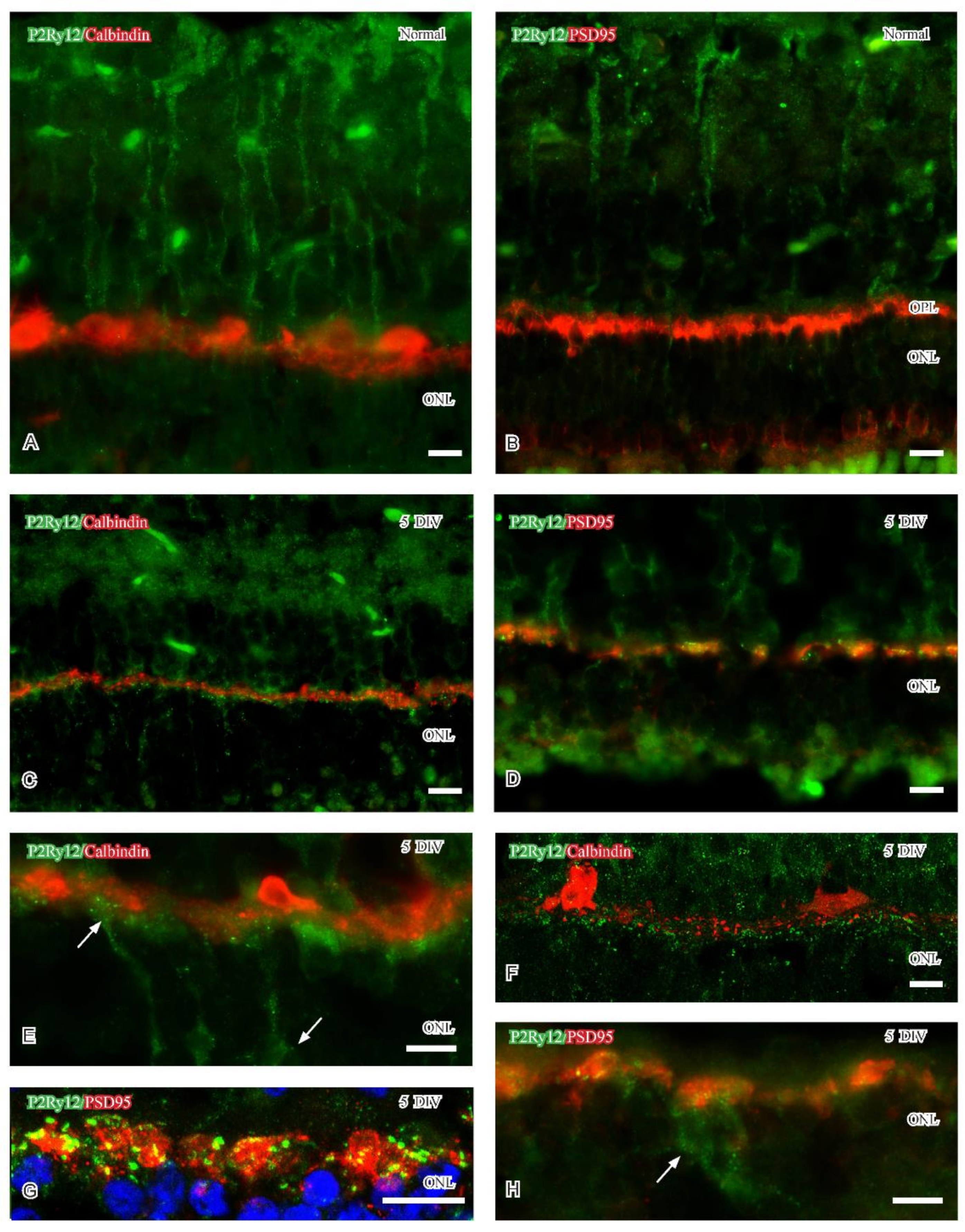
2.2.2. P2Ry12
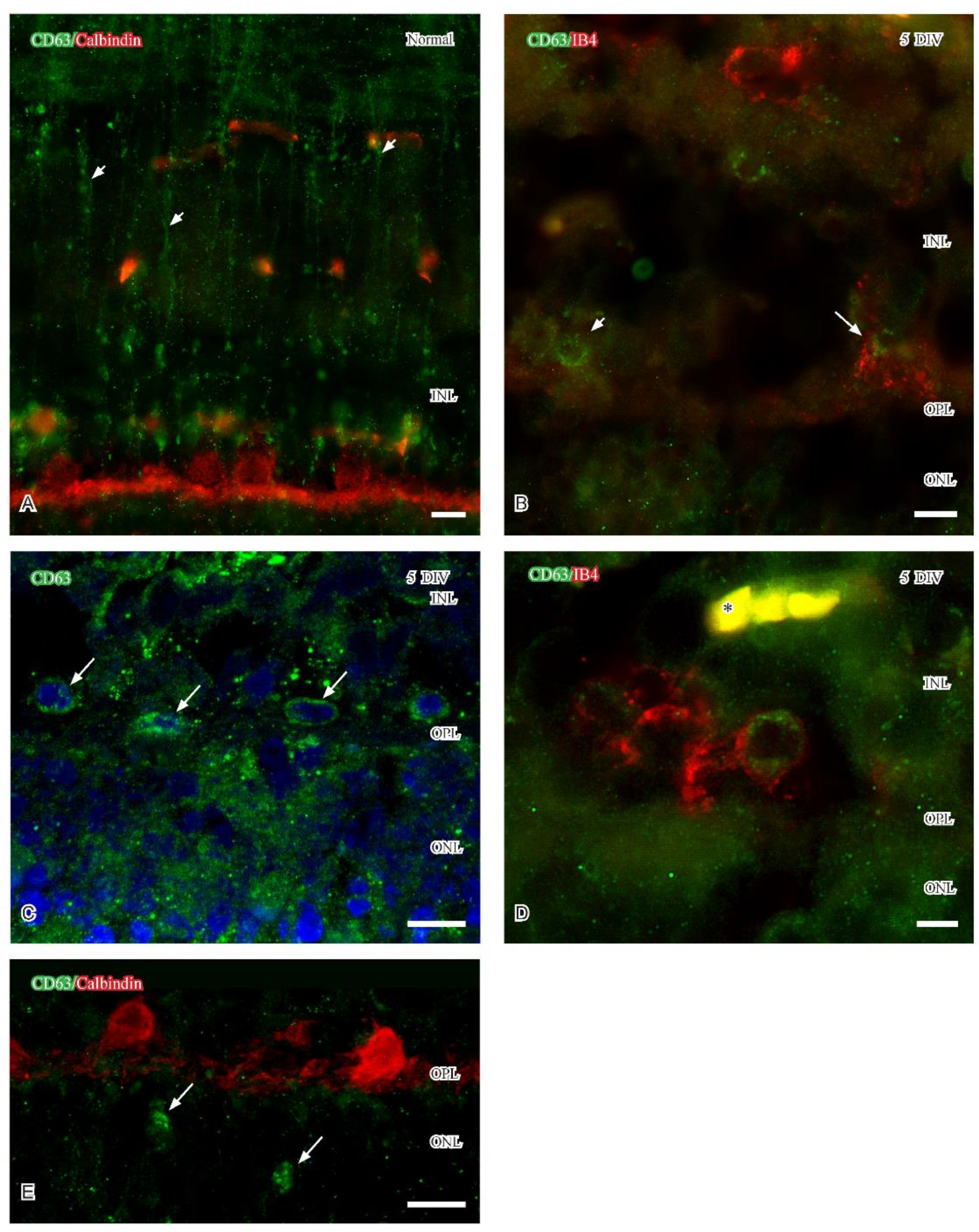
2.2.3. CD63
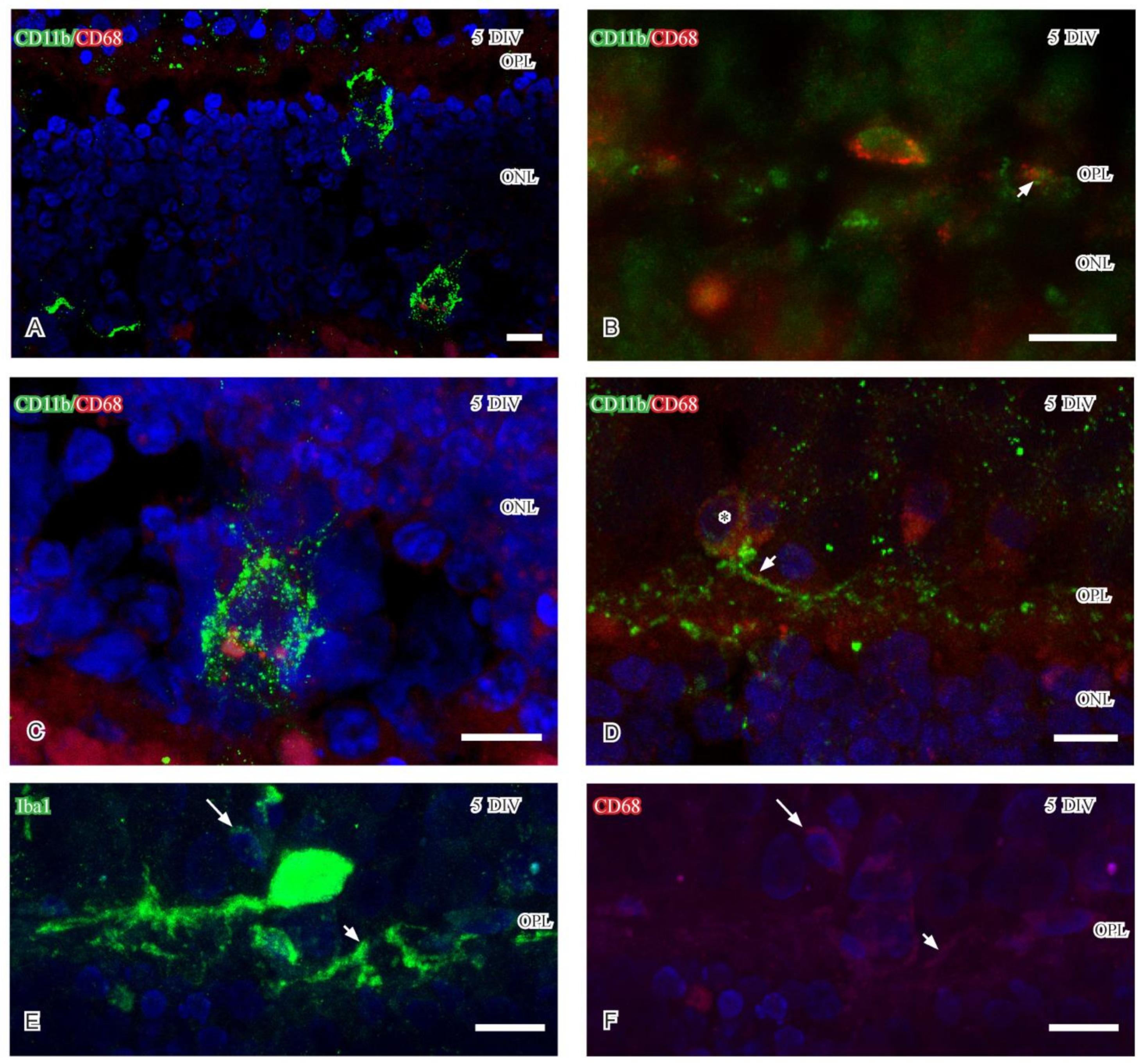
2.2.4. CD68
3. Discussion
3.1. General Considerations
3.2. Retinal Detachment in the Cultured Retina
3.3. Microglia Heterogeneity
4. Material and Methods
4.1. Animals and Culture Paradigm
4.2. Fluorescence and Confocal Microscopy
4.3. Lectin Histochemistry and Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia—A player in healthy and diseases retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Ret. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.L.; Anderson, J.R.; Dahal, J.; Garcia, J.C.; Yang, J.H.; Sigulinsky, C.L.; Rapp, K.; Emrich, D.P.; Watt, C.B.; Johnstun, H.A.; et al. A pathoconnectome of early neurodegeneration: Network changes in retinal degeneration. Exp. Eye Res. 2020, 99, 108196. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánches, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.A.; Jiang, D.; Mackin, R.D.; Samuel, M.A. Development and maintenance of vision´s first synapse. Devel. Biol. 2021, 476, 218–239. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Zhang, J.; Fariss, R.; Ma, W.; Kretschmer, F.; Wang, M.; Qian, H.H.; Badea, T.C.; Diamond, J.S.; et al. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J. Neurosci. 2016, 36, 2827–2842. [Google Scholar] [CrossRef]
- Johansson, K.; Allevang-Svensson, L.; Mohlin, C. Morphological analyzes of microglia heterogeneity and dynamics during photoreceptor degeneration in vitro: Presumptive dark microglia porcine retina. Exp. Eye Res. 2020, 200, 108217. [Google Scholar] [CrossRef]
- Zabel, M.K.; Zhao, L.; Zhang, Y.; Gonzalez, S.R.; Ma, W.; Wang, X.; Fariss, R.N.; Wong, W.T. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 2016, 64, 1479–1491. [Google Scholar] [CrossRef]
