Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes
Abstract
1. Introduction
2. Epidemiology of Lean/Normal-Weight T2D
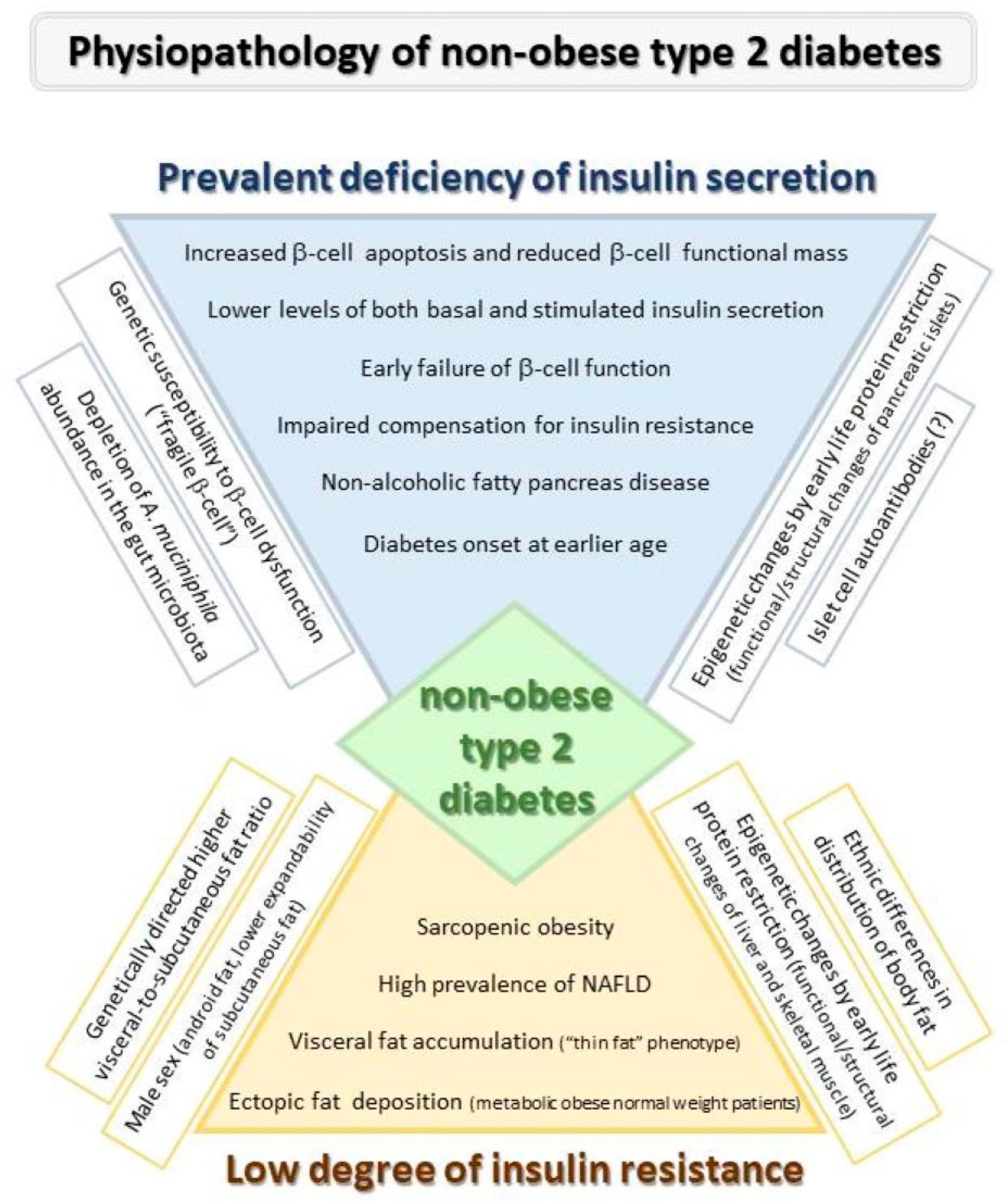
3. Pathophysiology of T2D Development in Absence of Excess Weight
3.1. Role of β-Cell Failure
3.2. Role of Insulin Resistance
4. Factors Influencing Β-Cell Function and Insulin Sensitivity in Non-Obese Diabetes
4.1. Distribution of Body Fat
4.1.1. Visceral Fat and T2D Incidence in Non-Obese Subjects
4.1.2. Fatty Liver Disease and Diabetes Risk in Non-Obese People
4.2. Reduced Mass of Skeletal Muscles
4.3. Genetic Architecture of Non-Obese T2D
4.3.1. Genetic Susceptibility to β-Cell Dysfunction
4.3.2. Genetics of Insulin Resistance and Adipose Tissue Distribution
4.4. Early Life Malnutrition
4.5. Influence of Sex
4.6. Islet Cells Auto-Antibodies and Metabolome Profiling
4.7. Role of Microbiota and Adipokines
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Anti-GAD65 | auto-antibodies against glutamic acid decarboxylase 65 |
| BMI | body mass index |
| CT | computed tomography |
| GWA | genome-wide association |
| HOMA-β | homeostatic model assessment of β-cell function |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| mBMI | metabolite fingerprint of BMI |
| NAFLD | non-alcoholic fatty liver disease |
| NHANES | National Health and Nutrition Examination Survey |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Yacamán-Méndez, D.; Trolle-Lagerros, Y.; Zhou, M.; Monteiro Ponce de Leon, A.; Gudjonsdottir, H.; Tynelius, P.; Lager, A. Life-course trajectories of weight and their impact on the incidence of type 2 diabetes. Sci Rep. 2021, 11, 12494, Erratum in: Sci. Rep. 2021, 11, 18602. [Google Scholar] [CrossRef] [PubMed]
- Hugh-Jones, P. Diabetes in Jamaica. Lancet 1955, 269, 891–897. [Google Scholar] [CrossRef] [PubMed]
- WHO Study Group on Diabetes Mellitus & World Health Organization. Diabetes Mellitus: Report of a WHO Study Group [Meeting Held in Geneva from 11 to 16 February 1985]; World Health Organization: Geneva, Switzerland, 1985; Available online: https://apps.who.int/iris/handle/10665/39592 (accessed on 30 August 2022).
