Acute Inflammation in Tissue Healing
Abstract
1. Introduction
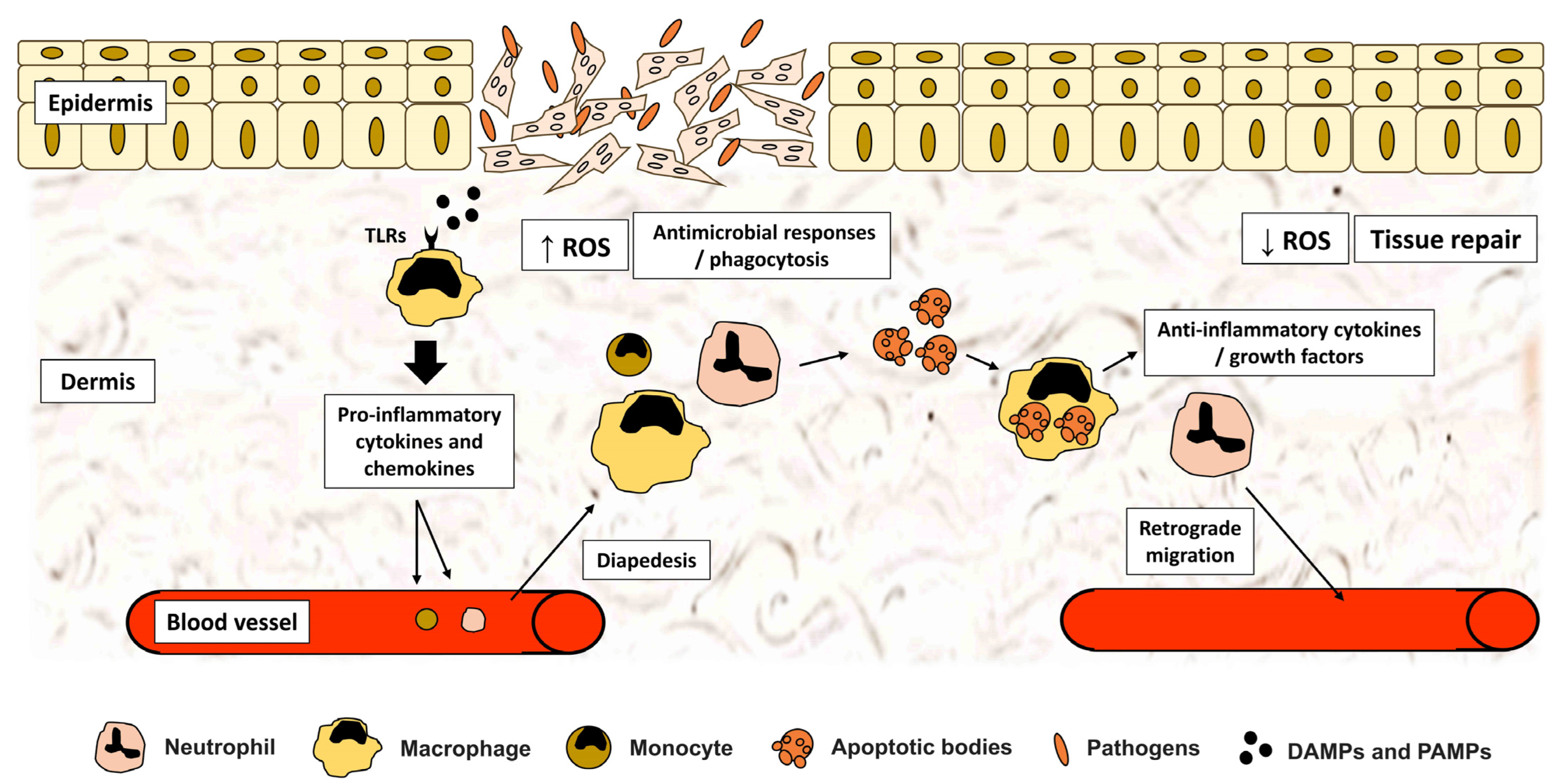
2. Induction of Inflammation Phase of Tissue Repair: Innate Immune Responses
2.1. Immune System Perception of Injury: The Role of DAMPs and PAMPs
2.2. Activation of PPRs and Downstream Inflammatory Pathways
2.3. Inflammatory Cytokines and Mediators
| Cytokine | Receptor | Source | Functions |
|---|---|---|---|
| TNF-α | TNFR1 (p55) and TNFR2 (p75) | PMN, macrophages and mast cells |
|
| IL-1β | IL-1R1, IL-1R2 and IL-1RAcP (IL-1R3) | keratinocytes, PMN and macrophages |
|
| CXCL8 | CXCR1 | Platelets, PMN and macrophages | |
| IL-6 | gp130 and IL-6R | Myeloid cells, lymphocytes and fibroblasts |
|
| IFN-γ | IFNGR1 and IFNGR2 | Natural killer cells, plasmacytoid DCs and T cells | |
| IL-10 | IL-10R | Macrophages, DCs, PMN, mast cells and T cells |
|
| TGF-β | type II TGF-β receptor | Macrophages, keratinocytes, fibroblasts and platelets |
2.4. Cellular Recruitment to Injury Site
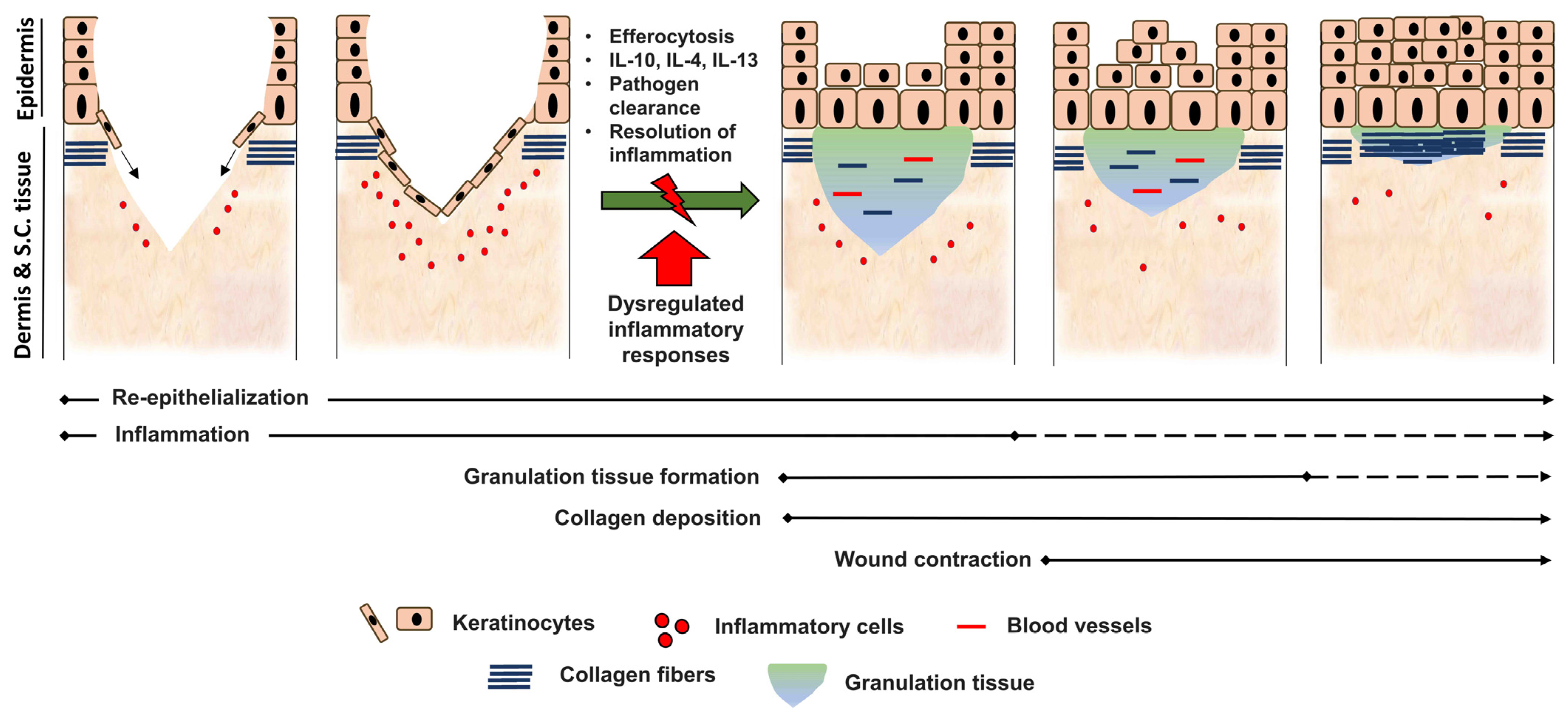
3. Role of Inflammatory Cells during Tissue Repair
3.1. Neutrophils
3.2. Macrophages
3.3. Dendritic (DCs) and Langerhans Cells (LCs)
3.4. Mast Cells
3.5. T Cells
4. Suppression of Inflammation
5. Dysregulation of Inflammatory Responses and Its Outcome
6. Current Tissue Engineering Strategies Managing Chronic Injuries
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galis, F.; Wagner, G.P.; Jockusch, E.L. Why Is Limb Regeneration Possible in Amphibians but Not in Reptiles, Birds, and Mammals? Evol. Dev. 2003, 5, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Tissue Repair. In Inflammation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1517–1538. ISBN 978-3-527-69215-6. [Google Scholar]
- Soliman, A.M.; Yoon, T.; Wang, J.; Stafford, J.L.; Barreda, D.R. Isolation of Skin Leukocytes Uncovers Phagocyte Inflammatory Responses During Induction and Resolution of Cutaneous Inflammation in Fish. Front. Immunol. 2021, 12, 725063. [Google Scholar] [CrossRef]
- Krafts, K.P. Tissue Repair. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef]
- Clark, R.A.F. Fibrin Is a Many Splendored Thing. J. Investig. Dermatol. 2003, 121. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Barbul, A. Understanding the Role of Immune Regulation in Wound Healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and Molecular Basis of Wound Healing in Diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A.F. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF Guides Angiogenic Sprouting Utilizing Endothelial Tip Cell Filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Kaltalioglu, K.; Coskun-Cevher, S. A Bioactive Molecule in a Complex Wound Healing Process: Platelet-Derived Growth Factor. Int. J. Dermatol. 2015, 54, 972–977. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of Acute Wound Healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Singhal, P.K.; Sassi, S.; Lan, L.; Au, P.; Halvorsen, S.C.; Fukumura, D.; Jain, R.K.; Seed, B. Mouse Embryonic Fibroblasts Exhibit Extensive Developmental and Phenotypic Diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 122–127. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Fries, K.M.; Blieden, T.; Looney, R.J.; Sempowski, G.D.; Silvera, M.R.; Willis, R.A.; Phipps, R.P. Evidence of Fibroblast Heterogeneity and the Role of Fibroblast Subpopulations in Fibrosis. Clin. Immunol. Immunopathol. 1994, 72, 283–292. [Google Scholar] [CrossRef]
- Donaldson, D.J.; Mahan, J.T. Fibrinogen and Fibronectin as Substrates for Epidermal Cell Migration during Wound Closure. J. Cell Sci. 1983, 62, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.L.; Arpey, C.J. Normal Cutaneous Wound Healing: Clinical Correlation with Cellular and Molecular Events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-Epithelialization of Adult Skin Wounds: Cellular Mechanisms and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Gaudino, G. Cellular and Molecular Facets of Keratinocyte Reepithelization during Wound Healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Jung, K.; Covington, S.; Sen, C.K.; Januszyk, M.; Kirsner, R.S.; Gurtner, G.C.; Shah, N.H. Rapid Identification of Slow Healing Wounds. Wound Repair Regen. 2016, 24, 181–188. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef] [PubMed]
- Pradeu, T.; Cooper, E.L. The Danger Theory: 20 Years Later. Front. Immunol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How Dying Cells Alert the Immune System to Danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.J.; Suzuki, K.; Coban, C.; Takeshita, F.; Itoh, Y.; Matoba, H.; Kohn, L.D.; Klinman, D.M. Genomic DNA Released by Dying Cells Induces the Maturation of APCs. J. Immunol. 2001, 167, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but Not Apoptotic Cell Death Releases Heat Shock Proteins, Which Deliver a Partial Maturation Signal to Dendritic Cells and Activate the NF-Kappa B Pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular Identification of a Danger Signal That Alerts the Immune System to Dying Cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Wenceslau, C.F.; McCarthy, C.G.; Szasz, T.; Spitler, K.; Goulopoulou, S.; Webb, R.C. Working Group on DAMPs in Cardiovascular Disease Mitochondrial Damage-Associated Molecular Patterns and Vascular Function. Eur. Heart J. 2014, 35, 1172–1177. [Google Scholar] [CrossRef]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A Novel Peptide CXCR Ligand Derived from Extracellular Matrix Degradation during Airway Inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan Fragments Stimulate Endothelial Recognition of Injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef]