- Jiao, H.; Natoli, R.; Valter, K.; Provis, J.M.; Rutar, M. Spatiotemporal cadence of macrophage polarization in a model of light-induced retinal degeneration. PLoS ONE 2015, 10, e0143952. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Takata, K.; Ginhoux, F.; Shimohama, S. Roles of microglia in Alzheimer´s disease and impact of new findings on microglial heterogeneity as a target for therapeutic intervention. Biochem. Pharmacol. 2021, 192, 114754. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Young, A.M.H.; Kumasaka, N.; Calvert, F.; Hammond, T.R.; Knights, A.; Panousis, N.; Park, J.S.; Schwartzentruber, J.; Liu, J.; Kundu, K.; et al. A map of transcriptional heterogeneity and regulatory variation in human microglia. Nat. Genet. 2021, 53, 861–868. [Google Scholar] [CrossRef]
- Dixon, M.A.; Greferath, U.; Fletcher, E.L.; Jobling, A.I. The contribution of microglia to the development and maturation of the visual system. Front. Cell Neurosci. 2021, 15, 659843. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.; Lacoste, B.; Tremblay, M.E. Dark microglia: Why are they dark? Commun. Integr. Biol. 2016, 9, e1230575. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.P.; Lecours, C.; Sanchez, M.G.; Hajj, H.E.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, G.; et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 2016, 64, 826–839. [Google Scholar] [CrossRef]
- Makabe, K.; Sugita, S.; Mandai, M.; Fytatsugi, Y.; Takahashi, M. Microglia dynamics in retinitis pigmentosa model: Formation of fundus whitening and autofluorescence as an indicator of activity of retinal degeneration. Sci. Rep. 2020, 10, 14700. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in retinal degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef]
- Iandiev, I.; Uckermann, O.; Pannicke, T.; Wurm, A.; Tenckhoff, S.; Pietsch, U.C.; Reichenbach, A.; Weidemann, P.; Bringmann, A.; Uhlmann, S. Glial cell reactivity in a porcine model of retinal detachment. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2161–2171. [Google Scholar] [CrossRef]