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999; Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 30 August 2022).
- Maiti, S.; Sinha, N.K.; Khan, M.M.; Das, P.K.; Chattopadhyay, J.C. Diabetes in rural individuals of different nutritional status and the alarming situation demands focus more on its under-nutrition association. Arch. Physiol. Biochem. 2015, 121, 26–31. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef]
- Alemu, S.; Dessie, A.; Seid, E.; Bard, E.; Lee, P.T.; Trimble, E.R.; Phillips, D.I.; Parry, E.H. Insulin-requiring diabetes in rural Ethiopia: Should we reopen the case for malnutrition-related diabetes? Diabetologia 2009, 52, 1842–1845. [Google Scholar] [CrossRef]
- Coleman, N.J.; Miernik, J.; Philipson, L.; Fogelfeld, L. Lean versus obese diabetes mellitus patients in the United States minority population. J. Diabetes Complicat. 2014, 28, 500–505. [Google Scholar] [CrossRef]
- Zhu, Y.; Sidell, M.A.; Arterburn, D.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.A.; Koebnick, C.; McCormick, E.; Oshiro, C.; et al. Racial/Ethnic Disparities in the Prevalence of Diabetes and Prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) Multisite Cohort of Adults in the U.S. Diabetes Care 2019, 42, 2211–2219. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- International Diabetes Federation IDF Diabetes Atlas, 10th Edition [Internet]. 2021. Available online: http://www.diabetesatlas.org/ (accessed on 3 October 2022).
- US Census Bureau. American Community Survey 1-Year Estimates: ‘Asian Alone or in Any Combination by Selected Groups’. 2017. Available online: https://www.census.gov/history/pdf/acs15yr-korean62017.pdf (accessed on 30 August 2022).
- Office of National Statistics (2011) UK Population by Ethnicity: Population of England and Wales. Available online: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-andwales/latest#data-sources (accessed on 30 August 2022).
- Carnethon, M.R.; De Chavez, P.J.; Biggs, M.L.; Lewis, C.E.; Pankow, J.S.; Bertoni, A.G.; Golden, S.H.; Liu, K.; Mukamal, K.J.; Campbell-Jenkins, B.; et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012, 308, 581–590, Erratum in: JAMA 2012, 308, 2085. [Google Scholar] [CrossRef]
- Doehner, W.; Erdmann, E.; Cairns, R.; Clark, A.L.; Dormandy, J.A.; Ferrannini, E.; Anker, S.D. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: An analysis of the PROactive study population. Int. J. Cardiol. 2012, 162, 20–26. [Google Scholar] [CrossRef]
- McEwen, L.N.; Karter, A.J.; Waitzfelder, B.E.; Crosson, J.C.; Marrero, D.G.; Mangione, C.M.; Herman, W.H. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD). Diabetes Care 2012, 35, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Dhalwani, N.N.; Papamargaritis, D.; Webb, D.R.; Murphy, G.J.; Davies, M.J.; Khunti, K. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: A systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia 2017, 60, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Li, Y.H.; Hsieh, C.H.; Liu, P.Y.; Lin, G.M. Association of body mass index with all-cause mortality in patients with diabetes: A systemic review and meta-analysis. Cardiovasc. Diagn. Ther. 2016, 6, 109–119. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.J.; Park, S.; Park, Y.G.; Cho, K.H. Body Mass Index-Related Mortality in Patients with Type 2 Diabetes and Heterogeneity in Obesity Paradox Studies: A Dose-Response Meta-Analysis. PLoS ONE 2017, 12, e0168247. [Google Scholar] [CrossRef]
- Tobias, D.K.; Pan, A.; Jackson, C.L.; O’Reilly, E.J.; Ding, E.L.; Willett, W.C.; Manson, J.E.; Hu, F.B. Body-mass index and mortality among adults with incident type 2 diabetes. N. Engl. J. Med. 2014, 370, 233–244, Erratum in: N. Engl. J. Med. 2014, 370, 1368. [Google Scholar] [CrossRef]
- Han, S.J.; Boyko, E.J. The Evidence for an Obesity Paradox in Type 2 Diabetes Mellitus. Diabetes Metab. J. 2018, 42, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Banack, H.R.; Stokes, A. The ‘obesity paradox’ may not be a paradox at all. Int. J. Obes. 2017, 41, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Drucker, D.J. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 891–900. [Google Scholar] [CrossRef]
- Dalton, M.; Cameron, A.J.; Zimmet, P.Z.; Shaw, J.E.; Jolley, D.; Dunstan, D.W.; Welborn, T.A.; AusDiab Steering Committee. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J. Intern. Med. 2003, 254, 555–563. [Google Scholar] [CrossRef]
- Skarfors, E.T.; Selinus, K.I.; Lithell, H.O. Risk factors for developing non-insulin dependent diabetes: A 10 year follow up of men in Uppsala. BMJ 1991, 303, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Austin, P.C.; Manuel, D.G.; Shah, B.R.; Tu, J.V. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011, 34, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Caleyachetty, R.; Barber, T.M.; Mohammed, N.I.; Cappuccio, F.P.; Hardy, R.; Mathur, R.; Banerjee, A.; Gill, P. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 419–426, Erratum in: Lancet Diabetes Endocrinol. 2021, 9, e2. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.L.; Ayles, H.; Beyers, N.; Godfrey-Faussett, P.; Muyoyeta, M.; du Toit, E.; Yudkin, J.S.; Floyd, S. Diabetes mellitus in Zambia and the Western Cape province of South Africa: Prevalence, risk factors, diagnosis and management. Diabetes Res. Clin. Pract. 2016, 118, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Fang, Z.; Huang, W.; Liu, Z.; Chen, Y.; Li, Z.; Zhu, T.; Wang, Q.; Simpson, S.; Taylor, B.V.; et al. Non-Obese Diabetes and Its Associated Factors in an Underdeveloped Area of South China, Guangxi. Int. J. Environ. Res. Public Health 2016, 13, 976. [Google Scholar] [CrossRef]
- Kashima, S.; Inoue, K.; Matsumoto, M.; Akimoto, K. Prevalence and characteristics of non-obese diabetes in Japanese men and women: The Yuport Medical Checkup Center Study. J. Diabetes 2015, 7, 523–530. [Google Scholar] [CrossRef]
- International Diabetes Federation (2017) Clinical Practice Recommendations fo Rmanaging Type 2 Diabetes in Primary Care. Available online: https://www.idf.org/e-library/guidelines/128-idf-clinicalpractice-recommendations-for-managing-type-2-diabetes-inprimary-care.html (accessed on 30 August 2022).
