Transcriptome Analysis Reveals the Mechanisms of Tolerance to High Concentrations of Calcium Chloride Stress in Parachlorella kessleri
Abstract
:1. Introduction
2. Results
2.1. Physiological Analysis
2.1.1. Algal Growth
2.1.2. Photosynthetic Activity
2.1.3. Chlorophyll Content
2.1.4. Antioxidant Enzyme Activity
2.2. Morphological Observation
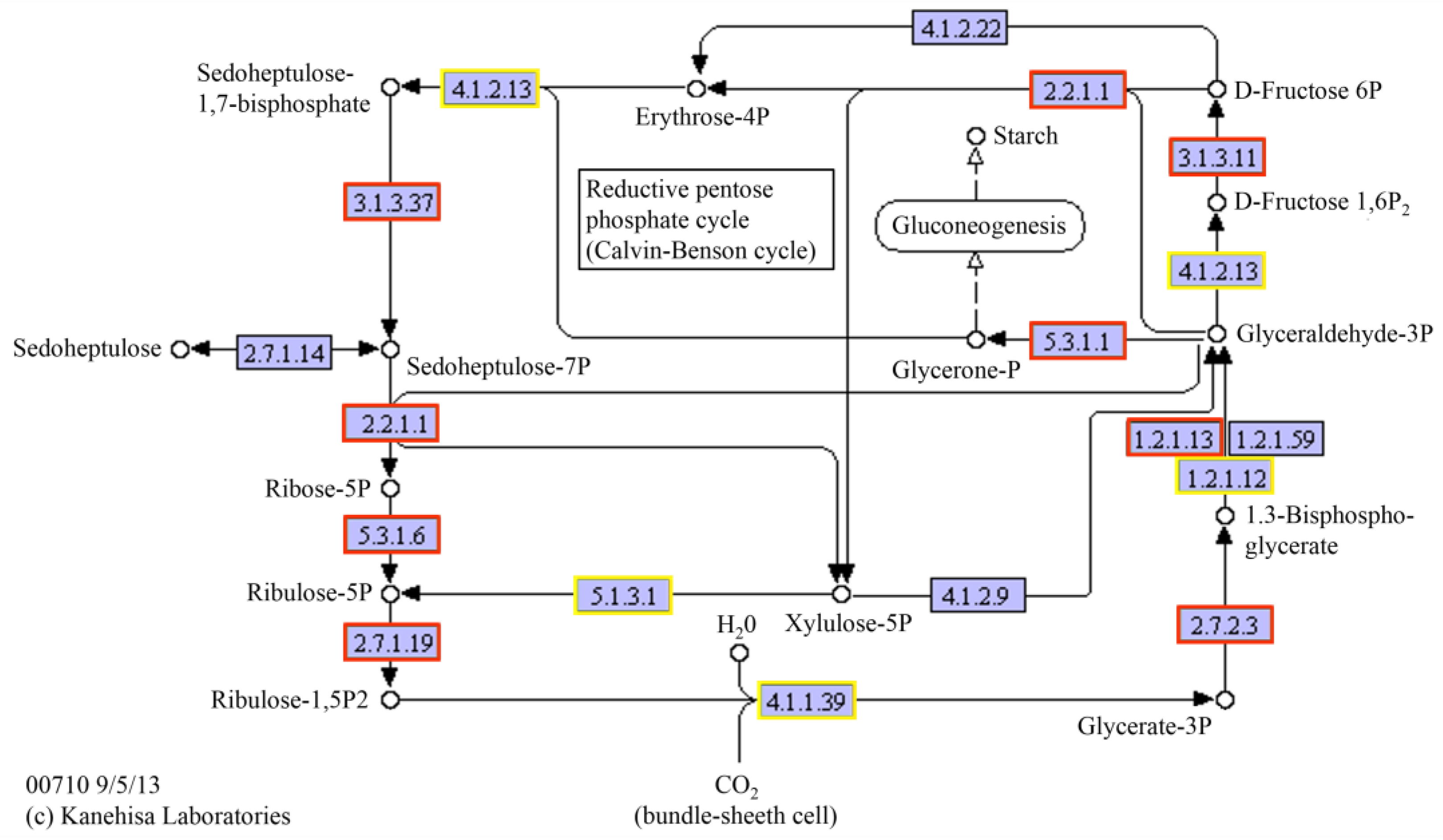
2.3. Transcriptome Analysis
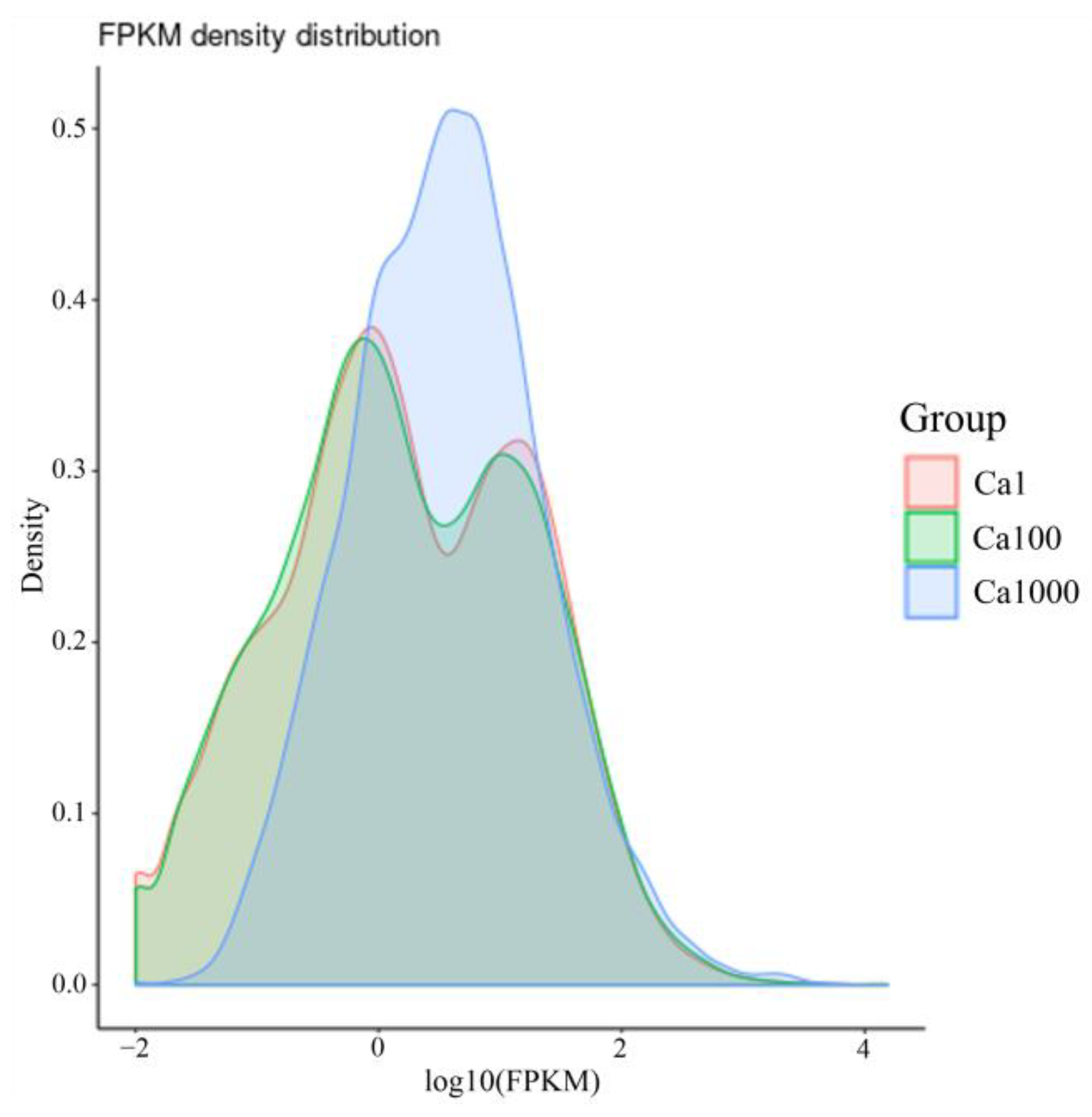
2.3.1. Transcriptome Assembly
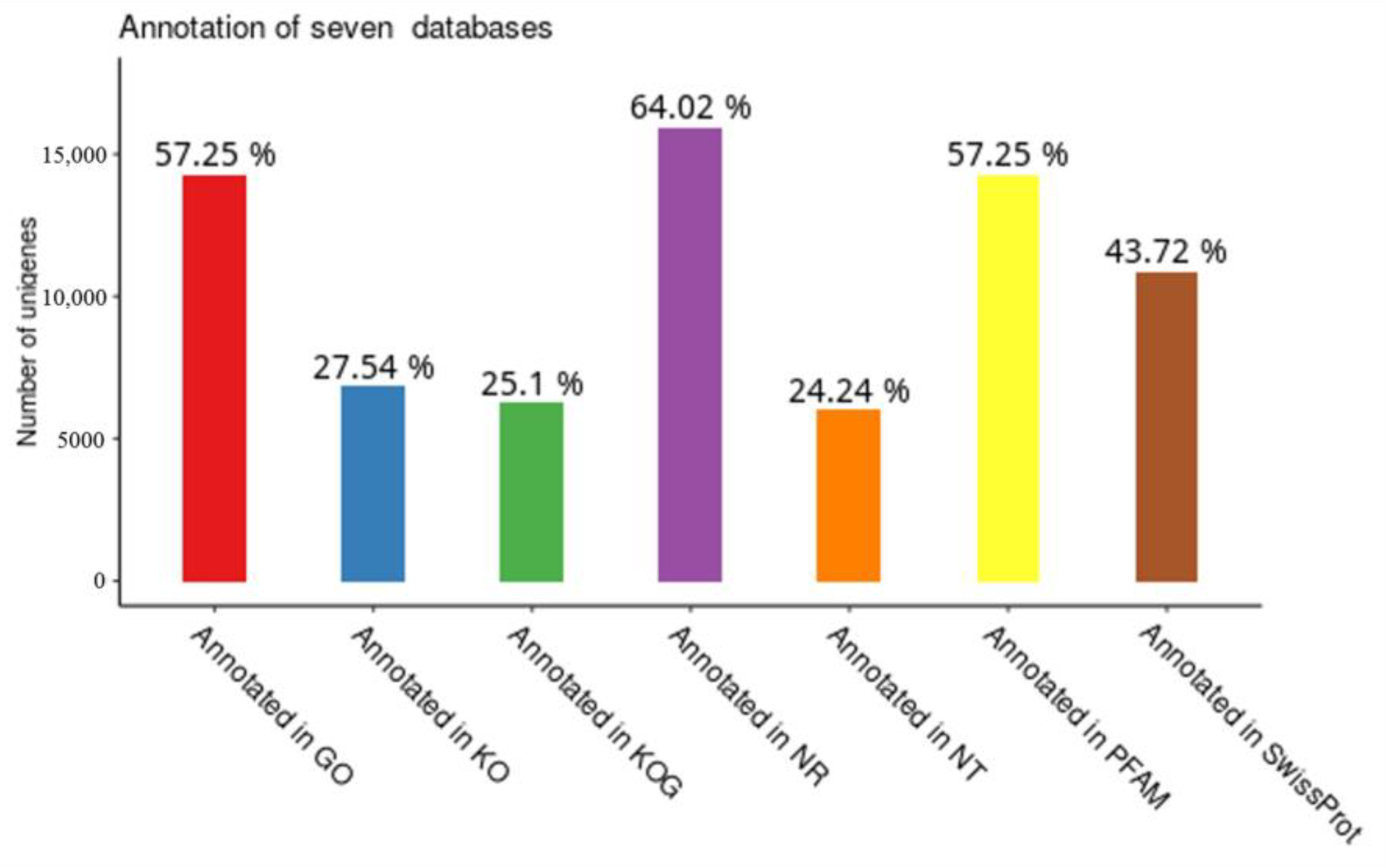
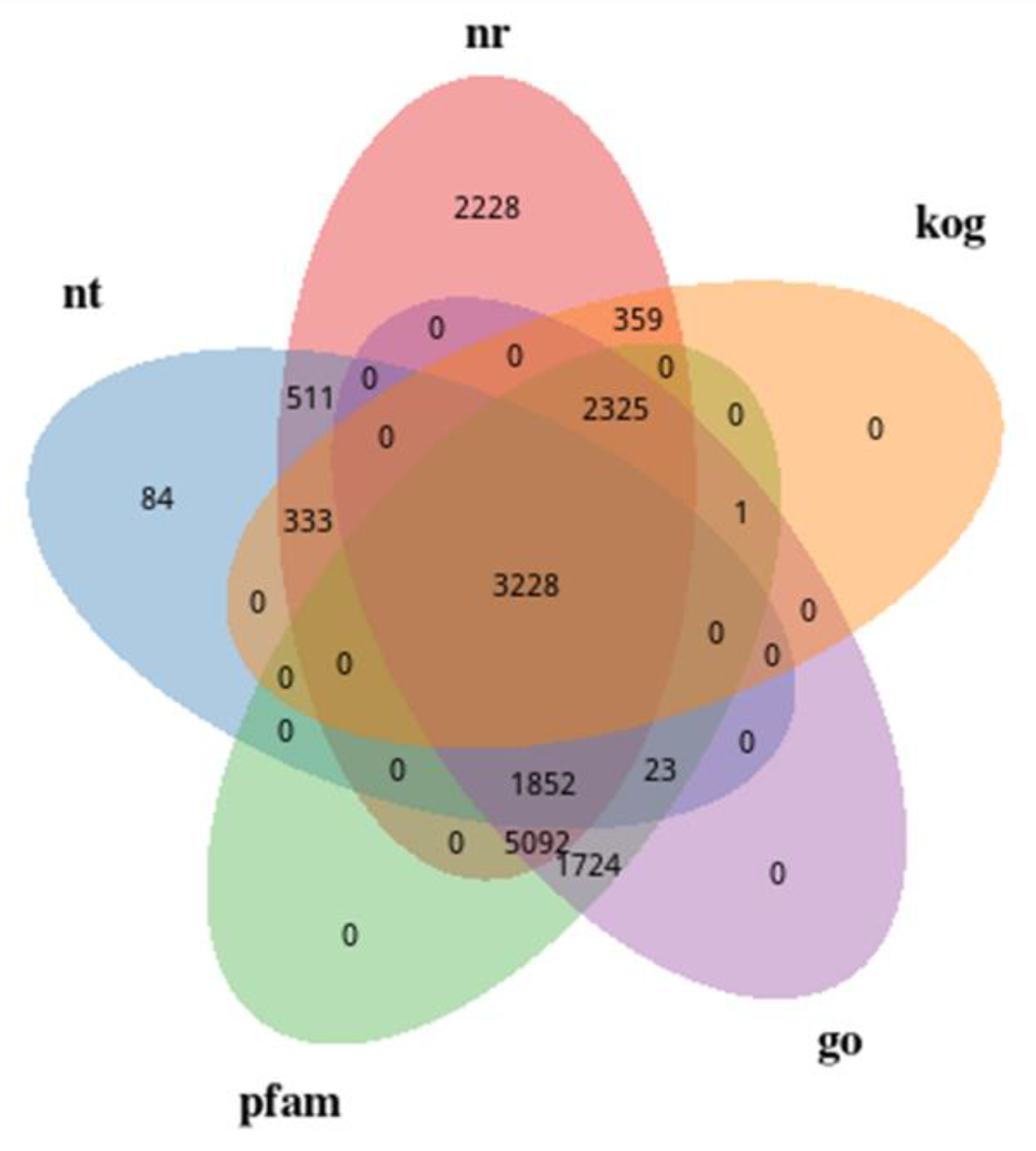
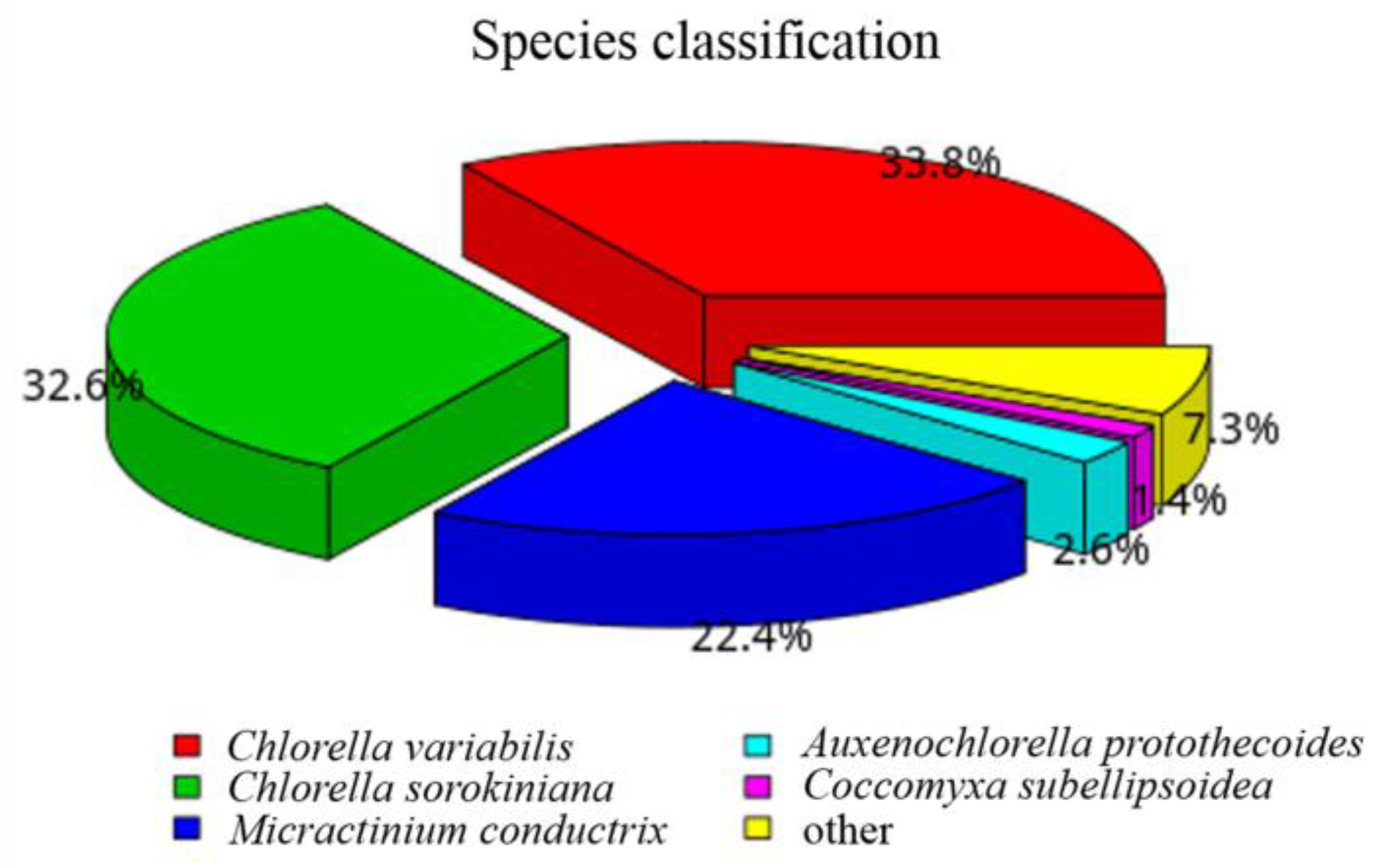
2.3.2. Gene Functional Annotation
2.3.3. Differential Gene Expression Analysis
2.3.4. Real-Time Quantitative PCR Analysis
3. Discussion
4. Materials and Methods
4.1. Algal Isolation and Pre-Cultivation
4.2. Experimental Set Up
4.3. Physiological Characteristics Determination
4.3.1. Cell Growth
4.3.2. Photosynthetic Efficiency
4.3.3. Chlorophyll Content
4.3.4. Superoxide Dismutase (SOD) Activity
4.4. Transmission Electron Microscopy Observation
4.5. Transcriptome Sequencing and Analysis
4.5.1. RNA Extraction and cDNA Library Preparation
4.5.2. Data Processing, Functional Annotation, and Metabolic Pathway Analysis
4.5.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majeed, A.; Nisar, M.F.; Hussain, K. Effect of saline culture on the concentration of Na+, K+ and Cl− in Agrostis tolonifera. Curr. Res. J. Biol. Sci. 2010, 2, 76–82. [Google Scholar]
- Zhou, H.; Guo, S.; Shao, H.; Chen, X.; Wei, B.; Sun, J. Effects of iso-smotic Ca(NO3)2 and NaCl stress on growth and physiological characteristics of cucumber seedlings. Acta Ecol. Sin. 2014, 34, 1880–1890. [Google Scholar]
- Li, D.P.; Wu, Z.J.; Liang, C.H.; Chen, L. Characteristics and regulation of greenhouse soil environment. Chin. J. Ecol. 2004, 23, 192–197. [Google Scholar]
- Wu, X.G. Causes and preventive measures of salt accumulation on face soil in vegetable greenhouse. J. Zhejiang Wanli Univ. 2001, 14, 19–21. [Google Scholar]
