Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L.
Abstract
1. Introduction
2. Results
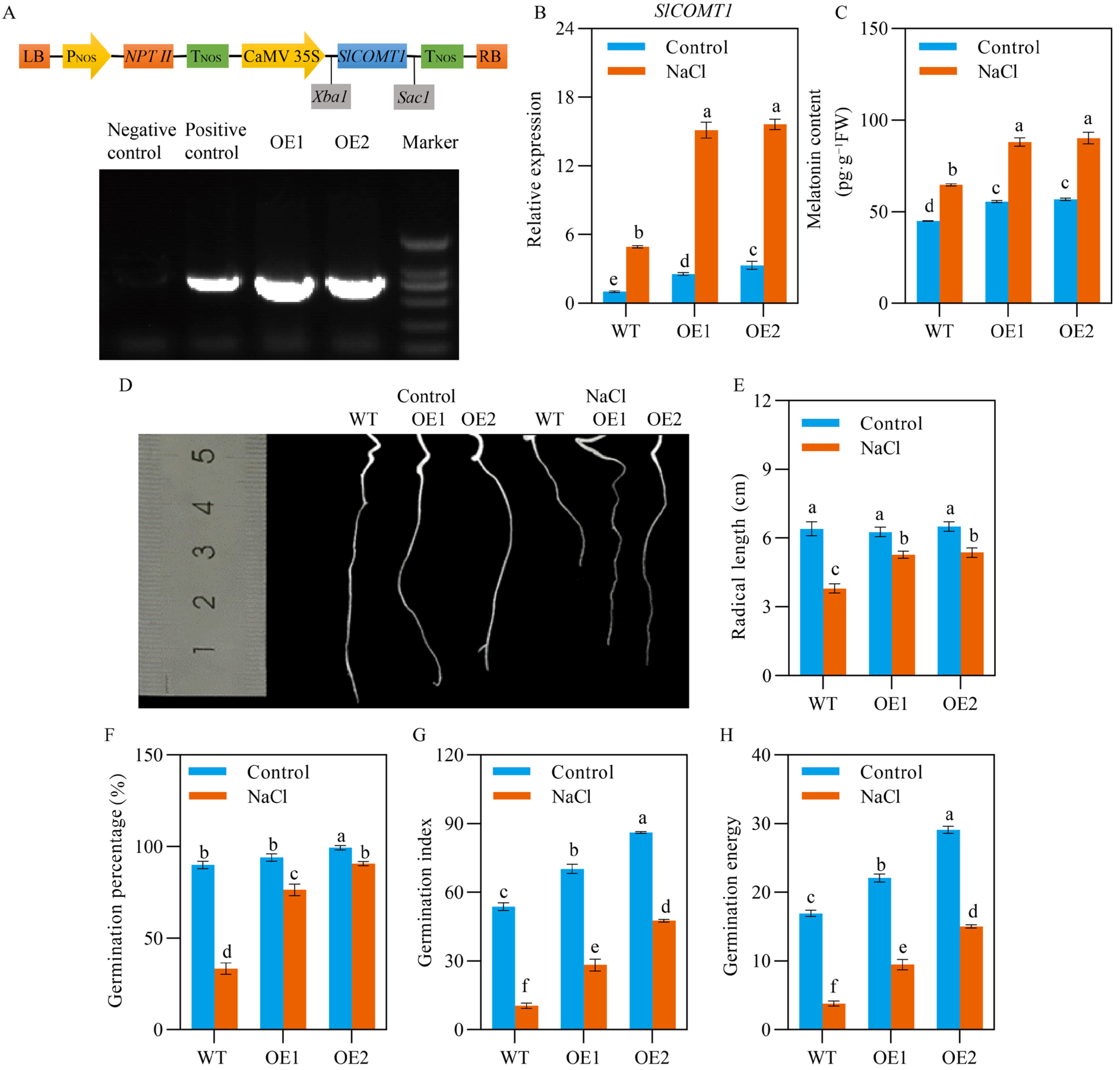
2.1. SlCOMT1 Ooverexpression Improves Tomato Seed Germination under Salt Stress
2.2. SlCOMT1 Overexpression Benefits Starch Metabolism in Tomato Seeds under Salt Stress
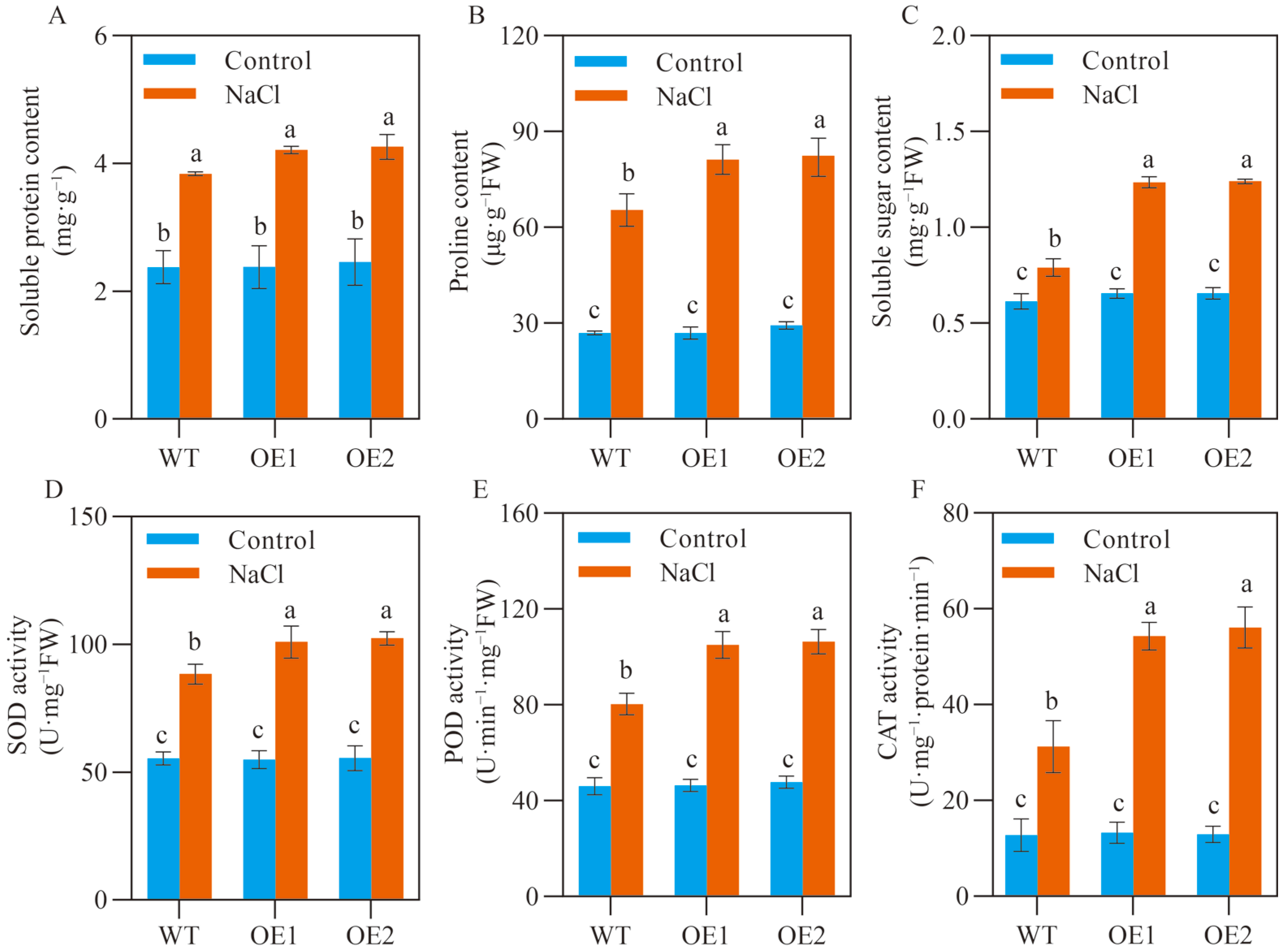
2.3. SlCOMT1 Overexpression Enhances Osmotic Adjustment and Antioxidant Capacity in Tomato Seeds under Salt Stress
2.4. Membrane Is Stablized by SlCOMT1 Overexpression in Tomato Seeds under Salt Stress
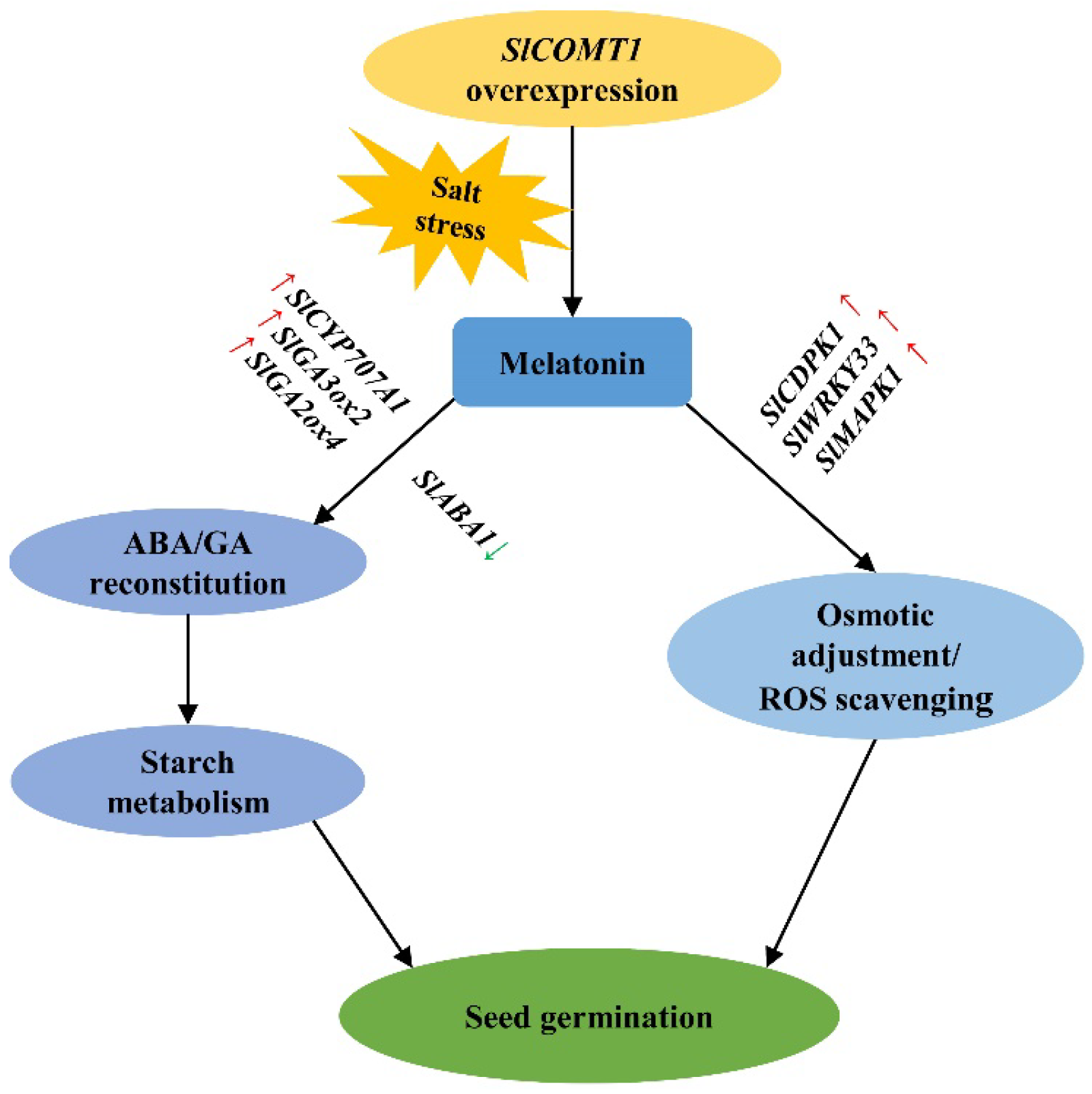
2.5. SlCOMT1 Overexpression Benefits the Expression of Germination- and Tolerance-Related Genes in Tomato Seeds under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Designs
4.2. Evaluation of Seed Germination
4.3. Determination of MT Content
4.4. Starch Metabolism Assay
4.5. Evaluation of Antioxidant Enzyme Activity
4.6. Determinaiton of ROS, MDA and Proline Contents