- Wrenshall, L.E.; Cerra, F.B.; Carlson, A.; Bach, F.H.; Platt, J.L. Regulation of Murine Splenocyte Responses by Heparan Sulfate. J. Immunol. 1991, 147, 455–459. [Google Scholar]
- Van der Vliet, A.; Janssen-Heininger, Y.M.W. Hydrogen Peroxide as a Damage Signal in Tissue Injury and Inflammation: Murderer, Mediator, or Messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Tlr4: Central Component of the Sole Mammalian LPS Sensor. Curr. Opin. Immunol. 2000, 12, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Aoyama, K.; Tamura, T.; Suda, Y. Lipoprotein Is a Predominant Toll-like Receptor 2 Ligand in Staphylococcus Aureus Cell Wall Components. Int. Immunol. 2006, 18, 355–362. [Google Scholar] [CrossRef]
- Levitz, S.M. Innate Recognition of Fungal Cell Walls. PLoS Pathog. 2010, 6, e1000758. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune Sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Deane, J.A.; Bolland, S. Nucleic Acid-Sensing TLRs as Modifiers of Autoimmunity. J. Immunol. 2006, 177, 6573–6578. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, e17023. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like Receptors and Innate Immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Tsirogianni, A.K.; Moutsopoulos, N.M.; Moutsopoulos, H.M. Wound Healing: Immunological Aspects. Injury 2006, 37, S5–S12. [Google Scholar] [CrossRef]
- Zhang, F.X.; Kirschning, C.J.; Mancinelli, R.; Xu, X.-P.; Jin, Y.; Faure, E.; Mantovani, A.; Rothe, M.; Muzio, M.; Arditi, M. Bacterial Lipopolysaccharide Activates Nuclear Factor-ΚB through Interleukin-1 Signaling Mediators in Cultured Human Dermal Endothelial Cells and Mononuclear Phagocytes. J. Biol. Chem. 1999, 274, 7611–7614. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like Receptor Function and Signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Kupper, T.S. Inflammatory Skin Diseases, T Cells, and Immune Surveillance. N. Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Calcium: A Potential Central Regulator in Wound Healing in the Skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Historical Review of Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Gethin, G. Understanding the Inflammatory Process in Wound Healing. Br. J. Community Nurs. 2012, 17, S17–S22. [Google Scholar] [CrossRef]
- Kishimoto, T. The Biology of Interleukin-6. Blood 1989, 74, 1–10. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential Involvement of IL-6 in the Skin Wound-Healing Process as Evidenced by Delayed Wound Healing in IL-6-Deficient Mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef]
- Weller, K.; Foitzik, K.; Paus, R.; Syska, W.; Maurer, M. Mast Cells Are Required for Normal Healing of Skin Wounds in Mice. FASEB J. 2006, 20, 2366–2368. [Google Scholar] [CrossRef]
- Garbuzenko, E.; Nagler, A.; Pickholtz, D.; Gillery, P.; Reich, R.; Maquart, F.-X.; Levi-Schaffer, F. Human Mast Cells Stimulate Fibroblast Proliferation, Collagen Synthesis and Lattice Contraction: A Direct Role for Mast Cells in Skin Fibrosis. Clin. Exp. Allergy 2002, 32, 237–246. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet Alpha-Granules: Basic Biology and Clinical Correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.-C.; He, Z.-X.; et al. Prostaglandin E2 Hydrogel Improves Cutaneous Wound Healing via M2 Macrophages Polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Tanigami, M.; Amagase, K.; Ochi, A.; Okuda, S.; Hatazawa, R. Endogenous Prostaglandin E2 Accelerates Healing of Indomethacin-Induced Small Intestinal Lesions through Upregulation of Vascular Endothelial Growth Factor Expression by Activation of EP4 Receptors. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Zhang, Q.-Z.; Su, W.-R.; Shi, S.-H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of M2 Macrophages and Enhance Cutaneous Wound Healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Regenerative Medicine: Inhibiting Prostaglandin Breakdown Triggers Tissue Regeneration. Nat. Rev. Drug Discov. 2015, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Ogletree, M.L. Overview of Physiological and Pathophysiological Effects of Thromboxane A2. Fed. Proc. 1987, 46, 133–138. [Google Scholar]
- Pierre, S.; Linke, B.; Suo, J.; Tarighi, N.; Del Turco, D.; Thomas, D.; Ferreiros, N.; Stegner, D.; Frölich, S.; Sisignano, M.; et al. GPVI and Thromboxane Receptor on Platelets Promote Proinflammatory Macrophage Phenotypes during Cutaneous Inflammation. J. Investig. Dermatol. 2017, 137, 686–695. [Google Scholar] [CrossRef]
- Daniel, T.O.; Liu, H.; Morrow, J.D.; Crews, B.C.; Marnett, L.J. Thromboxane A2 Is a Mediator of Cyclooxygenase-2-Dependent Endothelial Migration and Angiogenesis. Cancer Res. 1999, 59, 4574–4577. [Google Scholar]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-Protein-Coupled Receptor for Leukotriene B4 That Mediates Chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef]