- Yuan, L.; Shu, S.; Sun, J.; Guo, S.; Tezuka, T. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth. Res. 2012, 112, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Li, T.X.; Zhou, J.M. Secondary salinization of greenhouse soil and its effects on soil properties. Soils 2005, 37, 581–586. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Yuan, D.X. On the karst ecosystem. Acta Geol. Sin. 2001, 75, 336–338. [Google Scholar]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.K.; Goswami, T.K. Mechanical properties of cumin seed (Cuminum cyminum Linn.) under compressive loading. J. Food Eng. 1998, 36, 311–321. [Google Scholar] [CrossRef]
- Jin, C.Y.; Sun, J.; Guo, S.R. Effects of exogenous spermidine on growth and active oxygen metabolism in cucumber seedlings under Ca(NO3)2 stress. Acta Bot. Boreali-Occident. Sin. 2010, 30, 1627–1633. [Google Scholar]
- Sun, Y.D.; Luo, W.R.; Li, X.Z.; Qi, A. Effects of Ca(NO3)2 stress on the growth and physiological indexes of cucumber seedlings. Environ. Sci. Inf. Appl. Technol. 2009, 1, 268–271. [Google Scholar]
- Zhang, G.W.; Liu, Z.L.; Zhou, J.G.; Zhu, Y.L. Effects of Ca(NO3)2 stress on oxidative damage, antioxidant enzymes activities and polyamine contents in roots of grafted and non-grafted tomato plants. Plant Growth Regul. 2008, 56, 7–19. [Google Scholar] [CrossRef]
- Mettler, T.; Mühlhaus, T.; Hemme, D.; Schöttler, M.A.; Rupprecht, J.; Idoine, A.; Veyel, D.; Pal, S.K.; Yaneva-Roder, L.; Winck, F.V.; et al. Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell. 2014, 26, 2310–2350. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhou, W.C.; Zhang, F.G.; Chen, L.Z.; Wang, G.H. Effect of Salt Stress on Photosynthetic Activity and Metabolites of Chlamydomonas reinhardtii. Plant Physiol. J. 2015, 51, 1887–1894. [Google Scholar]
- Sithtisarn, S.; Yokthongwattana, K.; Mahong, B.; Roytrakul, S.; Paemanee, A.; Phaonakrop, N.; Yokthongwattana, C. Comparative proteomic analysis of Chlamydomonas reinhardtii control and a salinity-tolerant strain revealed a differential protein expression pattern. Planta 2017, 246, 843–856. [Google Scholar] [CrossRef]
- Zuo, Z.; Chen, Z.; Zhu, Y.; Bai, Y.; Wang, Y. Effects of NaCl and Na2CO3 stresses on photosynthetic ability of Chlamydomonas reinhardtii. Biologia 2014, 69, 1314–1322. [Google Scholar] [CrossRef]