- Gujral, U.P.; Pradeepa, R.; Weber, M.B.; Narayan, K.M.; Mohan, V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013, 1281, 51–63. [Google Scholar] [CrossRef]
- Ntuk, U.E.; Gill, J.M.; Mackay, D.F.; Sattar, N.; Pell, J.P. Ethnic-specific obesity cutoffs for diabetes risk: Cross-sectional study of 490,288 UK biobank participants. Diabetes Care 2014, 37, 2500–2507. [Google Scholar] [CrossRef]
- Gujral, U.P.; Mohan, V.; Pradeepa, R.; Deepa, M.; Anjana, R.M.; Narayan, K.M. Ethnic differences in the prevalence of diabetes in underweight and normal weight individuals: The CARRS and NHANES studies. Diabetes Res. Clin. Pract. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Yu, H.J.; Ho, M.; Liu, X.; Yang, J.; Chau, P.H.; Fong, D.Y.T. Association of weight status and the risks of diabetes in adults: A systematic review and meta-analysis of prospective cohort studies. Int. J. Obes. 2022, 46, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Hagopian, W.A.; Oram, R.; Steck, A.K.; Vehik, K.; Weedon, M.; Balasubramanyam, A.; Dabelea, D. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia 2020, 63, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Gentile, S.; Turco, S.; Guarino, G.; Oliviero, B.; Annunziata, S.; Cozzolino, D.; Torella, R. Effect of treatment with acarbose and insulin in patients with non-insulin-dependent diabetes mellitus associated with non-alcoholic liver cirrhosis. Diabetes Obes. Metab. 2001, 3, 33–40. [Google Scholar] [CrossRef]
- Fukushima, M.; Usami, M.; Ikeda, M.; Nakai, Y.; Taniguchi, A.; Matsuura, T.; Suzuki, H.; Kurose, T.; Yamada, Y.; Seino, Y. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: A cross-sectional study of Japanese type 2 diabetes. Metabolism 2004, 53, 831–835. [Google Scholar] [CrossRef]
- Møller, J.B.; Pedersen, M.; Tanaka, H.; Ohsugi, M.; Overgaard, R.V.; Lynge, J.; Almind, K.; Vasconcelos, N.M.; Poulsen, P.; Keller, C.; et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014, 37, 796–804. [Google Scholar] [CrossRef]
- Huh, K.B.; Lee, H.C.; Kim, H.M.; Cho, Y.W.; Kim, Y.L.; Lee, K.W.; Lee, E.J.; Lim, S.K.; Kim, D.H.; Yoon, J.W. Immunogenetic and nutritional profile in insulin-using youth-onset diabetics in Korea. Diabetes Res. Clin. Pract. 1992, 16, 63–70. [Google Scholar] [CrossRef]
- Abu-Bakare, A.; Taylor, R.; Gill, G.V.; Alberti, K.G. Tropical or malnutrition-related diabetes: A real syndrome? Lancet 1986, 1, 1135–1138. [Google Scholar] [CrossRef]
- Hagroo, A.A.; Verma, N.P.; Datta, P.; Ajmani, N.K.; Vaishnava, H. Observations on lipolysis in ketosis-resistant, growth-onset diabetes. Diabetes 1974, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.K.; Kumar, V.; Ahuja, M.M. Degree of acetonaemia following epinephrine infusion to determine biochemical characterization of diabetes mellitus. Indian J. Med. Res. 1974, 62, 80–85. [Google Scholar] [PubMed]
- Rao, R.H.; Vigg, B.L.; Rao, K.S. Suppressible glucagon secretion in young, ketosis-resistant, type “J” diabetic patients in India. Diabetes 1983, 32, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Bautista, F.P.; Jasul, G., Jr.; Dampil, O.A. Insulin Resistance and β-Cell Function of Lean versus Overweight or Obese Filipino Patients with Newly Diagnosed Type 2 Diabetes Mellitus. J. ASEAN Fed. Endocr. Soc. 2019, 34, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kibirige, D.; Sekitoleko, I.; Lumu, W.; Jones, A.G.; Hattersley, A.T.; Smeeth, L.; Nyirenda, M.J. Understanding the pathogenesis of lean non-autoimmune diabetes in an African population with newly diagnosed diabetes. Diabetologia 2022, 65, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Ewing, S.K.; Liu, K.; Blaha, M.J.; Dave, S.S.; Qureshi, F.; Kandula, N.R. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care 2014, 37, 1621–1628. [Google Scholar] [CrossRef]
- Hulman, A.; Simmons, R.K.; Brunner, E.J.; Witte, D.R.; Færch, K.; Vistisen, D.; Ikehara, S.; Kivimaki, M.; Tabák, A.G. Trajectories of glycaemia, insulin sensitivity and insulin secretion in South Asian and white individuals before diagnosis of type 2 diabetes: A longitudinal analysis from the Whitehall II cohort study. Diabetologia 2017, 60, 1252–1260. [Google Scholar] [CrossRef]
- Siddiqui, M.K.; Anjana, R.M.; Dawed, A.Y.; Martoeau, C.; Srinivasan, S.; Saravanan, J.; Madanagopal, S.K.; Taylor, A.; Bell, S.; Veluchamy, A.; et al. Young-onset diabetes in Asian Indians is associated with lower measured and genetically determined beta cell function. Diabetologia 2022, 65, 973–983. [Google Scholar] [CrossRef]
- Zaharia, O.P.; Strassburger, K.; Strom, A.; Bönhof, G.J.; Karusheva, Y.; Antoniou, S.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Müssig, K.; et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: A 5-year follow-up study. Lancet Diabetes Endocrinol. 2019, 7, 684–694. [Google Scholar] [CrossRef]
- Prasad, R.B.; Asplund, O.; Shukla, S.R.; Wagh, R.; Kunte, P.; Bhat, D.; Parekh, M.; Shah, M.; Phatak, S.; Käräjämäki, A.; et al. Subgroups of patients with young-onset type 2 diabetes in India reveal insulin deficiency as a major driver. Diabetologia 2022, 65, 65–78. [Google Scholar] [CrossRef]