- Brandt, S.L.; Wang, S.; Dejani, N.N.; Klopfenstein, N.; Winfree, S.; Filgueiras, L.; McCarthy, B.P.; Territo, P.R.; Serezani, C.H. Excessive Localized Leukotriene B4 Levels Dictate Poor Skin Host Defense in Diabetic Mice. JCI Insight 2018, 3, e120220. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Nosenko, M.A.; Ambaryan, S.G.; Drutskaya, M.S. Proinflammatory Cytokines and Skin Wound Healing in Mice. Mol. Biol. 2019, 53, 653–664. [Google Scholar] [CrossRef]
- Frank, J.; Born, K.; Barker, J.H.; Marzi, I. In Vivo Effect of Tumor NecrosisFactor Alpha on Wound Angiogenesis AndEpithelialization. Eur. J. Trauma 2003, 29, 208–219. [Google Scholar] [CrossRef]
- Shinozaki, M.; Okada, Y.; Kitano, A.; Ikeda, K.; Saika, S.; Shinozaki, M. Impaired Cutaneous Wound Healing with Excess Granulation Tissue Formation in TNFalpha-Null Mice. Arch Dermatol. Res. 2009, 301, 531–537. [Google Scholar] [CrossRef]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of Mast Cell Secretory Granules on Skin Inflammation Boosts Dendritic Cell Migration and Priming Efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864.e4. [Google Scholar] [CrossRef]
- Qing, C. The Molecular Biology in Wound Healing & Non-Healing Wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [CrossRef]
- Xue, X.; Falcon, D.M. The Role of Immune Cells and Cytokines in Intestinal Wound Healing. Int. J. Mol. Sci. 2019, 20, 6097. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.-K.; Ji, R.-R. Cytokine Mechanisms of Central Sensitization: Distinct and Overlapping Role of Interleukin-1beta, Interleukin-6, and Tumor Necrosis Factor-Alpha in Regulating Synaptic and Neuronal Activity in the Superficial Spinal Cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- Werner, S.; Peters, K.G.; Longaker, M.T.; Fuller-Pace, F.; Banda, M.J.; Williams, L.T. Large Induction of Keratinocyte Growth Factor Expression in the Dermis during Wound Healing. Proc. Natl. Acad. Sci. USA 1992, 89, 6896–6900. [Google Scholar] [CrossRef]
- Tang, A.; Gilchrest, B.A. Regulation of Keratinocyte Growth Factor Gene Expression in Human Skin Fibroblasts. J. Dermatol. Sci. 1996, 11, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Gund, R.; Dutta, A.; Pincha, N.; Rana, I.; Ghosh, S.; Witherden, D.; Kandyba, E.; MacLeod, A.; Kobielak, K.; et al. Stimulation of Hair Follicle Stem Cell Proliferation through an IL-1 Dependent Activation of ΓδT-Cells. eLife 2017, 6, e28875. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A. Effects of Interleukin-1 on Cardiac Fibroblast Function: Relevance to Post-Myocardial Infarction Remodelling. Vasc. Pharmacol. 2014, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Laird, R.E.; Brown, R.D.; Long, C.S. IL-1beta Stimulates Rat Cardiac Fibroblast Migration via MAP Kinase Pathways. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1139–H1147. [Google Scholar] [CrossRef]
- De Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, Â. Cxcl8 (Interleukin-8) Mediates Neutrophil Recruitment and Behavior in the Zebrafish Inflammatory Response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Das, S.T.; Rajagopalan, L.; Guerrero-Plata, A.; Sai, J.; Richmond, A.; Garofalo, R.P.; Rajarathnam, K. Monomeric and Dimeric CXCL8 Are Both Essential for In Vivo Neutrophil Recruitment. PLoS ONE 2010, 5, e11754. [Google Scholar] [CrossRef] [PubMed]
- Paccaud, J.P.; Schifferli, J.A.; Baggiolini, M. NAP-1/IL-8 Induces up-Regulation of CR1 Receptors in Human Neutrophil Leukocytes. Biochem. Biophys. Res. Commun. 1990, 166, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and Granules of Human Neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef]
- Rabe, B.; Chalaris, A.; May, U.; Waetzig, G.H.; Seegert, D.; Williams, A.S.; Jones, S.A.; Rose-John, S.; Scheller, J. Transgenic Blockade of Interleukin 6 Transsignaling Abrogates Inflammation. Blood 2008, 111, 1021–1028. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Yang, R.; Masters, A.R.; Fortner, K.A.; Champagne, D.P.; Yanguas-Casás, N.; Silberger, D.J.; Weaver, C.T.; Haynes, L.; Rincon, M. IL-6 Promotes the Differentiation of a Subset of Naive CD8+ T Cells into IL-21-Producing B Helper CD8+ T Cells. J. Exp. Med. 2016, 213, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Bosurgi, L.; Cao, Y.G.; Cabeza-Cabrerizo, M.; Tucci, A.; Hughes, L.D.; Kong, Y.; Weinstein, J.S.; Licona-Limon, P.; Schmid, E.T.; Pelorosso, F.; et al. Macrophage Function in Tissue Repair and Remodeling Requires IL-4 or IL-13 with Apoptotic Cells. Science 2017, 356, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Luckett-Chastain, L.R.; Gallucci, R.M. Interleukin (IL)-6 Modulates Transforming Growth Factor-β Expression in Skin and Dermal Fibroblasts from IL-6-Deficient Mice. Br. J. Dermatol. 2009, 161, 237–248. [Google Scholar] [CrossRef] [PubMed]
- McFarland-Mancini, M.M.; Funk, H.M.; Paluch, A.M.; Zhou, M.; Giridhar, P.V.; Mercer, C.A.; Kozma, S.C.; Drew, A.F. Differences in Wound Healing in Mice with Deficiency of IL-6 versus IL-6 Receptor. J. Immunol. 2010, 184, 7219–7228. [Google Scholar] [CrossRef]