- Juárez, Á.B.; Vélez, C.G.; Iñiguez, A.R.; Martínez, D.E.; Rodríguez, M.C.; Vigna, M.S.; del Carmen Ríos de Molina, M.A. Parachlorella kessleri (Trebouxiophyceae, Chlorophyta) strain from an extremely acidic geothermal pond in Argentina. Phycologia 2011, 50, 413–421. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Xie, T.; Xie, S. A Parachlorella kessleri (Trebouxiophyceae, Chlorophyta) strain tolerant to high concentration of calcium chloride. J. Eukaryotic Microbiol. 2022, 69, e12872. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, S.; Yin, Y. Transcriptome analysis of the Taxodium ‘Zhongshanshan 405’ roots in response to salinity stress. Plant Physiol. Biochem. 2016, 100, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Amirbakhtiar, N.; Ismaili, A.; Ghaffari, M.R.; Nazarian Firouzabadi, F.; Shobbar, Z.S. Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS ONE 2019, 14, e0213305. [Google Scholar] [CrossRef]
- Goyal, E.; Amit, S.K.; Singh, R.S.; Mahato, A.K.; Chand, S.; Kanika, K. Transcriptome profiling of the salt-stress response in Triticum aestivum cv. Kharchia Local. Sci. Rep. 2016, 6, 27752. [Google Scholar] [CrossRef] [Green Version]
- Sicilia, A.; Testa, G.; Santoro, D.F.; Cosentino, S.L.; Lo Piero, A.R. RNASeq analysis of giant cane reveals the leaf transcriptome dynamics under long-term salt stress. BMC Plant Biol. 2019, 19, 355. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.R.; Cheeseman, J.M. The effects of external NaCl on thylakoid stacking in lettuce plants. Plant Cell Environ. 1993, 16, 215–222. [Google Scholar] [CrossRef]
- Jeanjean, R.; Matthijs, H.C.; Onana, B.; Havaux, M.; Joset, F. Exposure of the cyanobacterium Synechocystis PCC6803 to salt stress induces concerted changes in respiration and photosynthesis. Plant Cell Physiol. 1993, 34, 1073–1079. [Google Scholar]
- Tong, H.; Zhang, Z.; Li, B.; Wang, J.; Guo, S. Effects of iso-osmotic Ca(NO3)2 and NaCl stress on chloroplast ultrastructure and photosynthesis in cucumber leaves. China Veg. 2012, 18, 160–165. [Google Scholar]
- Li, Q.Y.; Ge, H.B.; Hu, S.M.; Wang, H.Y. Effects of sodium and calcium salt stresses on strawberry photosynthesis. Acta Bot. Boreali-Occident. Sin. 2006, 26, 1713–1717. [Google Scholar]
- Rao, G.G.; Rao, G.R. Pigment composition and chlorophyllase activity in pigeon pea (Cajanus indicus Spreng) and Gingelley (Sesamum indicum L.) under NaCl salinity. Indian J. Exp. Biol. 1981, 19, 768–780. [Google Scholar]