4.7. Gene Expression Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bizouerne, E.; Buitink, J.; Vu, B.L.; Vu, J.L.; Esteban, E.; Pasha, A.; Provart, N.; Verdier, J.; Leprince, O. Gene co-expression analysis of tomato seed maturation reveals tissue-specific regulatory networks and hubs associated with the acquisition of desiccation tolerance and seed vigour. BMC Plant Biol. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Pagano, A.; Leonetti, P.; Carbonera, D.; Balestrazzi, A.; Araujo, S.S. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: Implications on seed technology traits. Plant Cell Rep. 2017, 36, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying biochemical and molecular mechanisms for seed germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Real, C.; Adalid-Martinez, A.M.; Gregori-Montaner, A.; Prohens, J.; Rodriguez-Burruezo, A.; Fita, A. Factors affecting germination of Diplotaxis erucoides and their effect on selected quality properties of the germinated products. Sci. Hortic. 2020, 261, 109013. [Google Scholar] [CrossRef]
- Tian, A.; Zhao, J.; Tang, B.; Zhu, D.; Fu, C.; Xiong, H. Study on the pretreatment of soil hyperspectral and Na+ ion data under different degrees of human activity stress by fractional-order derivatives. Remote Sens. 2021, 13, 3974. [Google Scholar] [CrossRef]