- Luckett, L.R.; Gallucci, R.M. Interleukin-6 (IL-6) Modulates Migration and Matrix Metalloproteinase Function in Dermal Fibroblasts from IL-6KO Mice. Br. J. Dermatol. 2007, 156, 1163–1171. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Sugawara, T.; Yucesoy, B.; Berryann, K.; Simeonova, P.P.; Matheson, J.M.; Luster, M.I. Interleukin-6 Treatment Augments Cutaneous Wound Healing in Immunosuppressed Mice. J. Interferon Cytokine Res. 2001, 21, 603–609. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; van de Water, L. Expression of Vascular Permeability Factor (Vascular Endothelial Growth Factor) by Epidermal Keratinocytes during Wound Healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of Interferon-γ During Innate and Adaptive Immune Responses. In Advances in Immunology; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 41–101. [Google Scholar]
- Miles, R.H.; Paxton, T.P.; Zacheis, D.; Dries, D.J.; Gamelli, R.L. Systemic Administration of Interferon-Gamma Impairs Wound Healing. J. Surg. Res. 1994, 56, 288–294. [Google Scholar] [CrossRef]
- Ishida, Y.; Kondo, T.; Takayasu, T.; Iwakura, Y.; Mukaida, N. The Essential Involvement of Cross-Talk between IFN-γ and TGF-β in the Skin Wound-Healing Process. J. Immunol. 2004, 172, 1848–1855. [Google Scholar] [CrossRef]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2019, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Le, L.D.; Marsh, E.; Crombleholme, T.M.; Keswani, S.G. Interleukin-10 Regulates Fetal Extracellular Matrix Hyaluronan Production. J. Pediatr. Surg. 2013, 48, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-H.; Guan, H.; Shi, S.; Cai, W.-X.; Bai, X.-Z.; Hu, X.-L.; Fang, X.-B.; Liu, J.-Q.; Tao, K.; Zhu, X.-X.; et al. Protection against TGF-Β1-Induced Fibrosis Effects of IL-10 on Dermal Fibroblasts and Its Potential Therapeutics for the Reduction of Skin Scarring. Arch. Dermatol. Res. 2013, 305, 341–352. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming Growth Factor-Beta Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Reibman, J.; Meixler, S.; Lee, T.C.; Gold, L.I.; Cronstein, B.N.; Haines, K.A.; Kolasinski, S.L.; Weissmann, G. Transforming Growth Factor Beta 1, a Potent Chemoattractant for Human Neutrophils, Bypasses Classic Signal-Transduction Pathways. Proc. Natl. Acad. Sci. USA 1991, 88, 6805–6809. [Google Scholar] [CrossRef]
- White, L.A.; Mitchell, T.I.; Brinckerhoff, C.E. Transforming Growth Factor Beta Inhibitory Element in the Rabbit Matrix Metalloproteinase-1 (Collagenase-1) Gene Functions as a Repressor of Constitutive Transcription. Biochim. Biophys. Acta 2000, 1490, 259–268. [Google Scholar] [CrossRef]
- Evrard, S.M.; d’Audigier, C.; Mauge, L.; Israël-Biet, D.; Guerin, C.L.; Bieche, I.; Kovacic, J.C.; Fischer, A.-M.; Gaussem, P.; Smadja, D.M. The Profibrotic Cytokine Transforming Growth Factor-Β1 Increases Endothelial Progenitor Cell Angiogenic Properties. J. Thromb. Haemost. 2012, 10, 670–679. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFβ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef]
- Martins-Green, M.; Petreaca, M.; Wang, L. Chemokines and Their Receptors Are Key Players in the Orchestra That Regulates Wound Healing. Adv. Wound Care 2013, 2, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Knaut, H. Chemokine Signaling in Development and Disease. Development 2014, 141, 4199–4205. [Google Scholar] [CrossRef] [PubMed]
- Clark-Lewis, I.; Kim, K.S.; Rajarathnam, K.; Gong, J.H.; Dewald, B.; Moser, B.; Baggiolini, M.; Sykes, B.D. Structure-Activity Relationships of Chemokines. J. Leukoc. Biol. 1995, 57, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Morales, J.; Hedrick, J.A. Recent Advances in Chemokines and Chemokine Receptors. Crit. Rev. Immunol. 1999, 19, 47. [Google Scholar] [CrossRef]
- Moser, B.; Dewald, B.; Barella, L.; Schumacher, C.; Baggiolini, M.; Clark-Lewis, I. Interleukin-8 Antagonists Generated by N-Terminal Modification. J. Biol. Chem. 1993, 268, 7125–7128. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Chemokines in Ischemia and Reperfusion. Thromb. Haemost. 2007, 97, 738–747. [Google Scholar] [CrossRef]
- Miller, M.D.; Krangel, M.S. The Human Cytokine I-309 Is a Monocyte Chemoattractant. Proc. Natl. Acad. Sci. USA 1992, 89, 2950–2954. [Google Scholar] [CrossRef]
- D’Ambrosio, D.; Iellem, A.; Bonecchi, R.; Mazzeo, D.; Sozzani, S.; Mantovani, A.; Sinigaglia, F. Cutting Edge: Selective Up-Regulation of Chemokine Receptors CCR4 and CCR8 upon Activation of Polarized Human Type 2 Th Cells. J. Immunol. 1998, 161, 5111–5115. [Google Scholar]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and Cytokine Levels in Inflammatory Bowel Disease Patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef]
- Jin, T.; Xu, X.; Hereld, D. Chemotaxis, Chemokine Receptors and Human Disease. Cytokine 2008, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gerard, C.; Rollins, B.J. Chemokines and Disease. Nat. Immunol. 2001, 2, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Sim, R.H.; Das, S.; Mahakkanukrauh, P. Therapeutic Targeting of Inflammatory Pathways with Emphasis on NLRP3 Inflammasomes by Natural Products: A Novel Approach for the Treatment of Inflammatory Eye Diseases. Curr. Med. Chem. 2022, 29, 2891–2912. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Das, S.; Mahakkanukrauh, P. Inflammatory Molecular Mediators and Pathways Involved in Vascular Aging and Stroke: A Comprehensive Review. Curr. Med. Chem. 2021, 28, 5522–5542. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.C.; Kosco-Vilbois, M.H. Glycosaminoglycan Binding and Oligomerization Are Essential for the in Vivo Activity of Certain Chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [CrossRef]