- Reaven, G.M.; Doberne, L.; Greenfield, M.S. Comparison of insulin secretion and in vivo insulin action in nonobese and moderately obese individuals with non-insulin-dependent diabetes mellitus. Diabetes 1982, 31 Pt 1, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, C.B.; Chen, Y.D.; Reaven, G.M. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes 1984, 33, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Sasso, F.C. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Shulman, G.I. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Conus, F.; Rabasa-Lhoret, R.; Péronnet, F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl. Physiol. Nutr. Metab. 2007, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Masharani, U.B.; Maddux, B.A.; Li, X.; Sakkas, G.K.; Mulligan, K.; Schambelan, M.; Goldfine, I.D.; Youngren, J.F. Insulin resistance in non-obese subjects is associated with activation of the JNK pathway and impaired insulin signaling in skeletal muscle. PLoS ONE 2011, 6, e19878. [Google Scholar] [CrossRef]
- Abate, N.; Garg, A.; Peshock, R.M.; Stray-Gundersen, J.; Adams-Huet, B.; Grundy, S.M. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes 1996, 45, 1684–1693. [Google Scholar] [CrossRef]
- Taniguchi, A.; Nakai, Y.; Sakai, M.; Yoshii, S.; Hayashi, M.; Nishitani, K.; Hamanaka, D.; Nakaishi, S.; Kamamoto, T.; Nagata, I.; et al. Relationship of regional adiposity to insulin resistance in nonobese Japanese type 2 diabetic patients. Diabetes Care 2001, 24, 966–968. [Google Scholar] [CrossRef][Green Version]
- Banerji, M.A.; Chaiken, R.L.; Gordon, D.; Kral, J.G.; Lebovitz, H.E. Does intra-abdominal adipose tissue in black men determine whether NIDDM is insulin-resistant or insulin-sensitive? Diabetes 1995, 44, 141–146. [Google Scholar] [CrossRef]
- Sharp, P.S.; Mohan, V.; Levy, J.C.; Mather, H.M.; Kohner, E.M. Insulin resistance in patients of Asian Indian and European origin with non-insulin dependent diabetes. Horm. Metab. Res. 1987, 19, 84–85. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Godsland, I.F.; Whincup, P.; Forouhi, N.G.; Welsh, P.; Sattar, N.; McKeigue, P.M.; Chaturvedi, N. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: The Southall And Brent REvisited (SABRE) cohort. Diabetes Care 2013, 36, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Anoop, S.; Misra, A.; Bhatt, S.P.; Gulati, S.; Mahajan, H. High fasting C-peptide levels and insulin resistance in non-lean & non-obese (BMI>19 to <25 kg/m2) Asian Indians with type 2 diabetes are independently associated with high intra-abdominal fat and liver span. Diabetes Metab. Syndr. 2019, 13, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Parkinson, J.R.; Frost, G.S.; Goldstone, A.P.; Doré, C.J.; McCarthy, J.P.; Collins, A.L.; Fitzpatrick, J.A.; Durighel, G.; Taylor-Robinson, S.D.; et al. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity 2012, 20, 76–87. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A system¬atic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 956–966. [Google Scholar] [CrossRef]
- Ding, C.; Chan, Z.; Magkos, F. Lean, but not healthy: The ‘metabolically obese, normal-weight’ phenotype. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 408–417. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim. Biophys. Acta 2010, 1801, 338–349. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Gentile, S.; Turco, S.; Guarino, G.; Sasso, C.F.; Amodio, M.; Magliano, P.; Torella, R. Comparative efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes Obes. Metab. 2000, 2, 355–362. [Google Scholar] [CrossRef]
- Sasso, F.C.; Salvatore, T.; Tranchino, G.; Cozzolino, D.; Caruso, A.A.; Persico, M.; Torella, R. Cochlear dysfunction in type 2 diabetes: A complication independent of neuropathy and acute hyperglycemia. Metabolism 1999, 48, 1346–1350. [Google Scholar] [CrossRef]
- Torella, D.; Iaconetti, C.; Tarallo, R.; Marino, F.; Giurato, G.; Veneziano, C.; Indolfi, C. miRNA Regulation of the Hyperproliferative Phenotype of Vascular Smooth Muscle Cells in Diabetes. Diabetes 2018, 67, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Yudkin, J.S. The Y-Y paradox. Lancet 2004, 363, 163. [Google Scholar] [CrossRef] [PubMed]
- Yaghootkar, H.; Scott, R.A.; White, C.C.; Zhang, W.; Speliotes, E.; Munroe, P.B.; Ehret, G.B.; Bis, J.C.; Fox, C.S.; Walker, M.; et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 2014, 63, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Fall, T.; Pasko, D.; Barker, A.; Sharp, S.J.; Arriola, L.; Balkau, B.; Barricarte, A.; Barroso, I.; Boeing, H.; et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes 2014, 63, 4378–4387. [Google Scholar] [CrossRef] [PubMed]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef]
- Rydén, M.; Andersson, D.P.; Bergström, I.B.; Arner, P. Adipose tissue and metabolic alterations: Regional differences in fat cell size and number matter, but differently: A cross-sectional study. J. Clin. Endocrinol. Metab. 2014, 99, E1870–E1876. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Jerosch-Herold, M.; Lima, J.A.; Ding, J.; Allison, M.A. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef]
- Kuwahara, K.; Honda, T.; Nakagawa, T.; Yamamoto, S.; Hayashi, T.; Mizoue, T. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci. Rep. 2017, 7, 43521. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Saldana, S.; Carnethon, M.R.; Chen, H.; Effoe, V.; Golden, S.H.; Joseph, J.; Kalyani, R.R.; Bertoni, A.G. Determinants of Metabolic Syndrome and Type 2 Diabetes in the Absence of Obesity: The Jackson Heart Study. J. Endocr. Soc. 2022, 6, bvac059. [Google Scholar] [CrossRef]