- Okunuki, Y.; Mukai, R.; Pearsall, E.A.; Klokman, G.; Husain, D.; Park, D.H.; Korobkina, E.; Weiner, H.L.; Butovsky, O.; Ksander, B.R. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc. Natl. Acad. Sci. USA 2019, 115, E6264–E6273. [Google Scholar] [CrossRef]
- Belgio, B.; Boschetti, F.; Mantero, S. Towards an in vitro retinal model to study and develop new therapies for age-related macular degeneration. Bioengineering 2021, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef] [PubMed]
- Potic, J.; Mbefo, M.; Berger, A.; Nicolas, M.; Wanner, D.; Kostic, C.; Matet, A.; Behar-Cohen, F.; Moulin, A.; Arsenijevic, Y. An in vitro model of human retinal detachment reveals successive death pathway activations. Front. Neurosci. 2020, 14, 571293. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Safaei, A.; Vogt, P.A.; Gammel, M.R.; Buckhard Dick, D.; Schnichels, S.; Joachim, S.C. Coculture of ARPE-19 cells and porcine neural retina as an ex vivo retinal model. Altern. Lab. Anim. 2022, 50, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Mohlin, C.; Kvanta, A.; Delbro, D.; Johansson, K. Evaluation of Congo red staining in degenerating porcine photoreceptors in vitro: Protective effects by structural and trophic support. J. Histochem. Cytochem. 2018, 66, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Mohlin, C.; Sandholm, K.; Kvanta, A.; Ekdahl, K.N.; Johansson, K. A model to study complement involvement in experimental retinal degeneration. Ups. J. Med. Sci. 2018, 123, 28–42. [Google Scholar] [CrossRef]
- Mollick, T.; Mohlin, C.; Johansson, K. Human neural progenitor cells decrease photoreceptor degeneration, normalize opsin distribution and support synapse structure in cultured porcine retina. Brain Res. 2016, 1646, 522–534. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Guduric-Fuchs, J.; Ringland, L.J.; Gu, P.; Dellett, M.; Archer, D.B.; Cogliati, T. Immunohistochemical study of pig retinal development. Mol. Vis. 2009, 15, 1915–1928. [Google Scholar]