- Olson, T.S.; Ley, K. Chemokines and Chemokine Receptors in Leukocyte Trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 Chemokine Family and Its Receptors in Inflammatory Diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Zaja-Milatovic, S.; Richmond, A. CXC Chemokines and Their Receptors: A Case for a Significant Biological Role in Cutaneous Wound Healing. Histol. Histopathol. 2008, 23, 1399–1407. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Abkowitz, J.L.; Robinson, A.E.; Kale, S.; Long, M.W.; Chen, J. Mobilization of Hematopoietic Stem Cells during Homeostasis and after Cytokine Exposure. Blood 2003, 102, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Ley, K.; McEver, R.P.; Hidalgo, A. Leukocyte Ligands for Endothelial Selectins: Specialized Glycoconjugates That Mediate Rolling and Signaling under Flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kefer, J.; Bando, M.; Niles, W.D.; Malik, A.B. E-Selectin Expression in Human Endothelial Cells by TNF-Alpha-Induced Oxidant Generation and NF-KappaB Activation. Am. J. Physiol. 1998, 275, L533–L544. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The Role of Integrins in Inflammation and Angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Lämmermann, T.; Bader, B.L.; Monkley, S.J.; Worbs, T.; Wedlich-Söldner, R.; Hirsch, K.; Keller, M.; Förster, R.; Critchley, D.R.; Fässler, R.; et al. Rapid Leukocyte Migration by Integrin-Independent Flowing and Squeezing. Nature 2008, 453, 51–55. [Google Scholar] [CrossRef]
- Herter, J.; Zarbock, A. Integrin Regulation during Leukocyte Recruitment. J. Immunol. 2013, 190, 4451–4457. [Google Scholar] [CrossRef]
- McEver, R.P.; Zhu, C. Rolling Cell Adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. [Google Scholar] [CrossRef]
- Kuijper, P.H.; Gallardo Torres, H.I.; Houben, L.A.; Lammers, J.W.; Zwaginga, J.J.; Koenderman, L. P-Selectin and MAC-1 Mediate Monocyte Rolling and Adhesion to ECM-Bound Platelets under Flow Conditions. J. Leukoc. Biol. 1998, 64, 467–473. [Google Scholar] [CrossRef]
- Wolf, D.; Anto-Michel, N.; Blankenbach, H.; Wiedemann, A.; Buscher, K.; Hohmann, J.D.; Lim, B.; Bäuml, M.; Marki, A.; Mauler, M.; et al. A Ligand-Specific Blockade of the Integrin Mac-1 Selectively Targets Pathologic Inflammation While Maintaining Protective Host-Defense. Nat. Commun. 2018, 9, 525. [Google Scholar] [CrossRef]
- Detmers, P.A.; Lo, S.K.; Olsen-Egbert, E.; Walz, A.; Baggiolini, M.; Cohn, Z.A. Neutrophil-Activating Protein 1/Interleukin 8 Stimulates the Binding Activity of the Leukocyte Adhesion Receptor CD11b/CD18 on Human Neutrophils. J. Exp. Med. 1990, 171, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal Crawling of Neutrophils to Emigration Sites: A Molecularly Distinct Process from Adhesion in the Recruitment Cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Voisin, M.-B.; Larbi, K.Y.; Dangerfield, J.; Scheiermann, C.; Tran, M.; Maxwell, P.H.; Sorokin, L.; Nourshargh, S. Venular Basement Membranes Contain Specific Matrix Protein Low Expression Regions That Act as Exit Points for Emigrating Neutrophils. J. Exp. Med. 2006, 203, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.; Toksoy, A.; Goebeler, M.; Debus, S.; Bröcker, E.B.; Gillitzer, R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig Are Sequentially and Differentially Expressed during Phase-Specific Infiltration of Leukocyte Subsets in Human Wound Healing. Am. J. Pathol. 1998, 153, 1849–1860. [Google Scholar] [CrossRef]
- Ng, M.F.Y. The Role of Mast Cells in Wound Healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef]
- Younan, G.; Suber, F.; Xing, W.; Shi, T.; Kunori, Y.; Abrink, M.; Pejler, G.; Schlenner, S.M.; Rodewald, H.-R.; Moore, F.D.; et al. The Inflammatory Response after an Epidermal Burn Depends on the Activities of Mouse Mast Cell Proteases 4 and 5. J. Immunol. 2010, 185, 7681–7690. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil Migration in Infection and Wound Repair: Going Forward in Reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Gillitzer, R.; Goebeler, M. Chemokines in Cutaneous Wound Healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [CrossRef]
- Su, Y.; Richmond, A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv. Wound Care 2015, 4, 631–640. [Google Scholar] [CrossRef]
- Lämmermann, T. In the Eye of the Neutrophil Swarm-Navigation Signals That Bring Neutrophils Together in Inflamed and Infected Tissues. J. Leukoc. Biol. 2016, 100, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Qin, J.S.; Roediger, B.; Wang, Y.; Jain, R.; Cavanagh, L.L.; Smith, A.L.; Jones, C.A.; de Veer, M.; Grimbaldeston, M.A.; et al. Visualizing the Neutrophil Response to Sterile Tissue Injury in Mouse Dermis Reveals a Three-Phase Cascade of Events. J. Investig. Dermatol. 2011, 131, 2058–2068. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.N.; Vachon, D.J.; Gould, L.J. Proteolytic Activity in Wound Fluids and Tissues Derived from Chronic Venous Leg Ulcers. Wound Repair Regen. 2009, 17, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Degradation of the Epidermal-Dermal Junction by Proteolytic Enzymes from Human Skin and Human Polymorphonuclear Leukocytes. J. Exp. Med. 1984, 160, 1027–1042. [CrossRef]
