Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression
Abstract
1. Introduction
2. Results
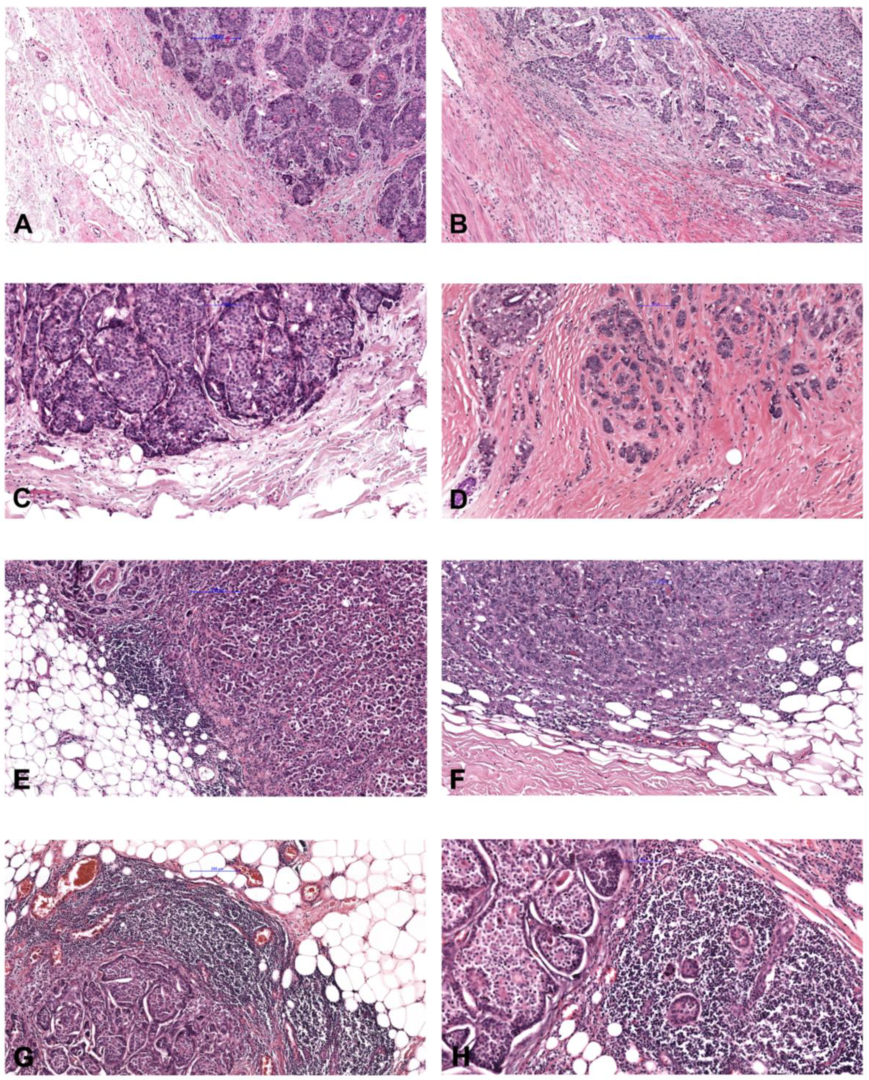
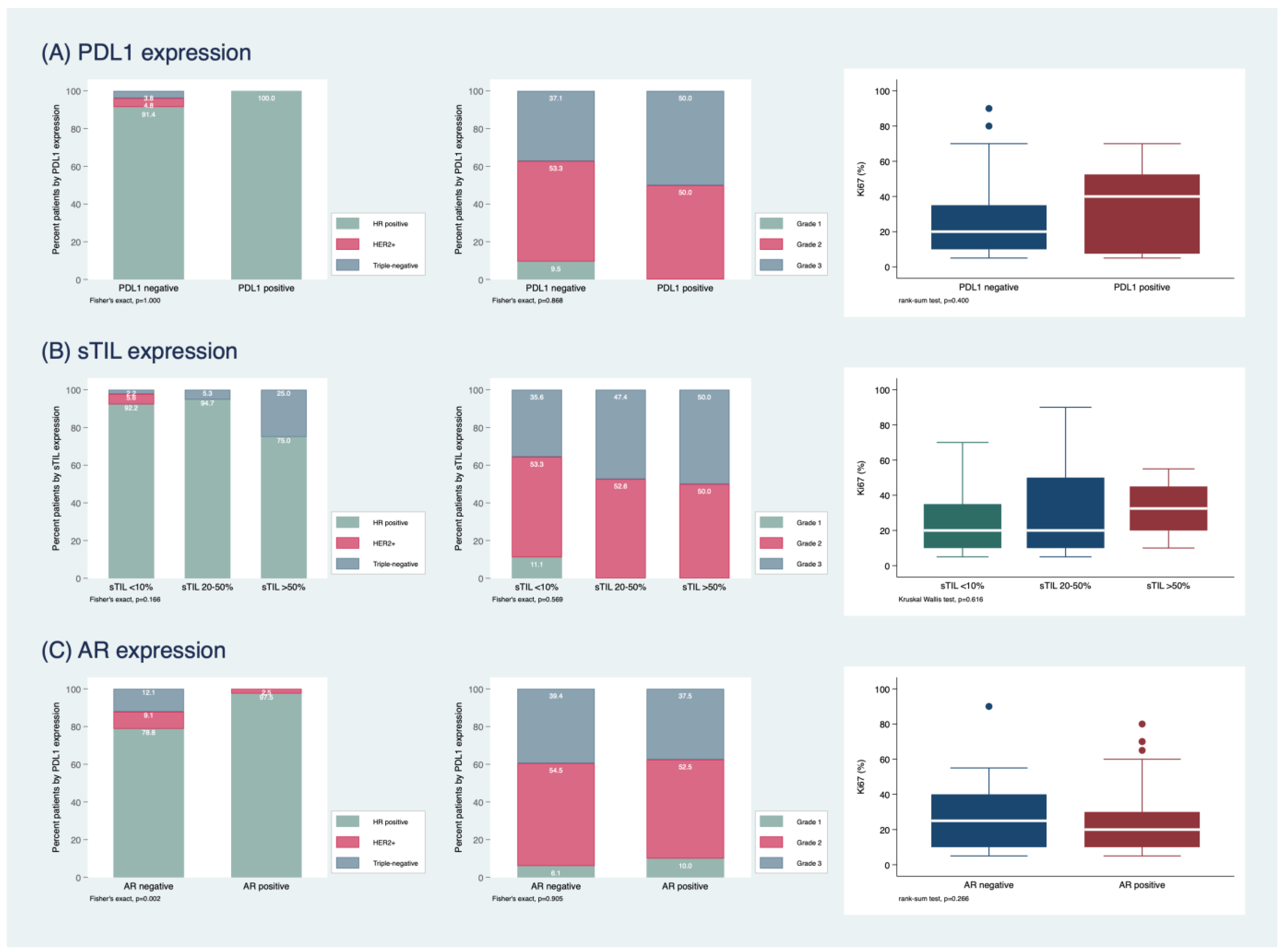
2.1. Study Population and Histopathological Characterization
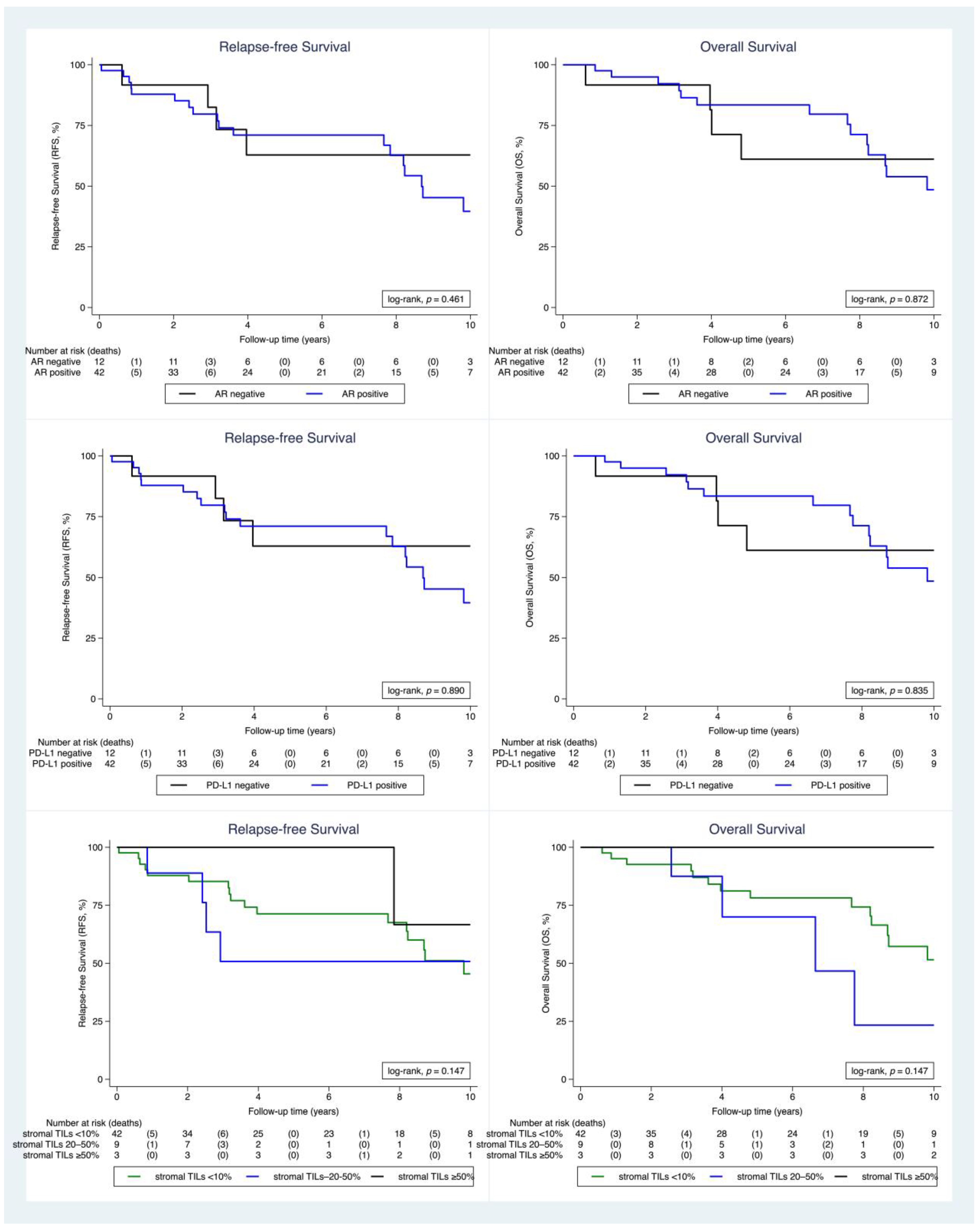
2.2. Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Evaluation of Stromal Tumor-Infiltrating Lymphocytes (TILs)
4.2. Tissue Microarray Construction
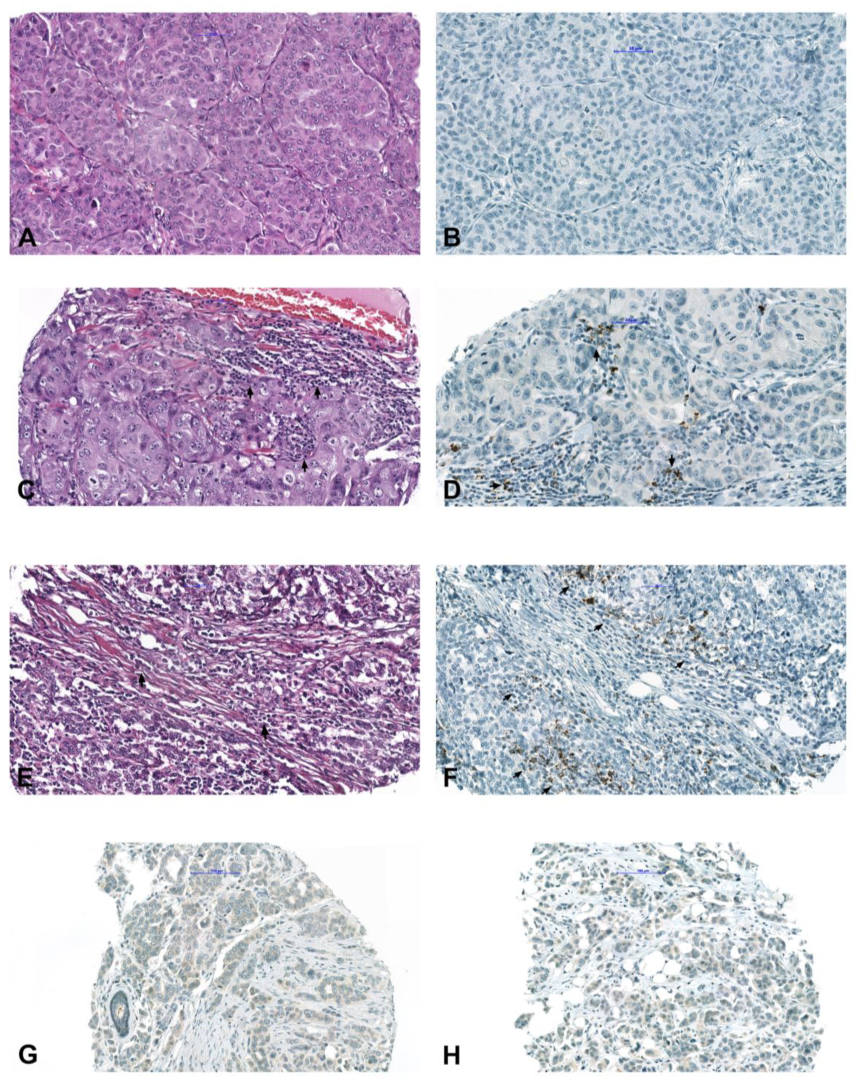
4.3. Immunohistochemical Analysis
4.4. Molecular Analysis
4.4.1. RNA Workflow
4.4.2. Targeted Next-Generation Sequencing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H. A review of the diagnosis and management of male breast cancer. Oncologist 2005, 10, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pritzlaff, M.; Summerour, P.; McFarland, R.; Li, S.; Reineke, P.; Dolinsky, J.S.; Goldgar, D.E.; Shimelis, H.; Couch, F.J.; Chao, E.C.; et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res. Treat. 2017, 161, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Bartlett, J.; Slaets, L.; van Deurzen, C.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann. Oncol. 2018, 29, 405–417. [Google Scholar] [CrossRef]