- Koulen, P.; Fletcher, E.L.; Craven, S.E.; Bredt, D.S.; Wässle, H. Immunohistochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J. Neurosci. 1998, 18, 10136–10149. [Google Scholar] [CrossRef]
- Boscia, F.; Esposito, C.L.; Casamassa, A.; de Franciscis, V.; Annunziato, L.; Cerchia, L. The isolectin IB4 binds RET receptor tyrosin kinase in microglia. J. Neurochem. 2013, 124, 428–436. [Google Scholar] [CrossRef]
- Mighty, J.; Zhou, J.; Benito-Martin, A.; Sauma, S.; Hanna, S.; Onwumere, O.; Shi, C.; Muntzel, M.; Sauane, M.; Young, M.; et al. Analysis of adult neural retina extracellular vesicle release, RNA transport and proteomic cargo. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef]
- Morillas, A.G.; Besson, V.C.; Lerouet, D. Microglia and neuroinflammation: What place for P2RY12? Int. J. Mol. Sci. 2021, 22, 1636. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulations of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkratsky, A. Physiology of microglia. Physiol. Rev. 2010, 91, 461–553. [Google Scholar] [CrossRef]
- Ohsawa, K.; Irino, Y.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. P2Y12 receptor-mediated integrin-β1 activation regulates microglial processes extension induced by ATP. Glia 2010, 58, 790–801. [Google Scholar]
- Mohlin, C.; Taylor, L.; Ghosh, F.; Johansson, K. Autophagy and ER-stress contribute to photoreceptor degeneration in cultured adult porcine retina. Brain Res. 2014, 1585, 167–185. [Google Scholar] [CrossRef]
- Arroyo, J.G.; Yang, L.; Bula, D.; Chen, D.F. Photoreceptor apoptosis in human retinal detachment. Am. J. Ophthalmol. 2005, 139, 605–610. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Sun, Y.J.; Song, J.Y.; Fan, B.; Li, G.Y. Experimental models and examination methods of retinal detachment. Brain Res. Bull. 2021, 169, 51–62. [Google Scholar] [CrossRef]
- Halász, E.; Zarbin, M.A.; Davidow, A.L.; Frishman, L.J.; Gombkoto, P.; Townes-Anderson, E. ROCK inhibition reduces morphological and functional damage to rod synapses after retinal injury. Sci. Rep. 2021, 11, 692. [Google Scholar] [CrossRef]