- Pirilä, E.; Korpi, J.T.; Korkiamäki, T.; Jahkola, T.; Gutierrez-Fernandez, A.; Lopez-Otin, C.; Saarialho-Kere, U.; Salo, T.; Sorsa, T. Collagenase-2 (MMP-8) and Matrilysin-2 (MMP-26) Expression in Human Wounds of Different Etiologies. Wound Repair Regen. 2007, 15, 47–57. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-Induced Genomic Instability Impedes Resolution of Inflammation and Wound Healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Levin, R.; Grinstein, S.; Canton, J. The Life Cycle of Phagosomes: Formation, Maturation, and Resolution. Immunol. Rev. 2016, 273, 156–179. [Google Scholar] [CrossRef]
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by Neutrophils. Microbes Infect. 2003, 5, 1299–1306. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.M.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing Activity of Neutrophils Is Mediated through Activation of Proteases by K+ Flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The Paradox of the Neutrophil’s Role in Tissue Injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, A.; Amati, F.; Terranova, L.; Sotgiu, G.; Tarsia, P.; Miglietta, D.; Calderazzo, M.A.; Aliberti, S.; Blasi, F. Neutrophil Elastase in Bronchiectasis. Respir. Res. 2017, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.A.; Campbell, M.A.; Sannes, P.L.; Boukedes, S.S.; Campbell, E.J. Cell Surface-Bound Elastase and Cathepsin G on Human Neutrophils: A Novel, Non-Oxidative Mechanism by Which Neutrophils Focus and Preserve Catalytic Activity of Serine Proteinases. J. Cell Biol. 1995, 131, 775–789. [Google Scholar] [CrossRef]
- Sagel, S.D.; Wagner, B.D.; Anthony, M.M.; Emmett, P.; Zemanick, E.T. Sputum Biomarkers of Inflammation and Lung Function Decline in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 857–865. [Google Scholar] [CrossRef]
- Döring, G.; Frank, F.; Boudier, C.; Herbert, S.; Fleischer, B.; Bellon, G. Cleavage of Lymphocyte Surface Antigens CD2, CD4, and CD8 by Polymorphonuclear Leukocyte Elastase and Cathepsin G in Patients with Cystic Fibrosis. J. Immunol. 1995, 154, 4842–4850. [Google Scholar]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal Neutrophil Polarization Following Myocardial Infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J. Immunol. 2017, 198, 1387–1394. [Google Scholar] [CrossRef]
- Havixbeck, J.J.; Rieger, A.M.; Wong, M.E.; Hodgkinson, J.W.; Barreda, D.R. Neutrophil Contributions to the Induction and Regulation of the Acute Inflammatory Response in Teleost Fish. J. Leukoc. Biol. 2016, 99, 241–252. [Google Scholar] [CrossRef]
- Devalaraja, R.M.; Nanney, L.B.; Du, J.; Qian, Q.; Yu, Y.; Devalaraja, M.N.; Richmond, A. Delayed Wound Healing in CXCR2 Knockout Mice. J. Investig. Dermatol. 2000, 115, 234–244. [Google Scholar] [CrossRef]
- Nishio, N.; Okawa, Y.; Sakurai, H.; Isobe, K. Neutrophil Depletion Delays Wound Repair in Aged Mice. Age 2008, 30, 11–19. [Google Scholar] [CrossRef]
- Ortmann, W.; Kolaczkowska, E. Age Is the Work of Art? Impact of Neutrophil and Organism Age on Neutrophil Extracellular Trap Formation. Cell Tissue Res. 2018, 371, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Liu, G.Y. Expanding Roles of Neutrophils in Aging Hosts. Curr. Opin. Immunol. 2014, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dovi, J.V.; He, L.-K.; DiPietro, L.A. Accelerated Wound Closure in Neutrophil-Depleted Mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-Specific Contribution of Macrophages to Wound Healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The Role of Macrophages in Skin Homeostasis. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 455–463. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The Chemotaxis of M1 and M2 Macrophages Is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Polverini, P.J.; Rahbe, S.M.; Kovacs, E.J. Modulation of JE/MCP-1 Expression in Dermal Wound Repair. Am. J. Pathol. 1995, 146, 868–875. [Google Scholar]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Song, E.; Ouyang, N.; Hörbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of Alternatively and Classically Activated Macrophages on Fibrogenic Activities of Human Fibroblasts. Cell. Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Macrophage Phenotypes during Tissue Repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Slauch, J.M. How Does the Oxidative Burst of Macrophages Kill Bacteria? Still an Open Question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in Wound Healing: Activation and Plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Hart, P.H.; Jones, C.A.; Finlay-Jones, J.J. Monocytes Cultured in Cytokine-Defined Environments Differ from Freshly Isolated Monocytes in Their Responses to IL-4 and IL-10. J. Leukoc. Biol. 1995, 57, 909–918. [Google Scholar] [CrossRef]
- Goren, I.; Allmann, N.; Yogev, N.; Schürmann, C.; Linke, A.; Holdener, M.; Waisman, A.; Pfeilschifter, J.; Frank, S. A Transgenic Mouse Model of Inducible Macrophage Depletion: Effects of Diphtheria Toxin-Driven Lysozyme M-Specific Cell Lineage Ablation on Wound Inflammatory, Angiogenic, and Contractive Processes. Am. J. Pathol. 2009, 175, 132–147. [Google Scholar] [CrossRef]