- Vermeulen, M.A.; Slaets, L.; Cardoso, F.; Giordano, S.H.; Tryfonidis, K.; van Diest, P.J.; Dijkstra, N.H.; Schröder, C.P.; van Asperen, C.J.; Linderholm, B.; et al. Pathological characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international male breast Cancer program. Eur. J. Cancer 2017, 82, 219–227. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Swaika, A.; Hammond, W.A.; Joseph, R.W. Current state of anti-PD-L1 and antiPD-1 agents in cancer therapy. Mol. Immunol. 2015, 67, 4–17. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Brčić, I.; Godschachner, T.M.; Bergovec, M.; Igrec, J.; Till, H.; Lackner, H.; Scheipl, S.; Kashofer, K.; Brodowicz, T.; Leithner, A.; et al. Broadening the spectrum of NTRK rearranged mesenchymal tumors and usefulness of pan-TRK immunohistochemistry for identification of NTRK fusions. Mod. Pathol. 2021, 34, 396–407. [Google Scholar] [CrossRef]
- Hung, Y.P.; Fletcher, C.D.M.; Hornick, J.L. Evaluation of pan-TRK immunohistochemistry in infantile fibrosarcoma, lipofibromatosis-like neural tumour and histological mimics. Histopathology 2018, 73, 634–644. [Google Scholar] [CrossRef]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Humphries, M.P.; Sundara Rajan, S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of male breast cancer: A descriptive biomarker study from a large patient series. Sci. Rep. 2017, 7, 45293. [Google Scholar] [CrossRef]