- Shelden, M.C.; Dias, D.A.; Jayasinghe, N.S.; Bacic, A.; Roessner, U. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J. Exp. Bot. 2016, 67, 3731–3745. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Han, G.; Song, J.; Wang, B. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 2018, 45, 350–361. [Google Scholar] [CrossRef]
- Kong, C.; Ren, C.; Li, R.; Xie, Z.; Wang, J. Hydrogen peroxide and strigolactones signaling are involved in alleviation of salt stress induced by arbuscular mycorrhizal fungus in Sesbania cannabina Seedlings. J. Plant Growth Regul. 2017, 36, 734–742. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Masciarelli, O.; Alemano, S.; Luna, V. Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci. Res. 2016, 26, 1–13. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules 2018, 23, 1887. [Google Scholar] [CrossRef] [PubMed]
- Rehaman, A.; Mishra, A.K.; Ferdose, A.; Per, T.S.; Hanief, M.; Jan, A.T.; Asgher, M. Melatonin in plant defense against abiotic stress. Forests 2021, 12, 1404. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C.; et al. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef]
- Li, M.; Zhang, K.; Sun, Y.; Cui, H.; Cao, S.; Yan, L.; Xu, M. Growth, physiology, and transcriptional analysis of two contrasting carex rigescens genotypes under salt stress reveals salt-tolerance mechanisms. J. Plant Physiol. 2018, 229, 77–88. [Google Scholar] [CrossRef]
- Liu, D.; Sun, X.; Liu, L.; Shi, H.; Chen, S.; Zhao, D. Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt Stress. Molecules 2019, 24, 1514. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, H.; Cao, S.; Yan, L.; Li, M.; Sun, Y. Overexpression of CrCOMT from Carex rigescens increases salt stress and modulates melatonin synthesis in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Yan, J.; Zhang, Z.; Yuan, L.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic. Res. 2021, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. 2016. Available online: http://www.fao.org (accessed on 1 June 2022).