- Townes-Anderson, E.; Halasz, E.; Wang, W.; Zarbin, M. Coming of age for the photoreceptor synapse. Investig. Ophthalmol. Vis. Sci. 2021, 62, 24. [Google Scholar] [CrossRef] [PubMed]
- Specht, D.; tom Dieck, S.; Ammermüller, J.; Regus-Leidig, H.; Gundelfinger, E.D.; Brandstätter, J.H. Structural and functional remodeling in the retina of a mouse with photoreceptor synaptopathy: Plasticity in the rod and degeneration in the cone system. Eur. J. Neurosci. 2007, 26, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Striebel, J.F.; Race, B.; Leung, J.M.; Schwartz, C.; Chesebro, B. Prion-induced photoreceptor degeneration begins with misfolded prion protein accumulation in cones at two distinct sites: Cilia and ribbon synapses. Acta Neuropathol. Commun. 2021, 17, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vijayasarathy, C.; Zeng, Y.; Brooks, M.J.; Fariss, R.N.; Sieving, P.A. Genetic rescue of X-linked retinoschisis mouse (Rs1-/y) retina induces quiescence of retinal microglial inflammatory state following AAV8-RS1 gene transfer and identifies gene networks underlying retinal recovery. Hum. Gene Ther. 2021, 32, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, P.; Zhang, M.; Wang, X.; Li, G.; Xu, G.; Ni, Y. Synaptic changes and response of microglia in a light-induced photoreceptor degeneration model. Mol. Vis. 2021, 27, 206–220. [Google Scholar]
- Wang, Y.; Zhao, X.; Gao, M.; Wan, X.; Guo, Y.; Qu, Y.; Chen, Y.; Li, T.; Liu, H.; Jiang, M.; et al. Myosin 1f-mediated activation of microglia contributes to photoreceptor degeneration in a mouse model of retinal detachment. Cell Death Dis. 2021, 12, 926. [Google Scholar] [CrossRef]
- Wagner, N.; Reinehr, S.; Gammel, M.R.; Greulich, A.; Hurst, J.; Dick, H.B.; Schnichels, S.; Joachim, S.C. Novel porcine retina cultivation techniques provide improved photoreceptor preservation. Front. Neurosci. 2020, 71, 527–544. [Google Scholar] [CrossRef]