- Herbst, R.S.; de Marinis, F.; Giaccone, G.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Clinical efficacy of atezolizumab (atezo) in biomarker subgroups by SP142, SP263 and 22C3 PD-L1 immunohistochemistry (IHC) assays and by blood tumour mutational burden (bTMB): Results from the IMpower110 study. Ann. Oncol. 2019, 30 (Suppl. 11), xi62–xi68. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, P.; Liu, Z.; Maimaiti, Y.; Wang, S.; Yin, X.; Liu, C.; Huang, T. Prognostic and Clinicopathological Value of Programmed Death Ligand-1 in Breast Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0156323. [Google Scholar] [CrossRef]
- Li, Z.; Dong, P.; Ren, M.; Song, Y.; Qian, X.; Yang, Y.; Li, S.; Zhang, X.; Liu, F. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J. Cancer 2016, 7, 784–793. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Somerfield, M.R.; Baker, E.R.; Cardoso, F.; Kansal, K.J.; Kwait, D.C.; Plichta, J.K.; Ricker, C.; Roshal, A.; Ruddy, K.J.; et al. Management of Male Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Remoué, A.; Conan-Charlet, V.; Bourhis, A.; Flahec, G.L.; Lambros, L.; Marcorelles, P.; Uguen, A. Non-secretory breast carcinomas lack NTRK rearrangements and TRK protein expression. Pathol. Int. 2019, 69, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 2017, 4, 1547–1551. [Google Scholar] [CrossRef]