- Miqyass, M.; Van Gorkom, H.J.; Yocum, C.F. The PSII calcium site revisited. Photosynth. Res. 2007, 92, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popelková, H.; Yocum, C.F. Current status of the role of Cl− ion in the oxygen-evolving complex. Photosynth. Res. 2007, 93, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, I.; Malec, P.; Sallai, A.; Kovács, L.; Itoh, K.; Shen, G.; Ughy, B.; Bogos, B.; Sakurai, I.; Kis, M.; et al. Phosphatidylglycerol is essential for oligomerization of photosystem I reaction center. Plant Physiol. 2004, 134, 1471–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liska, A.J.; Shevchenko, A.; Pick, U.; Katz, A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol. 2004, 136, 2806–2817. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Cui, R.; Xu, P.; Wu, J.; Mao, J.L.; Chen, Y.; Zhou, C.Z.; Yu, L.H.; Xiang, C.B. ATHB17 enhances stress tolerance by coordinating photosynthesis associated nuclear gene and ATSIG5 expression in response to abiotic stress. Sci. Rep. 2017, 7, 45492. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Wang, S.J.; Zhang, G.L.; Wang, C.Y.; Yang, H.Y.; Liao, X.R. Effects of calcium concentration on photosynthesis characteristics of two fern plants. Ecol. Environ. Sci. 2013, 22, 258–262. [Google Scholar]
- Ahmad, P.; Nabi, G.; Jeleel, C.A.; Umar, S. Free Radical Production, Oxidative Damage and Antioxidant Defense Mechanisms in Plants under Abiotic Stress. Oxidative Stress: Role of Antioxidants in Plants; Studium Press: New Delhi, India, 2011; pp. 19–53. [Google Scholar]
- Lindermayr, C.; Durner, J. Interplay of reactive oxygen species and nitric oxide: Nitric oxide coordinates reactive oxygen species homeostasis. Plant Physiol. 2015, 167, 1209–1210. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Jha, A.B.; Dubey, R.S. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 2011, 248, 565–577. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, H.M.; Yang, W. Effect of different Ca2+ concentrations on the growth, photosynthetic characteristics, protective enzyme activity of watermelon seedling. North Hortic. 2012, 14, 144–146. [Google Scholar]
- Floyd, R.A.; Nagy, I.Z. Formation of long-lived hydroxyl free radical adducts of proline and hydroxyproline in a Fenton reaction. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1984, 790, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Alia Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Huang, L.; Peng, L.; Yan, X. Multi-omics responses of red algae Pyropia haitanensis to intertidal desiccation during low tides. Algal Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Dalla Vecchia, F. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE 2014, 9, e112270. [Google Scholar] [CrossRef] [Green Version]