- Xu, R.; Pan, J.; Zhou, W.; Ji, G.; Dang, Y. Recent advances in lean NAFLD. Biomed. Pharmacother. 2022, 153, 113331. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Morone, M.V.; Silvestri, C.; Giordano, M.; Salvatore, T.; Sasso, F.C. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants 2021, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Caturano, A.; Vetrano, E.; Cesaro, A.; Rinaldi, L.; Salvatore, T.; Sasso, F.C. Pathophysiological mechanisms and clinical evidence of relationship between Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev. Cardiovasc. Med. 2021, 22, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef]
- Cozzolino, D.; Sessa, G.; Salvatore, T.; Sasso, F.C.; Giugliano, D.; Lefebvre, P.J.; Torella, R. The involvement of the opioid system in human obesity: A study in normal weight relatives of obese people. J. Clin. Endocrinol. Metab. 1996, 81, 713–718. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Wei, Z.M.; Li, N.; Sun, L.L.; Jin, Z.Y.; Wu, Z.; Sun, H. Quantitative analysis of the risk of type 2 diabetes and fatty liver in non-obese individuals by computed tomography. Abdom. Radiol. 2022, 47, 2099–2105. [Google Scholar] [CrossRef]
- Wei, L.; Cheng, X.; Luo, Y.; Yang, R.; Lei, Z.; Jiang, H.; Chen, L. Lean non-alcoholic fatty liver disease and risk of incident diabetes in a euglycaemic population undergoing health check-ups: A cohort study. Diabetes Metab. 2021, 47, 101200. [Google Scholar] [CrossRef]
- Misra, A.; Anoop, S.; Gulati, S.; Mani, K.; Bhatt, S.P.; Pandey, R.M. Body Fat Patterning, Hepatic Fat and Pancreatic Volume of Non-Obese Asian Indians with Type 2 Diabetes in North India: A Case-Control Study. PLoS ONE 2015, 10, e0140447, Erratum in: PLoS ONE 2015, 10, e0142749. [Google Scholar] [CrossRef]
- Hill, J.O.; Sidney, S.; Lewis, C.E.; Tolan, K.; Scherzinger, A.L.; Stamm, E.R. Racial differences in amounts of visceral adipose tissue in young adults: The CARDIA (Coronary Artery Risk Development in Young Adults) study. Am. J. Clin. Nutr. 1999, 69, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Chiapa, A.L.; Rodriquez, M.; Phelps, D.R.; Cardarelli, K.M.; Vishwanatha, J.K.; Cardarelli, R. Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity 2008, 16, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Golden, S.H.; Allison, M.A.; Ding, J.; Ouyang, P.; Schreiner, P.J.; Anderson, C.A. The sex and race specific relationship between anthropometry and body fat composition determined from computed tomography: Evidence from the Multi-Ethnic Study of Atherosclerosis. PLoS ONE 2015, 10, e0139559. [Google Scholar] [CrossRef] [PubMed]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas 2010, 65, 315–319. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in: Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Gil, M.J.; Valentí, V.; Rotellar, F.; Ramírez, B.; Salvador, J.; et al. Body adiposity and type 2 diabetes: Increased risk with a high body fat percentage even having a normal BMI. Obesity 2011, 19, 1439–1444. [Google Scholar] [CrossRef]
- Florez, H.; Castillo-Florez, S. Beyond the obesity paradox in diabetes: Fitness, fatness, and mortality. JAMA 2012, 308, 619–620. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988, 255 Pt 1, E769–E774. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Sasso, F.C. Metformin: An old drug against old age and associated morbidities. Diabetes Res. Clin. Pract. 2020, 160, 108025. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Jones, J.D.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 National Health and Nutrition Examination Survey. Clin. Nutr. 2016, 35, 1472–1483. [Google Scholar] [CrossRef]
- Bower, J.K.; Meadows, R.J.; Foster, M.C.; Foraker, R.E.; Shoben, A.B. The Association of Percent Body Fat and Lean Mass With HbA1c in US Adults. J. Endocr. Soc. 2017, 1, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Chang, Y.; Jung, H.S.; Yun, K.E.; Shin, H.; Ryu, S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PLoS ONE 2017, 12, e0188650. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, Y.; Misumi, M.; Kim, Y.M.; Yamada, M.; Ohishi, W.; Fujiwara, S.; Nakanishi, S.; Yoneda, M. Body composition and development of diabetes: A 15-year follow-up study in a Japanese population. Eur. J. Clin. Nutr. 2018, 72, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wittert, G.A.; Vincent, A.; Atlantis, E.; Shi, Z.; Appleton, S.L.; Hill, C.L.; Jenkins, A.J.; Januszewski, A.S.; Adams, R.J. Muscle grip strength predicts incident type 2 diabetes: Population-based cohort study. Metabolism 2016, 65, 883–892. [Google Scholar] [CrossRef]
- Larsen, B.A.; Wassel, C.L.; Kritchevsky, S.B.; Strotmeyer, E.S.; Criqui, M.H.; Kanaya, A.M.; Fried, L.F.; Schwartz, A.V.; Harris, T.B.; Ix, J.H.; et al. Association of Muscle Mass, Area, and Strength With Incident Diabetes in Older Adults: The Health ABC Study. J. Clin. Endocrinol. Metab. 2016, 101, 1847–1855. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Braun, W.; Geisler, C.; Norman, K.; Müller, M.J. Body composition and cardiometabolic health: The need for novel concepts. Eur. J. Clin. Nutr. 2018, 72, 638–644. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Y.; Zhang, H.; Chen, C.; Wang, Y.; Zhang, J.; Xia, F.; Benedict, C.; Tan, X.; Lu, Y. Total and regional fat-to-muscle mass ratio measured by bioelectrical impedance and risk of incident type 2 diabetes. J. Cachexia Sarcopenia Muscle. 2021, 12, 2154–2162. [Google Scholar] [CrossRef]