- Harrison, B.T.; Fowler, E.; Krings, G.; Chen, Y.Y.; Bean, G.R.; Vincent-Salomon, A.; Fuhrmann, L.; Barnick, S.E.; Chen, B.; Hosfield, E.M.; et al. Pan-TRK Immunohistochemistry: A Useful Diagnostic Adjunct For Secretory Carcinoma of the Breast. Am. J. Surg. Pathol. 2019, 43, 1693–1700. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
| Total n = 113 (%) | ||
|---|---|---|
| Age at diagnosis | 69.0 (60.0–77.0) | |
| Histologic grade | Grade 1 | 10 (8.8%) |
| Grade 2 | 60 (53.1%) | |
| Grade 3 | 43 (38.1%) | |
| ER | Positive | 104 (92%) |
| Negative | 9 (8%) | |
| PR | Positive | 97 (85.8%) |
| Negative | 16 (14.2%) | |
| HER2 | Positive | 5 (4.4%) |
| “low” | 63 (55.8%) | |
| Negative | 45 (39.8%) | |
| AR | Positive | 80 (70.8%) |
| Negative | 33 (29.2%) | |
| TNBC | 4 (3.6%) | |
| PD-L1 | Positive | 8 (7.1%) |
| Negative | 105 (92.9%) | |
| Pan-TRK | Positive | 10 (8.8%) |
| Negative | 103 (91.2%) | |
| sTILs | <10 | 90 (79.6%) |
| 10–50 | 19 (16.8%) | |
| >50 | 4 (3.6%) |
| Antibody | Source | Clone | Dilution |
|---|---|---|---|
| ER | Dako/Agilent | EP1 | RTU |
| PR | Dako/Agilent | PgR 1294 | RTU |
| AR | Dako/Agilent | AR441 | 1:50 |
| Ki-67 | Dako/Agilent | MIB-1 | RTU |
| HER2 | Dako/Agilent | Hercep Test | RTU |