- Kerepesi, I.; Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Zhang, Z.; Liu, X.; Hsu, C.C.; Du, Y.; Sang, T.; Zhu, C.; Wang, Y.; Satheesh, V.; et al. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 2020, 11, 613. [Google Scholar] [CrossRef] [Green Version]
- Zhen, A.; Hu, X.H.; Ren, W.Q.; Su, C.J.; Jin, X.Q.; Sun, X.P. Effect of exogenous γ-aminobutyric acid on NO3−-N assimilation in muskmelon under Ca (NO3)2 stress. Chin. J. Appl. Ecol. 2016, 27, 3987–3995. [Google Scholar]
- Liu, L.Y.; Wang, M.Y. Effect of CaCl2 on germination of wheat seeds under salt stress. J. Henan Agric. Sci. 2010, 1, 5–7. [Google Scholar]
- Tripathi, B.N.; Singh, V.; Ezaki, B.; Sharma, V.; Gaur, J.P. Mechanism of Cu-and Cd-induced proline hyperaccumulation in Triticum aestivum (wheat). J. Plant Growth Regul. 2013, 32, 799–808. [Google Scholar] [CrossRef]
- Xu, J.; Yin, H.X.; Li, X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009, 28, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhao, F.; Fang, Y.P.; Chen, J. Effects of calcium and water stress on physiological and biochemical indexes of bryophytes. Environ. Sci. Technol. 2010, 33, 70–74. [Google Scholar]
- Buayam, N.; Davey, M.P.; Smith, A.G.; Pumas, C. Effects of Copper and pH on the Growth and Physiology of Desmodesmus sp. AARLG074. Metabolites 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Avron, M. Dunaliella: Physiology, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Pick, U. Adaptation of the halotolerant alga Dunaliella to high salinity. In Salinity: Environment-Plants-Molecules; Tanji, K.K., Ed.; Boston Kluwer Academic Publisher: Boston, MA, USA, 2002; pp. 97–112. [Google Scholar]
- Hadi, M.R.; Shariati, M.; Afsharzadeh, S. Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. BiotechnSol. Bioprocess Eng. 2008, 13, 540–544. [Google Scholar] [CrossRef]
- Mishra, A.; Mandoli, A.; Jha, B. Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J. Ind. Microbiol. Biotechnol. 2008, 35, 1093. [Google Scholar] [CrossRef]