- Wang, J.; Zarbin, M.; Sugino, I.; Whitehead, I.; Townes-Anderson, E. RhoA signaling and synaptic damage occur withing hours in a live pig model of CNS injury, retinal detachment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3892–3906. [Google Scholar] [CrossRef]
- Fontainhas, A.M.; Townes-Anderson, E. RhoA inactivation prevents photoreceptor axon retraction in an in vitro model of acute retinal detachment. Investig. Ophthalmol. Vis. Sci. 2011, 52, 579–587. [Google Scholar] [CrossRef]
- Khodair, M.A.; Zarbin, M.A.; Townes-Anderson, E. Synaptic plasticity in mammalian photoreceptors prepared as sheets for retinal transplantation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4976–4988. [Google Scholar] [CrossRef]
- Lin, J.B.; Apte, R.A. Visualizing the heterogeneity of retinal microglia. Immunity 2019, 50, 544–546. [Google Scholar] [CrossRef]
- Anderson, S.R.; Roberts, J.M.; Ghena, N.; Irvin, E.A.; Schwakopf, J.; Cooperstein, I.B.; Bosco, A.; Vetter, M.L. Neuronal apoptosis drives remodeling states of microglia and shifts in survival pathway dependence. eLife 2022, 11, e76564. [Google Scholar] [CrossRef]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.Y.; Chen, H.R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANZ1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef]
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girão, H.; Ambrósio, A.F.; Santiago, A.R. Microglial extracellular vesicles as vehicles for neurodegenerative spreading. Biomolecules 2021, 11, 770. [Google Scholar] [CrossRef]
- Wooff, Y.; Cioanca, A.V.; Chu-Tan, J.A.; Aggio-Bruce, R.; Schumann, U.; Natoli, R. Small-medium extracellular vesicles and their miRNA cargo in retinal health and degeneration: Mediators of homeostasis, and vehicles for targeted gene therapy. Front. Cell. Neurosci. 2020, 14, 160. [Google Scholar] [CrossRef]
- Lim, R.R.; Hainsworth, D.P.; Mohan, R.R.; Chaurasia, S.S. Characterization of a functionally active primary microglial cell culture from the pig retina. Exp. Eye Res. 2019, 185, 107670. [Google Scholar] [CrossRef]