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jing, X.; Tang, H.; Li, X.; Gong, B.; Shi, Q. Using Transcriptome to Discover a novel melatonin-induced sodic alkaline stress resistant pathway in Solanum lycopersicum L. Plant Cell Physiol. 2019, 60, 2051–2064. [Google Scholar] [CrossRef]
- Sun, S.; Wen, D.; Yang, W.; Meng, Q.; Shi, Q.; Gong, B. Overexpression of caffeic acid O-methyltransferase 1 (COMT1) increases melatonin level and salt stress tolerance in tomato plant. J. Plant Growth Regul. 2020, 39, 1221–1235. [Google Scholar] [CrossRef]
- Liu, B.; Lin, R.; Jiang, Y.; Jiang, S.; Xiong, Y.; Lian, H.; Zeng, Q.; Liu, X.; Liu, Z.; Chen, S. Transcriptome analysis and identification of genes associated with starch metabolism in Castanea henryi seed (Fagaceae). Int. J. Mol. Sci. 2020, 21, 1431. [Google Scholar] [CrossRef]
- Li, W.; Meng, R.; Liu, Y.; Chen, S.; Jiang, J.; Wang, L.; Zhao, S.; Wang, Z.; Fang, W.; Chen, F.; et al. Heterografted chrysanthemums enhance salt stress tolerance by integrating reactive oxygen species, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Lang, D.; Cui, J.; Li, Y. Silicon improves salt tolerance of Glycyrrhiza uralensis Fisch by ameliorating osmotic and oxidative stresses and improving phytohormonal balance. Environ. Sci. Pollut. Res. 2018, 25, 25916–25932. [Google Scholar] [CrossRef]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-destructive determination of malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 2016, 6, 35393. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Zhang, N.; Li, H.; Yang, S.; Liu, Y. SlSOM inhibits seed germination by regulating the expression of ABA/GA metabolic genes and SlABI5 in Solanum lycopersicum. J. Integr. Agric. 2015, 14, 326–336. [Google Scholar] [CrossRef]
- Khan, S.A.; Li, M.; Wang, S.; Yin, H. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yan, Y.; Wen, D.; Shi, Q. Hydrogen peroxide produced by NADPH oxidase: A novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant. 2017, 160, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Delormel, T.Y.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Price, L.; Han, Y.; Angessa, T.; Li, C. Molecular pathways of WRKY genes in regulating plant salinity tolerance. Int. J. Mol. Sci. 2022, 23, 10947. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, Y.; Xu, Y.; Qi, Z.; Li, M.; Ahammed, G.J.; Xia, X.; Shi, K.; Zhou, Y.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wen, X.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, S.; Zhao, N.; Yang, W.; Shi, Q.; Gong, B. COMT1 overexpression resulting in increased melatonin biosynthesis contributes to the alleviation of carbendazim phytotoxicity and residues in tomato plants. Environ. Pollut. 2019, 252, 51–61. [Google Scholar] [CrossRef]
- Kobylinska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.J.; Alghamdi, S.S.; Siddique, K.H.M. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, T.; Liu, Y.; Wang, J.; Wang, Q.; Zhu, W. Effect of exogenous glycine betaine on the germination of tomato seeds under cold stress. Int. J. Mol. Sci. 2022, 23, 10474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Dong, Y.; Zhang, F.; He, Q.; Chen, J.; Zhu, S.; Zhao, T. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crops Prod. 2021, 169, 113671. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing alpha-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Wei, J.; Liang, J.; Liu, D.; Liu, Y.; Liu, G.; Wei, S. Melatonin-induced physiology and transcriptome changes in banana seedlings under salt stress conditions. Front. Plant Sci. 2022, 13, 938262. [Google Scholar] [CrossRef]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y. Exogenous melatonin improves seed germination of wheat (Triticum aestivum L.) under salt stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef]