- Pocivavsek, L.; Gavrilov, K.; Cao, K.D.; Chi, E.Y.; Li, D.; Lin, B.; Meron, M.; Majewski, J.; Lee, K.Y.C. Glycerol-induced membrane stiffening: The role of viscous fluid adlayers. Biophys. J. 2011, 101, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.A., Jr. Lipids and membrane function in green algae. Biochim. Biophys. Acta-Lipids Lipid Metab. 1996, 1302, 17–45. [Google Scholar] [CrossRef]
- Alkayal, F.; Albion, R.L.; Tillett, R.L.; Hathwaik, L.T.; Lemos, M.S.; Cushman, J.C. Expressed sequence tag (EST) profiling in hyper saline shocked Dunaliella salina reveals high expression of protein synthetic apparatus components. Plant Sci. 2010, 179, 437–449. [Google Scholar] [CrossRef]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007, 45, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A. Adaptation of the unicellular alga Dunaliella parva to a saline environment. J. Phycol. 1975, 11, 50–54. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Mera, R.; Torres, E.; Abalde, J. Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: Implications for the optimization of algal culture media. J. Phycol. 2016, 52, 75–88. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, X.; Nan, F.; Liu, Q.; Lv, J.; Feng, J.; Xie, S. Toxicological Effects of Mercuric Chloride Exposure on Scenedesmus quadricauda. Water 2022, 14, 3228. [Google Scholar] [CrossRef]
- Holmes, D.S.; Bonner, J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry 1973, 12, 2330–2338. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
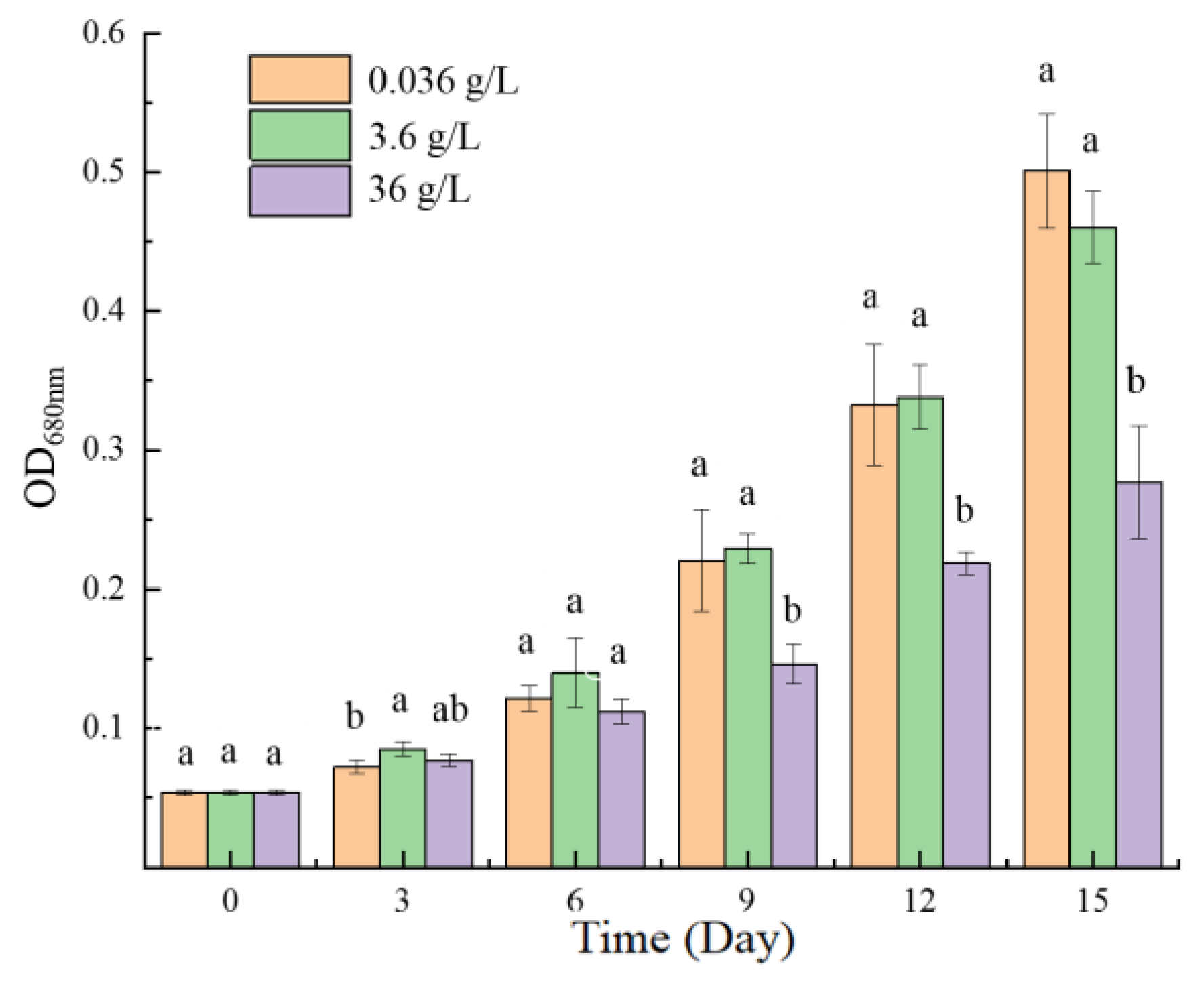
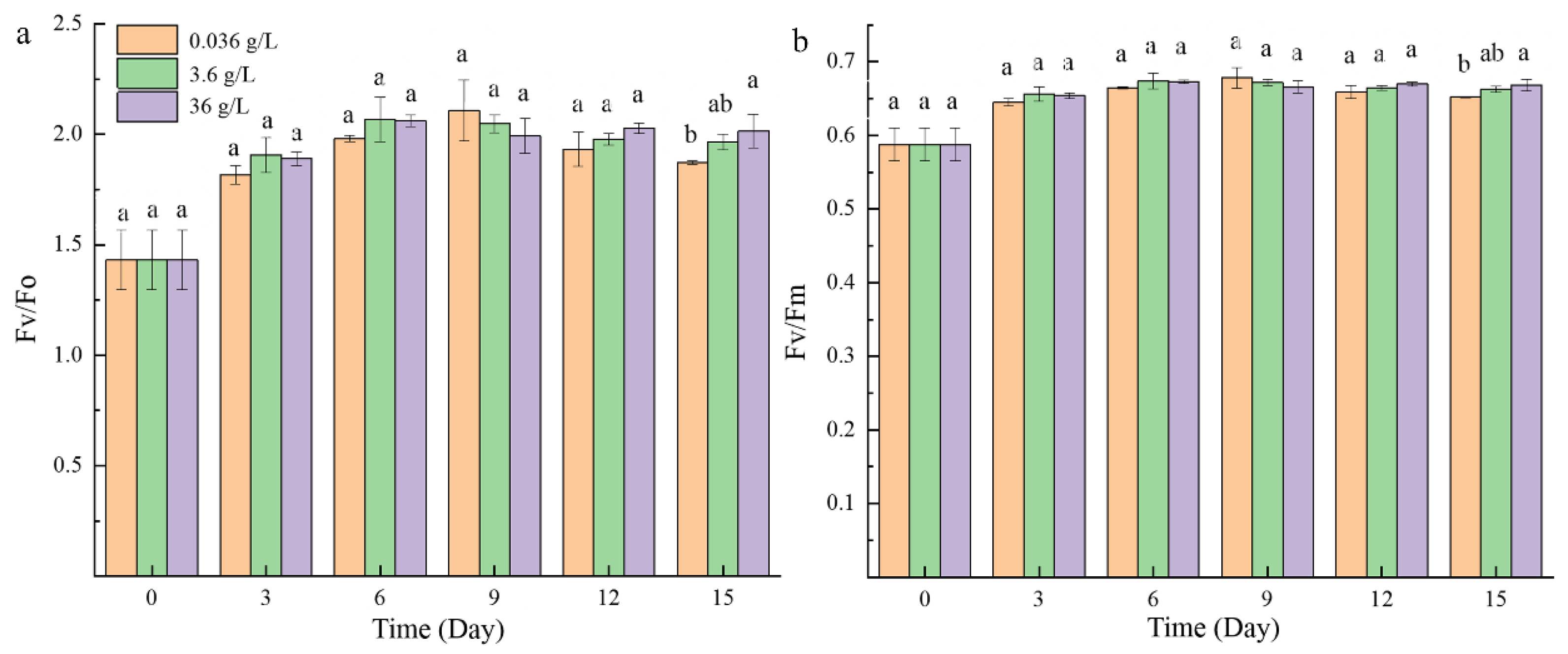
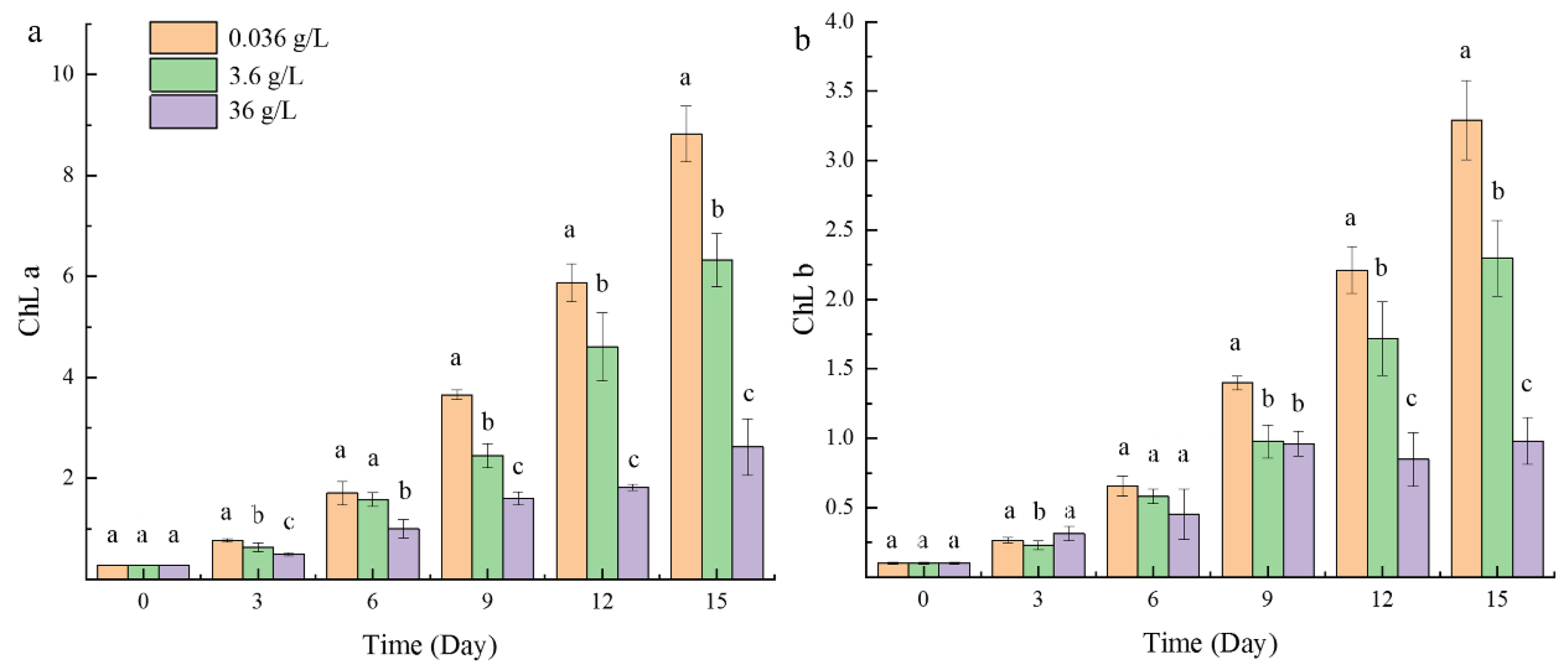


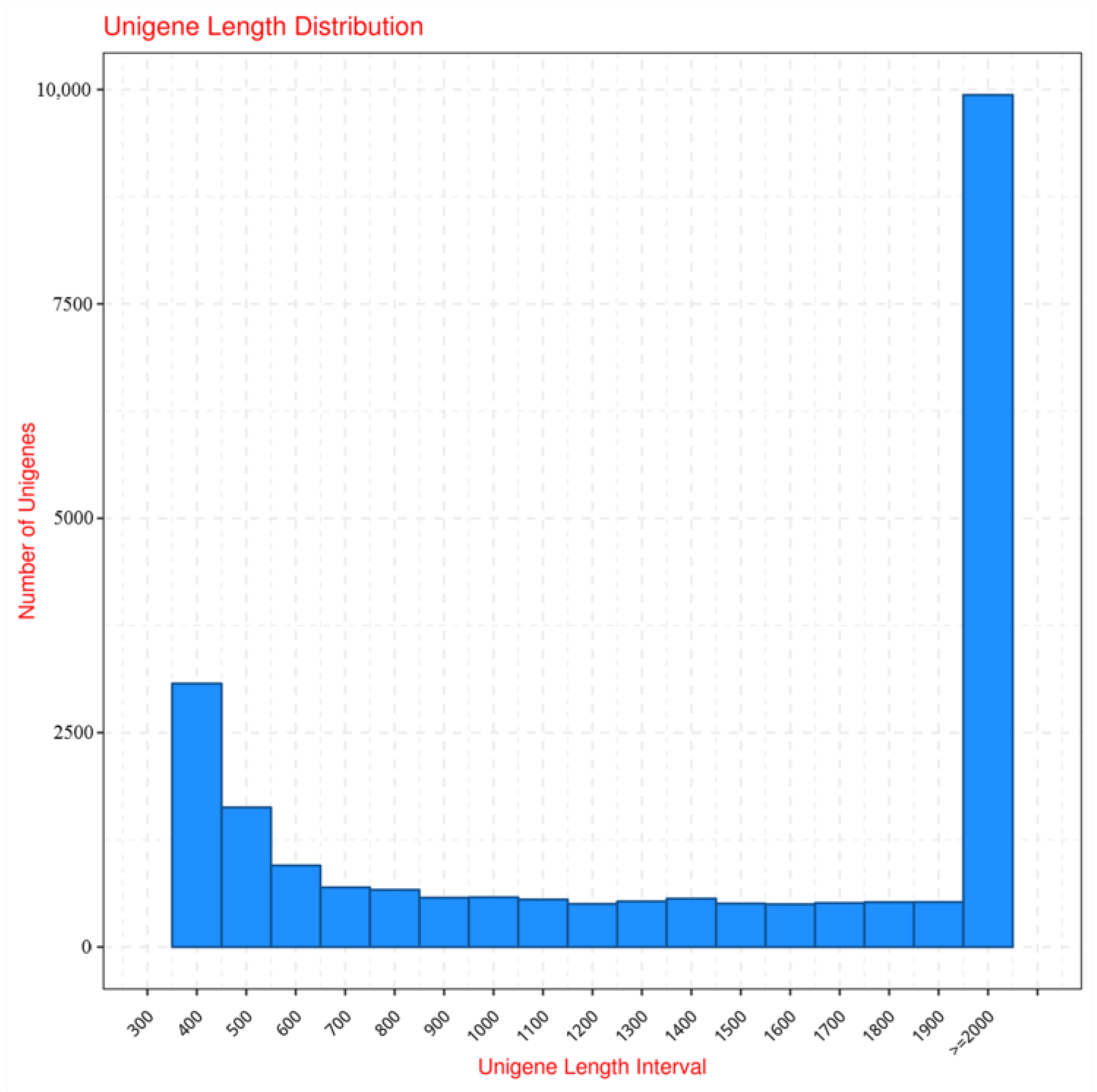







| Sample | Raw Read | Clean Reads | Clean Bases | Error (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| Ca1_1 | 24,757,741 | 23,970,283 | 7.2G | 0.03 | 97.56 | 93.65 | 61.1 |
| Ca1_2 | 23,767,023 | 22,861,583 | 6.9G | 0.03 | 97.46 | 93.47 | 61.01 |
| Ca1_3 | 23,361,638 | 22,330,665 | 6.7G | 0.02 | 97.91 | 94.44 | 61.18 |
| Ca100_1 | 22,090,363 | 21,354,250 | 6.4G | 0.02 | 97.84 | 94.31 | 61.25 |
| Ca100_2 | 23,129,013 | 22,043,108 | 6.6G | 0.03 | 97.84 | 94.31 | 61.25 |
| Ca100_3 | 24,195,434 | 23,402,511 | 7.0G | 0.03 | 97.71 | 93.89 | 61.08 |
| Ca1000_1 | 23,813,547 | 23,014,485 | 6.9G | 0.02 | 97.88 | 94.48 | 62.15 |