| PD-L1 | Roche/Ventana Medical Systems | SP142 | 1:50 |
| Pan-TRK | Roche/Ventana Medical Systems | EPR17341 | RTU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brcic, I.; Kluba, A.M.; Godschachner, T.M.; Suppan, C.; Regitnig, P.; Dandachi, N.; Lax, S.F.; Balić, M. Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression. Int. J. Mol. Sci. 2023, 24, 818. https://doi.org/10.3390/ijms24010818
Brcic I, Kluba AM, Godschachner TM, Suppan C, Regitnig P, Dandachi N, Lax SF, Balić M. Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression. International Journal of Molecular Sciences. 2023; 24(1):818. https://doi.org/10.3390/ijms24010818
Chicago/Turabian StyleBrcic, Iva, Andrea Maria Kluba, Theresa Marie Godschachner, Christoph Suppan, Peter Regitnig, Nadia Dandachi, Sigurd Friedwald Lax, and Marija Balić. 2023. "Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression" International Journal of Molecular Sciences 24, no. 1: 818. https://doi.org/10.3390/ijms24010818
APA StyleBrcic, I., Kluba, A. M., Godschachner, T. M., Suppan, C., Regitnig, P., Dandachi, N., Lax, S. F., & Balić, M. (2023). Tumor Microenvironment in Male Breast Carcinoma with Emphasis on Tumor Infiltrating Lymphocytes and PD-L1 Expression. International Journal of Molecular Sciences, 24(1), 818. https://doi.org/10.3390/ijms24010818