- Baker, C.F.; Overvad, K.; Dahm, C.C. Lean body mass and risk of type 2 diabetes—A Danish cohort study. J. Diabetes Metab. Disord. 2019, 18, 445–451. [Google Scholar] [CrossRef]
- Rehunen, S.K.J.; Kautiainen, H.; Korhonen, P.E.; Eriksson, J.G. A high lean body mass is not protecting from type 2 diabetes in the presence of a high body fat mass. Diabetes Metab. 2021, 47, 101219. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.S.; Leong, A.; Porneala, B.C.; Meigs, J.B.; Miller, K.K. Association between muscle mass and diabetes prevalence independent of body fat distribution in adults under 50 years old. Nutr. Diabetes 2022, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.; Dichtel, L.E.; Gerweck, A.V.; Valera, R.D.; Torriani, M.; Miller, K.K.; Bredella, M.A. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex Differ. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Li, L.S.; Glaser, A.; Ostenson, C.G.; Jiao, H.; Fakhrai-Rad, H.; Jacob, H.J.; Lander, E.S.; Luthman, H. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat. Genet. 1996, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Clark, A.; Rorsman, P. β-cell secretory dysfunction: A key cause of type 2 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 370. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589, Erratum in: Nat. Genet. 2011, 43, 388. [Google Scholar] [CrossRef]
- Perry, J.R.; Voight, B.F.; Yengo, L.; Amin, N.; Dupuis, J.; Ganser, M.; Grallert, H.; Navarro, P.; Li, M.; Qi, L.; et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012, 8, e1002741. [Google Scholar] [CrossRef]
- Okamoto, K.; Iwasaki, N.; Doi, K.; Noiri, E.; Iwamoto, Y.; Uchigata, Y.; Fujita, T.; Tokunaga, K. Inhibition of glucose-stimulated insulin secretion by KCNJ15, a newly identified susceptibility gene for type 2 diabetes. Diabetes 2012, 61, 1734–1741. [Google Scholar] [CrossRef]
- Ke, C.; Narayan, K.M.V.; Chan, J.C.N.; Jha, P.; Shah, B.R. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat. Rev. Endocrinol. 2022, 18, 413–432. [Google Scholar] [CrossRef]
- Cho, Y.S.; Chen, C.H.; Hu, C.; Long, J.; Ong, R.T.; Sim, X.; Takeuchi, F.; Wu, Y.; Go, M.J.; Yamauchi, T.; et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat. Genet. 2011, 44, 67–72. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Sim, X. Progress in Defining the Genetic Contribution to Type 2 Diabetes in Individuals of East Asian Ancestry. Curr. Diab. Rep. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.A.; Tekola-Ayele, F.; Doumatey, A.P.; Bentley, A.R.; Chen, G.; Huang, H.; Rotimi, C.N. Evaluation of Genome Wide Association Study Associated Type 2 Diabetes Susceptibility Loci in Sub Saharan Africans. Front. Genet. 2015, 6, 335. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.A.; Zaghloul, N.A.; Chen, G.; Doumatey, A.P.; Leitch, C.C.; Hostelley, T.L.; Nesmith, J.E.; Zhou, J.; Bentley, A.R.; Shriner, D.; et al. ZRANB3 is an African-specific type 2 diabetes locus associated with beta-cell mass and insulin response. Nat. Commun. 2019, 10, 3195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, L. Gene-Environment Interactions on Body Fat Distribution. Int. J. Mol. Sci. 2019, 20, 3690. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xing, X.; Hong, J.; Zhang, X.; Yang, W. Genetic variants associated with lean and obese type 2 diabetes in a Han Chinese population: A case-control study. Medicine 2016, 95, e3841, Erratum in: Medicine 2016, 95, e0916. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Whitcher, B.; Bell, J.D.; Thomas, E.L. Ethnic differences in adiposity and diabetes risk—Insights from genetic studies. J. Intern. Med. 2020, 288, 271–283. [Google Scholar] [CrossRef]
- Perumalsamy, S.; Wan Ahmad, W.A.; Zaman Huri, H. Single Nucleotide Polymorphism rs17173608 in the Chemerin Encoding Gene: Is It a Predictor of Insulin Resistance and Severity of Coronary Artery Disease in Non-Obese Type 2 Diabetes? Healthcare 2021, 9, 623. [Google Scholar] [CrossRef]
- Stein, A.D.; Obrutu, O.E.; Behere, R.V.; Yajnik, C.S. Developmental undernutrition, offspring obesity and type 2 diabetes. Diabetologia 2019, 62, 1773–1778. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Roseboom, T.J.; Painter, R.C. Famines in the last 100 years: Implications for diabetes. Curr. Diab. Rep. 2014, 14, 536. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Temple, N.; Woodside, J.V. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Blesson, C.S.; Schutt, A.K.; Balakrishnan, M.P.; Pautler, R.G.; Pedersen, S.E.; Sarkar, P.; Gonzales, D.; Zhu, G.; Marini, J.C.; Chacko, S.K.; et al. Novel lean type 2 diabetic rat model using gestational low-protein programming. Am. J. Obstet. Gynecol. 2016, 214, 540.e1. [Google Scholar] [CrossRef]
- Vipin, V.A.; Blesson, C.S.; Yallampalli, C. Maternal low protein diet and fetal programming of lean type 2 diabetes. World J. Diabetes 2022, 13, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Hoile, S.P.; Lillycrop, K.A.; Thomas, N.A.; Hanson, M.A.; Burdge, G.C. Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring. PLoS ONE 2011, 6, e21668. [Google Scholar] [CrossRef]
- Sandovici, I.; Smith, N.H.; Nitert, M.D.; Ackers-Johnson, M.; Uribe-Lewis, S.; Ito, Y.; Jones, R.H.; Marquez, V.E.; Cairns, W.; Tadayyon, M.; et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc. Natl. Acad. Sci. USA 2011, 108, 5449–5454. [Google Scholar] [CrossRef]