| Ca1000_2 | 23,964,132 | 23,158,172 | 6.9G | 0.02 | 97.87 | 94.44 | 62.18 |
| Ca1000_3 | 22,363,588 | 21,575,649 | 6.5G | 0.02 | 97.92 | 94.53 | 62.29 |
| Min Length | Mean Length | Median Length | Max Length | N50 | N90 | Total Nucleotide | |
|---|---|---|---|---|---|---|---|
| Unigenes (bp) | 301 | 2079 | 1463 | 32,200 | 3446 | 1018 | 51,724,505 |
| Pathway Term | Rich Factor | p-Value | Gene Number |
|---|---|---|---|
| Ribosome biogenesis in eukaryotes | 0.094340 | 0.000083 | 15 |
| Aminoacyl-tRNA biosynthesis | 0.069182 | 0.000644 | 11 |
| Alanine, aspartate and glutamate metabolism | 0.056604 | 0.000865 | 9 |
| RNA transport | 0.094340 | 0.003755 | 15 |
| Valine, leucine, and isoleucine biosynthesis | 0.031447 | 0.004667 | 5 |
| Photosynthesis-antenna proteins | 0.031447 | 0.006148 | 5 |
| 2-Oxocarboxylic acid metabolism | 0.044025 | 0.010170 | 7 |
| Biosynthesis of amino acids | 0.106918 | 0.015805 | 17 |
| One carbon pool by folate | 0.025157 | 0.019456 | 4 |
| Purine metabolism | 0.081761 | 0.021554 | 13 |
| Photosynthesis | 0.044025 | 0.041502 | 7 |
| Arginine and proline metabolism | 0.037736 | 0.045134 | 6 |
| Arginine biosynthesis | 0.025157 | 0.063898 | 4 |
| Lysine biosynthesis | 0.018868 | 0.078758 | 3 |
| Selenocompound metabolism | 0.018868 | 0.092687 | 3 |
| Histidine metabolism | 0.018868 | 0.100006 | 3 |
| Cysteine and methionine metabolism | 0.037736 | 0.115123 | 6 |
| Pyrimidine metabolism | 0.050314 | 0.122522 | 8 |
| Glycine, serine, and threonine metabolism | 0.031447 | 0.128750 | 5 |
| Steroid biosynthesis | 0.018868 | 0.139764 | 3 |
| Pathway Term | Rich Factor | p-Value | Gene Number |
|---|---|---|---|
| Photosynthesis | 0.024004 | 0.128858 | 53 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 0.012681 | 0.163888 | 28 |
| Ribosome biogenesis in eukaryotes | 0.032156 | 0.188577 | 71 |
| Porphyrin and chlorophyll metabolism | 0.024457 | 0.195093 | 54 |
| Alanine, aspartate and glutamate metabolism | 0.016757 | 0.265489 | 37 |
| Nitrogen metabolism | 0.007699 | 0.282168 | 17 |
| Folate biosynthesis | 0.006793 | 0.302803 | 15 |
| Aminoacyl-tRNA biosynthesis | 0.022192 | 0.317240 | 49 |
| Selenocompound metabolism | 0.007699 | 0.321789 | 17 |
| Fatty acid elongation | 0.004076 | 0.326930 | 9 |
| Apoptosis | 0.006341 | 0.336663 | 14 |