- Castañares, J.L.; Bouzob, A.C. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, S.; Liu, S.; Wang, W.; Sui, N. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica 2018, 56, 861–872. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Zhang, M.; Xu, H.; Ning, K.; Wang, B.; Chen, M. Melatonin increases growth and salt tolerance of Limonium bicolor by improving photosynthetic and antioxidant capacity. BMC Plant Biol. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2020, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, J.; Cui, X.; Guo, L.; Wang, Z.; Liu, Y.; Wang, F.; Qi, M.; Liu, Y.; Li, T. Melatonin mediates reactive oxygen species homeostasis via SlCV to regulate leaf senescence in tomato plants. J. Pineal Res. 2022, 73, e12810. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Yang, R.; Wang, L.; Sun, Q.; Li, D.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761. [Google Scholar] [CrossRef]
- Li, H.; Dong, Y.; Chang, J.; He, J.; Chen, H.; Liu, Q.; Wei, C.; Ma, J.; Zhang, Y.; Yang, J.; et al. High-throughput microRNA and mRNA sequencing reveals that microRNAs may be involved in melatonin-mediated cold tolerance in Citrullus lanatus L. Front. Plant Sci. 2016, 7, 1231. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Noli, E.; Barbanti, L. Assessing salicornia europaea tolerance to salinity at seed germination stage. Agriculture 2020, 10, 29. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Imtiaz, M.; Rizwan, M.; Dong, X.; Tu, S. Effect of vanadium on germination, growth and activities of amylase and antioxidant enzymes in genotypes of rice. Int. J. Environ. Sci. Technol. 2020, 17, 383–394. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, F.; Zhang, B. Study on preservation method and mechanism of peeling waxy corn kernels treated with composite film. J. Food Process. Preserv. 2022, 46, e16451. [Google Scholar] [CrossRef]
- Stewart, R.R.C.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Kar, M.; Mishre, D. Catalase Activity in Leaves and Cotyledons During Plant Development and Senescence. Biochem. Physiol. Pflanz. 1978, 172, 385–390. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef]
- Gong, B.; Li, X.; VandenLangenberg, K.M.; Wen, D.; Sun, S.; Wei, M.; Li, Y.; Yang, F.; Shi, Q.; Wang, X. Overexpression of S-adenosyl-L-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant Biotechnol. J. 2014, 12, 694–708. [Google Scholar] [CrossRef]
- Wang, H.; Liang, X.; Huang, J.; Zhang, D.; Lu, H.; Liu, Z.; Bi, Y. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiol. 2010, 51, 1754–1765. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2001–2007. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Actin | TTTGCTGGTGATGATGCC | CCTTAGGGTTGAGAGGTGCTT |
| NPTII | GTGGAGAGGCTATTCGGCTATGACTG | AGCTCTTCAGCAATATCACGGGTAGC |
| SlCOMT1 | TACCCTGGCGTTGAACACA | CCTTTCTTTGCCTCCTGGATTA |
| SlCYP707A1 | ATCACAACCCAGAGTTCTTTCCT | CAAGTTCATTCCCTGGACAAGC |
| SlGA3ox2 | GATAAGCTCATGTGGTCCGAAG | GCTTTTCCATTTCATTTTCGTA |
| SlGA2ox4 | TGGCAATAAGAAAATCGGACAA | ACACATAATCATTCACCGCAGC |
| SlABA1 | AGAGTCTGGAAGCCCTGTGGAT | AAGTCCGACGCCAAGATAAGC |
| SlMAPK1 | GGTGGCAGGTTCATTCAATAC | TTCTCTCTGTGGTGGTGGAA |
| SlWRKY33 | CTACAGTGTTGGCTAACCATTCTAAT | GTTAAGGAAAGAGCTGAAGAATAAATCA |
| SlCDPK1 | TCTTGTGATGGAGTTGTGTGG | AATGAATACCGACAGCCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Yang, X.; Liu, Y.; Tang, H.; Wang, Q.; Chu, S.; Hu, J.; Zhang, N.; Shi, Q. Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L. Int. J. Mol. Sci. 2023, 24, 734. https://doi.org/10.3390/ijms24010734
Ge L, Yang X, Liu Y, Tang H, Wang Q, Chu S, Hu J, Zhang N, Shi Q. Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L. International Journal of Molecular Sciences. 2023; 24(1):734. https://doi.org/10.3390/ijms24010734
Chicago/Turabian StyleGe, Lianjing, Xiaoyu Yang, Yue Liu, Huimeng Tang, Qifang Wang, Shunpeng Chu, Jinxiang Hu, Ning Zhang, and Qinghua Shi. 2023. "Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L." International Journal of Molecular Sciences 24, no. 1: 734. https://doi.org/10.3390/ijms24010734
APA StyleGe, L., Yang, X., Liu, Y., Tang, H., Wang, Q., Chu, S., Hu, J., Zhang, N., & Shi, Q. (2023). Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L. International Journal of Molecular Sciences, 24(1), 734. https://doi.org/10.3390/ijms24010734