- Jia, Y.; Cong, R.; Li, R.; Yang, X.; Sun, Q.; Parvizi, N.; Zhao, R. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. J. Nutr. 2012, 142, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, N.; Raychaudhuri, S.; Thamotharan, M.; Devaskar, S.U. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J. Biol. Chem. 2008, 283, 13611–13626. [Google Scholar] [CrossRef]
- Hardikar, A.A.; Satoor, S.N.; Karandikar, M.S.; Joglekar, M.V.; Puranik, A.S.; Wong, W.; Kumar, S.; Limaye, A.; Bhat, D.S.; Januszewski, A.S.; et al. Multigenerational Undernutrition Increases Susceptibility to Obesity and Diabetes that Is Not Reversed after Dietary Recuperation. Cell Metab. 2015, 22, 312–319. [Google Scholar] [CrossRef]
- Jia, Y.; Li, R.; Cong, R.; Yang, X.; Sun, Q.; Parvizi, N.; Zhao, R. Maternal low-protein diet affects epigenetic regulation of hepatic mitochondrial DNA transcription in a sex-specific manner in newborn piglets associated with GR binding to its promoter. PLoS ONE 2013, 8, e63855. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, X.; Li, Y.; Li, F.; Cheng, Y.; Peng, Y.; Song, D.; Hong, J.; Ning, G.; Cao, Y.; et al. Maternal Low Protein Isocaloric Diet Suppresses Pancreatic β-Cell Proliferation in Mouse Offspring via miR-15b. Endocrinology 2016, 157, 4782–4793. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, Q.; Wang, T.; Yu, M.; Xu, J. Maternal Low-Protein Diet Modulates Glucose Metabolism and Hepatic MicroRNAs Expression in the Early Life of Offspring. Nutrients 2017, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Yajnik, C.S. Developmental origins of diabetes-an Indian perspective. Eur. J. Clin. Nutr. 2017, 71, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Sathishkumar, K.; Chinnathambi, V.; Yallampalli, C. Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology 2014, 155, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Chinnathambi, V.; Kumar, S.; Yallampalli, C. Gestational Protein Restriction Impairs Glucose Disposal in the Gastrocnemius Muscles of Female Rats. Endocrinology 2017, 158, 756–767. [Google Scholar] [CrossRef][Green Version]
- Claycombe, K.J.; Roemmich, J.N.; Johnson, L.; Vomhof-DeKrey, E.E.; Johnson, W.T. Skeletal muscle Sirt3 expression and mitochondrial respiration are regulated by a prenatal low-protein diet. J. Nutr. Biochem. 2015, 26, 184–189. [Google Scholar] [CrossRef]
- Döring, F.; Lüersen, K.; Schmelzer, C.; Hennig, S.; Lang, I.S.; Görs, S.; Rehfeldt, C.; Otten, W.; Metges, C.C. Influence of maternal low protein diet during pregnancy on hepatic gene expression signature in juvenile female porcine offspring. Mol. Nutr. Food Res. 2013, 57, 277–290. [Google Scholar] [CrossRef]
- Blesson, C.S.; Schutt, A.; Chacko, S.; Marini, J.C.; Mathew, P.R.; Tanchico, D.; Balakrishnan, M.; Yallampalli, C. Sex Dependent Dysregulation of Hepatic Glucose Production in Lean Type 2 Diabetic Rats. Front. Endocrinol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Rake, A.; Melchior, K.; Rohde, W.; Dörner, G. Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J. Nutr. 2000, 130, 2582–2589. [Google Scholar] [CrossRef]
- Mericq, V.; Martinez-Aguayo, A.; Uauy, R.; Iñiguez, G.; Van der Steen, M.; Hokken-Koelega, A. Long-term metabolic risk among children born premature or small for gestational age. Nat. Rev. Endocrinol. 2017, 13, 50–62. [Google Scholar] [CrossRef]
- Durst, M.; Könczöl, K.; Ocskay, K.; Sípos, K.; Várnai, P.; Szilvásy-Szabó, A.; Fekete, C.; Tóth, Z.E. Hypothalamic Nesfatin-1 Resistance May Underlie the Development of Type 2 Diabetes Mellitus in Maternally Undernourished Non-obese Rats. Front. Neurosci. 2022, 16, 828571. [Google Scholar] [CrossRef]
- Sasaki, H.; Saisho, Y.; Inaishi, J.; Watanabe, Y.; Tsuchiya, T.; Makio, M.; Sato, M.; Kitago, M.; Yamada, T.; Itoh, H. Associations of birthweight and history of childhood obesity with beta cell mass in Japanese adults. Diabetologia 2020, 63, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, M.; Kharkwal, N.; Tiwari, A.; Gupta, K.K. A Cross Sectional Study of Type 2 Diabetes Mellitus Comparing Different Factors between Lean Body Weight, Non Obese and Obese Patients in Western Uttar Pradesh. J. Curr. Med. Res. Opin. 2020, 3, 405–409. [Google Scholar] [CrossRef]
- Camilleri, G.; Kiani, A.K.; Herbst, K.L.; Kaftalli, J.; Bernini, A.; Dhuli, K.; Manara, E.; Bonetti, G.; Stuppia, L.; Paolacci, S.; et al. Genetics of fat deposition. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 14–22. [Google Scholar] [CrossRef]
- Karpe, F.; Pinnick, K.E. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat. Rev. Endocrinol. 2015, 11, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Bloor, I.D.; Sébert, S.P.; Saroha, V.; Gardner, D.S.; Keisler, D.H.; Budge, H.; Symonds, M.E.; Mahajan, R.P. Sex differences in metabolic and adipose tissue responses to juvenile-onset obesity in sheep. Endocrinology 2013, 154, 3622–3631. [Google Scholar] [CrossRef]
- Wallace, J.M.; Milne, J.S.; Aitken, R.P.; Redmer, D.A.; Reynolds, L.P.; Luther, J.S.; Horgan, G.W.; Adam, C.L. Undernutrition and stage of gestation influence fetal adipose tissue gene expression. J. Mol. Endocrinol. 2015, 54, 263–275. [Google Scholar] [CrossRef]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Lyngman, L.K.; Mansouryar, M.; Dhakal, R.; Agerholm, J.S.; Khanal, P.; Nielsen, M.O. Depot and sex-specific implications for adipose tissue expandability and functional traits in adulthood of late prenatal and early postnatal malnutrition in a precocial sheep model. Physiol. Rep. 2020, 8, e14600. [Google Scholar] [CrossRef]