| Cell cycle | 0.031703 | 0.341372 | 70 |
| Pyrimidine metabolism | 0.032609 | 0.346717 | 72 |
| Protein digestion and absorption | 0.007246 | 0.354755 | 16 |
| Biotin metabolism | 0.007699 | 0.362715 | 17 |
| Base excision repair | 0.010417 | 0.364863 | 23 |
| Inositol phosphate metabolism | 0.010870 | 0.370652 | 24 |
| Ether lipid metabolism | 0.004529 | 0.396622 | 10 |
| Arginine and proline metabolism | 0.016757 | 0.398513 | 37 |
| Purine metabolism | 0.043931 | 0.402698 | 97 |
| Reaction Component | Dose (μL) |
|---|---|
| 1X SYBR Green Supermix | 5 |
| Primer-F | 0.5 |
| Primer-R | 0.5 |
| cDNA | 1 |
| ddH2O | 3 |
| Primers | Sequences (5′-3′) |
|---|---|
| 18S-F | TCCAGACATAGTGAGGACAGA |
| 18S-R | ACTCCACCAACTAAGAACGG |
| PsbO-F | CTGAGCGTTGCACATCAC |
| PsbO-R | AGACCTCCATGCTGAAGC |
| PsaF-F | CAAATGCCTTGCTCTCGGA |
| PsaF-R | GCCCTCACCTTTGGCTTT |
| rpiA-F | CGGTGTGCTGCTATGAAT |
| rpiA-R | TCGTCCAATATCCAGCCA |
| PRK-F | TGCGAGAAGAGAACTCCT |
| PRK-R | GTGGGAAGGTGTCAAGTG |
| hemY-F | GATGGTGAAGGTTTGGTA |
| hemY-R | CATTAGGACCCTCTCAAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, J.; Nan, F.; Liu, Q.; Lv, J.; Feng, J.; Xie, S. Transcriptome Analysis Reveals the Mechanisms of Tolerance to High Concentrations of Calcium Chloride Stress in Parachlorella kessleri. Int. J. Mol. Sci. 2023, 24, 651. https://doi.org/10.3390/ijms24010651
Liu X, Zhao J, Nan F, Liu Q, Lv J, Feng J, Xie S. Transcriptome Analysis Reveals the Mechanisms of Tolerance to High Concentrations of Calcium Chloride Stress in Parachlorella kessleri. International Journal of Molecular Sciences. 2023; 24(1):651. https://doi.org/10.3390/ijms24010651
Chicago/Turabian StyleLiu, Xudong, Jinli Zhao, Fangru Nan, Qi Liu, Junping Lv, Jia Feng, and Shulian Xie. 2023. "Transcriptome Analysis Reveals the Mechanisms of Tolerance to High Concentrations of Calcium Chloride Stress in Parachlorella kessleri" International Journal of Molecular Sciences 24, no. 1: 651. https://doi.org/10.3390/ijms24010651
APA StyleLiu, X., Zhao, J., Nan, F., Liu, Q., Lv, J., Feng, J., & Xie, S. (2023). Transcriptome Analysis Reveals the Mechanisms of Tolerance to High Concentrations of Calcium Chloride Stress in Parachlorella kessleri. International Journal of Molecular Sciences, 24(1), 651. https://doi.org/10.3390/ijms24010651