| Antibodies Used, Host Animal (Rb = Rabbit, Mo = Mouse), Dilution, Company and Reference | |
|---|---|
| Microglia markers. | |
| CD11b Rb 1:2000; Abcam, Cambridge, UK (general marker) | [28] |
| CD63 Rb 1:100, Abcam, Cambridge, UK (reactive microglia; EV marker) | [32] |
| CD68 Mo 1:100 Santa Cruz Biotechnology, Dallas, TX, USA (reactive microglia; lysosomal marker) | [28] |
| Iba1 Rb 1:1000; Proteintech Group, Chicago, IL, USA (general marker) | [28] |
| P2yR12 Rb 1:100 Novus Biological, Abingdon, UK (reactive microglia) | [28] |
| Neural markers | |
| Calbindin Mo 1:2000, Santa Cruz Biotechnology, Dallas, TX, USA (horizontal cells) | [29] |
| PSD95 Mo 1:1000; Millipore, Burlington, CA, USA (photoreceptor synapse) | [30] |
| Lectins | |
| Isolectin B4 FITC- or Texas red-conjugated lectin 1:1000, Vector Laboratories, Burlingame, CA, USA (general microglia marker but only in the degenerating porcine retina). | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, K.; Mohlin, C. Microglia in Cultured Porcine Retina: Qualitative Immunohistochemical Analyses of Reactive Microglia in the Outer Retina. Int. J. Mol. Sci. 2023, 24, 871. https://doi.org/10.3390/ijms24010871
Johansson K, Mohlin C. Microglia in Cultured Porcine Retina: Qualitative Immunohistochemical Analyses of Reactive Microglia in the Outer Retina. International Journal of Molecular Sciences. 2023; 24(1):871. https://doi.org/10.3390/ijms24010871
Chicago/Turabian StyleJohansson, Kjell, and Camilla Mohlin. 2023. "Microglia in Cultured Porcine Retina: Qualitative Immunohistochemical Analyses of Reactive Microglia in the Outer Retina" International Journal of Molecular Sciences 24, no. 1: 871. https://doi.org/10.3390/ijms24010871
APA StyleJohansson, K., & Mohlin, C. (2023). Microglia in Cultured Porcine Retina: Qualitative Immunohistochemical Analyses of Reactive Microglia in the Outer Retina. International Journal of Molecular Sciences, 24(1), 871. https://doi.org/10.3390/ijms24010871