- Vistisen, B.; Hellgren, L.I.; Vadset, T.; Scheede-Bergdahl, C.; Helge, J.W.; Dela, F.; Stallknecht, B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur. J. Endocrinol. 2008, 158, 61–68. [Google Scholar] [CrossRef]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010, 59, 89–97. [Google Scholar] [CrossRef]
- Perreault, L.; Bergman, B.C.; Hunerdosse, D.M.; Eckel, R.H. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity 2010, 18, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Capllonch-Amer, G.; Lladó, I.; Proenza, A.M.; García-Palmer, F.J.; Gianotti, M. Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J. Mol. Endocrinol. 2014, 52, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Vijayaprabha, R.; Rema, M.; Premalatha, G.; Poongothai, S.; Deepa, R.; Bhatia, E.; Mackay, I.R.; Zimmet, P. Clinical profile of lean NIDDM in South India. Diabetes Res. Clin. Pract. 1997, 38, 101–108, Erratum in: Diabetes Res. Clin. Pract. 1998, 41, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Song, E.H.; Lee, H.J.; Oh, Y.K.; Park, Y.S.; Park, J.W.; Kim, B.J.; Kim, D.J.; Lee, I.; Song, J.; et al. Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J. Biol. Chem. 2010, 285, 37251–37262. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.; Park, S.I. Associations of alcohol consumption and physical activity with lean type 2 diabetes mellitus among Korean adults: A prospective cohort study. PLoS ONE 2020, 15, e0238641. [Google Scholar] [CrossRef]
- Zhang, L.; Curhan, G.C.; Hu, F.B.; Rimm, E.B.; Forman, J.P. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care 2011, 34, 892–897. [Google Scholar] [CrossRef]
- Goswami, R.; Kochupillai, N.; Gupta, N.; Kukreja, A.; Lan, M.; Maclaren, N.K. Islet cell autoimmunity in youth onset diabetes mellitus in Northern India. Diabetes Res. Clin. Pract. 2001, 53, 47–54. [Google Scholar] [CrossRef]
- Unnikrishnan, A.G.; Singh, S.K.; Sanjeevi, C.B. Prevalence of GAD65 antibodies in lean subjects with type 2 diabetes. Ann. N. Y. Acad. Sci. 2004, 1037, 118–121. [Google Scholar] [CrossRef]
- Mahadeb, Y.P.; Gruson, D.; Buysschaert, M.; Hermans, M.P. What are the characteristics of phenotypic type 2 diabetic patients with low-titer GAD65 antibodies? Acta Diabetol. 2014, 51, 103–111. [Google Scholar] [CrossRef]
- Koopman, A.D.M.; Beulens, J.W.; Voerman, E.; Rauh, S.P.; van der Heijden, A.A.; McDonald, T.J.; Langendoen-Gort, M.; Rutters, F. The association between GAD65 antibody levels and incident Type 2 Diabetes Mellitus in an adult population: A meta-analysis. Metabolism 2019, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Smith, E.; Ericson, U.; Brunkwall, L.; Orho-Melander, M.; Di Somma, S.; Antonini, P.; Nilsson, P.M.; Fernandez, C.; Melander, O. Metabolome-Defined Obesity and the Risk of Future Type 2 Diabetes and Mortality. Diabetes Care 2022, 45, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, e2100536. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.S.; Wang, C.Y.; Hung, W.W.; Hung, W.C.; Tsai, H.J.; Chang, C.C.; Hwang, S.J.; Dai, C.Y.; Ho, W.Y.; Tsai, Y.C. Interrelationship of Gut Microbiota, Obesity, Body Composition and Insulin Resistance in Asians with Type 2 Diabetes Mellitus. J. Pers. Med. 2022, 12, 617. [Google Scholar] [CrossRef]
- Lee, S.H. Adipokine Profiles and Metabolic Health. Endocrinol. Metab. 2015, 30, 175–176. [Google Scholar] [CrossRef]
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef]
- Kocot, J.; Dziemidok, P.; Kiełczykowska, M.; Hordyjewska, A.; Szcześniak, G.; Musik, I. Adipokine Profile in Patients with Type 2 Diabetes Depends on Degree of Obesity. Med. Sci. Monit. 2017, 23, 4995–5004. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Zhang, S.; Cai, X.; Zhang, R.; Gong, S.; Han, X.; Ji, L. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus: A population-based study. Medicine 2020, 99, e19052. [Google Scholar] [CrossRef]
- Mir, M.M.; Mir, R.; Alghamdi, M.A.A.; Wani, J.I.; Sabah, Z.U.; Jeelani, M.; Marakala, V.; Sohail, S.K.; O’haj, M.; Alharthi, M.H.; et al. Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study. J. Pers. Med. 2022, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Criscuolo, L.; Di Martino, A.; Albanese, G.; Vetrano, E.; Catalini, C.; Sardu, C.; et al. Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 658. https://doi.org/10.3390/ijms24010658
Salvatore T, Galiero R, Caturano A, Rinaldi L, Criscuolo L, Di Martino A, Albanese G, Vetrano E, Catalini C, Sardu C, et al. Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes. International Journal of Molecular Sciences. 2023; 24(1):658. https://doi.org/10.3390/ijms24010658
Chicago/Turabian StyleSalvatore, Teresa, Raffaele Galiero, Alfredo Caturano, Luca Rinaldi, Livio Criscuolo, Anna Di Martino, Gaetana Albanese, Erica Vetrano, Christian Catalini, Celestino Sardu, and et al. 2023. "Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes" International Journal of Molecular Sciences 24, no. 1: 658. https://doi.org/10.3390/ijms24010658
APA StyleSalvatore, T., Galiero, R., Caturano, A., Rinaldi, L., Criscuolo, L., Di Martino, A., Albanese, G., Vetrano, E., Catalini, C., Sardu, C., Docimo, G., Marfella, R., & Sasso, F. C. (2023). Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes. International Journal of Molecular Sciences, 24(1), 658. https://doi.org/10.3390/ijms24010658