- Mirza, R.; DiPietro, L.A.; Koh, T.J. Selective and Specific Macrophage Ablation Is Detrimental to Wound Healing in Mice. Am. J. Pathol. 2009, 175, 2454–2462. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Ma, Z.; Iwashina, T.; Tredget, E.E. Systemic Depletion of Macrophages in the Subacute Phase of Wound Healing Reduces Hypertrophic Scar Formation. Wound Repair Regen. 2016, 24, 644–656. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Kreisel, D.; Nava, R.G.; Li, W.; Zinselmeyer, B.H.; Wang, B.; Lai, J.; Pless, R.; Gelman, A.E.; Krupnick, A.S.; Miller, M.J. In Vivo Two-Photon Imaging Reveals Monocyte-Dependent Neutrophil Extravasation during Pulmonary Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 18073–18078. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, L. The Impact of the Extracellular Matrix on Inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-Resident Macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and Macrophages in Tissue Repair: Implications for Immunoregenerative Biomaterial Design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Leitinger, N.; Schulman, I.G. Phenotypic Polarization of Macrophages in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1120–1126. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The Role of Macrophage Phenotype in Vascularization of Tissue Engineering Scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Filardy, A.A.; Pires, D.R.; Nunes, M.P.; Takiya, C.M.; Freire-de-Lima, C.G.; Ribeiro-Gomes, F.L.; DosReis, G.A. Proinflammatory Clearance of Apoptotic Neutrophils Induces an IL-12lowIL-10high Regulatory Phenotype in Macrophages. J. Immunol. 2010, 185, 2044–2050. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 Protooncogene Regulates IL-6 Expression in Macrophages and Promotes the Generation of M2d Macrophages. Cell Res. 2010, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-Associated Leukemia Inhibitory Factor and IL-6 Skew Monocyte Differentiation into Tumor-Associated Macrophage-like Cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Cohn, Z.A. Identification of a Novel Cell Type in Peripheral Lymphoid Organs of Mice. I. Morphology, Quantitation, Tissue Distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Gutchinov, B.; Witmer, M.D.; Nussenzweig, M.C. Dendritic Cells Are the Principal Stimulators of the Primary Mixed Leukocyte Reaction in Mice. J. Exp. Med. 1983, 157, 613–627. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking Dendritic Cells into Medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic Cell Subsets and Locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef]
- Gregorio, J.; Meller, S.; Conrad, C.; Di Nardo, A.; Homey, B.; Lauerma, A.; Arai, N.; Gallo, R.L.; Digiovanni, J.; Gilliet, M. Plasmacytoid Dendritic Cells Sense Skin Injury and Promote Wound Healing through Type I Interferons. J. Exp. Med. 2010, 207, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Jegalian, A.G.; Facchetti, F.; Jaffe, E.S. Plasmacytoid Dendritic Cells: Physiologic Roles and Pathologic States. Adv. Anat. Pathol. 2009, 16, 392–404. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.B.; Catron, D.M.; Moon, J.J.; Jenkins, M.K. Dendritic Cell Antigen Presentation Drives Simultaneous Cytokine Production by Effector and Regulatory T Cells in Inflamed Skin. Immunity 2009, 30, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Vinish, M.; Cui, W.; Stafford, E.; Bae, L.; Hawkins, H.; Cox, R.; Toliver-Kinsky, T. Dendritic Cells Modulate Burn Wound Healing by Enhancing Early Proliferation. Wound Repair Regen. 2016, 24, 6–13. [Google Scholar] [CrossRef]
- Gao, N.; Yin, J.; Yoon, G.S.; Mi, Q.-S.; Yu, F.-S.X. Dendritic Cell–Epithelium Interplay Is a Determinant Factor for Corneal Epithelial Wound Repair. Am. J. Pathol. 2011, 179, 2243–2253. [Google Scholar] [CrossRef]
- Romani, N.; Holzmann, S.; Tripp, C.H.; Koch, F.; Stoitzner, P. Langerhans Cells—Dendritic Cells of the Epidermis. APMIS 2003, 111, 725–740. [Google Scholar] [CrossRef]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External Antigen Uptake by Langerhans Cells with Reorganization of Epidermal Tight Junction Barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef]
- Joffre, O.; Nolte, M.A.; Spörri, R.; Reis e Sousa, C. Inflammatory Signals in Dendritic Cell Activation and the Induction of Adaptive Immunity. Immunol. Rev. 2009, 227, 234–247. [Google Scholar] [CrossRef]
- Tang, A.; Amagai, M.; Granger, L.G.; Stanley, J.R.; Udey, M.C. Adhesion of Epidermal Langerhans Cells to Keratinocytes Mediated by E-Cadherin. Nature 1993, 361, 82–85. [Google Scholar] [CrossRef]
- Ratzinger, G.; Stoitzner, P.; Ebner, S.; Lutz, M.B.; Layton, G.T.; Rainer, C.; Senior, R.M.; Shipley, J.M.; Fritsch, P.; Schuler, G.; et al. Matrix Metalloproteinases 9 and 2 Are Necessary for the Migration of Langerhans Cells and Dermal Dendritic Cells from Human and Murine Skin. J. Immunol. 2002, 168, 4361–4371. [Google Scholar] [CrossRef]
- Kabashima, K.; Shiraishi, N.; Sugita, K.; Mori, T.; Onoue, A.; Kobayashi, M.; Sakabe, J.-I.; Yoshiki, R.; Tamamura, H.; Fujii, N.; et al. CXCL12-CXCR4 Engagement Is Required for Migration of Cutaneous Dendritic Cells. Am. J. Pathol. 2007, 171, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human Epidermal Langerhans Cells Maintain Immune Homeostasis in Skin by Activating Skin Resident Regulatory T Cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef] [PubMed]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Yin, N.; Lehmann, J.; Pastar, I.; Kirsner, R.S.; Tomic-Canic, M. Increased Number of Langerhans Cells in the Epidermis of Diabetic Foot Ulcers Correlates with Healing Outcome. Immunol. Res. 2013, 57, 222–228. [Google Scholar] [CrossRef]
- Sonoda, T.; Kitamura, Y.; Haku, Y.; Hara, H.; Mori, K.J. Mast-Cell Precursors in Various Haematopoietic Colonies of Mice Produced in Vivo and in Vitro. Br. J. Haematol. 1983, 53, 611–620. [Google Scholar] [CrossRef]
- Artuc, M.; Hermes, B.; Steckelings, U.M.; Grützkau, A.; Henz, B.M. Mast Cells and Their Mediators in Cutaneous Wound Healing—Active Participants or Innocent Bystanders? Exp. Dermatol. 1999, 8, 1–16. [Google Scholar] [CrossRef]
- Trautmann, A.; Toksoy, A.; Engelhardt, E.; Bröcker, E.B.; Gillitzer, R. Mast Cell Involvement in Normal Human Skin Wound Healing: Expression of Monocyte Chemoattractant Protein-1 Is Correlated with Recruitment of Mast Cells Which Synthesize Interleukin-4 in Vivo. J. Pathol. 2000, 190, 100–106. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast Cells as Sources of Cytokines, Chemokines, and Growth Factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Bjermer, L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin. Rev. Allergy Immunol. 2019, 56, 234–247. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Rambasek, T.; Wöhrl, S. Mastocytosis: From a Molecular Point of View. Clin. Rev. Allergy Immunol. 2018, 54, 397–411. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Rambasek, T.; Bielory, L. Clinical Implications of Mast Cell Involvement in Allergic Conjunctivitis. Allergy 2018, 73, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Wulff, B.C. The Importance of Mast Cells in Dermal Scarring. Adv. Wound Care 2014, 3, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Brown, M.A. Mast Cells: Multifaceted Immune Cells with Diverse Roles in Health and Disease. Ann. N. Y. Acad. Sci. 2008, 1143, 83–104. [Google Scholar] [CrossRef]
- Nakano, T.; Sonoda, T.; Hayashi, C.; Yamatodani, A.; Kanayama, Y.; Yamamura, T.; Asai, H.; Yonezawa, T.; Kitamura, Y.; Galli, S.J. Fate of Bone Marrow-Derived Cultured Mast Cells after Intracutaneous, Intraperitoneal, and Intravenous Transfer into Genetically Mast Cell-Deficient W/Wv Mice. Evidence That Cultured Mast Cells Can Give Rise to Both Connective Tissue Type and Mucosal Mast Cells. J. Exp. Med. 1985, 162, 1025–1043. [Google Scholar] [CrossRef]
- Wojta, J.; Kaun, C.; Zorn, G.; Ghannadan, M.; Hauswirth, A.W.; Sperr, W.R.; Fritsch, G.; Printz, D.; Binder, B.R.; Schatzl, G.; et al. C5a Stimulates Production of Plasminogen Activator Inhibitor-1 in Human Mast Cells and Basophils. Blood 2002, 100, 517–523. [Google Scholar] [CrossRef]
- Oschatz, C.; Maas, C.; Lecher, B.; Jansen, T.; Björkqvist, J.; Tradler, T.; Sedlmeier, R.; Burfeind, P.; Cichon, S.; Hammerschmidt, S.; et al. Mast Cells Increase Vascular Permeability by Heparin-Initiated Bradykinin Formation in Vivo. Immunity 2011, 34, 258–268. [Google Scholar] [CrossRef]
- Kennelly, R.; Conneely, J.B.; Bouchier-Hayes, D.; Winter, D.C. Mast Cells in Tissue Healing: From Skin to the Gastrointestinal Tract. Curr. Pharm. Des. 2011, 17, 3772–3775. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Wan, S.-W.; Wu-Hsieh, B.A.; Lin, Y.-S.; Chen, W.-Y.; Huang, Y.; Anderson, R. The Monocyte-Macrophage-Mast Cell Axis in Dengue Pathogenesis. J. Biomed. Sci. 2018, 25, 77. [Google Scholar] [CrossRef]
- Egozi, E.I.; Ferreira, A.M.; Burns, A.L.; Gamelli, R.L.; Dipietro, L.A. Mast Cells Modulate the Inflammatory but Not the Proliferative Response in Healing Wounds. Wound Repair Regen. 2003, 11, 46–54. [Google Scholar] [CrossRef]
- Iba, Y.; Shibata, A.; Kato, M.; Masukawa, T. Possible Involvement of Mast Cells in Collagen Remodeling in the Late Phase of Cutaneous Wound Healing in Mice. Int. Immunopharmacol. 2004, 4, 1873–1880. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kanda, N.; Hau, C.S.; Tada, Y.; Watanabe, S. Histamine Induces Human Beta-Defensin-3 Production in Human Keratinocytes. J. Dermatol. Sci. 2009, 56, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.; Aalto, M.L.; Harvima, R.J.; Horsmanheimo, M.; Harvima, I.T. Alterations in Mast Cells Showing Tryptase and Chymase Activity in Epithelializating and Chronic Wounds. Exp. Dermatol. 2000, 9, 258–265. [Google Scholar] [CrossRef]
- Sivamani, R.K. Eicosanoids and Keratinocytes in Wound Healing. Adv. Wound Care 2014, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.; Hyttinen, M.; Nilsson, G.; Butterfield, J.H.; Horsmanheimo, M.; Harvima, I.T. Inhibition of Keratinocyte Growth in Cell Culture and Whole Skin Culture by Mast Cell Mediators. Exp. Dermatol. 2001, 10, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast Cells in Tumor Growth: Angiogenesis, Tissue Remodelling and Immune-Modulation. Biochim. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A Role for Skin Gammadelta T Cells in Wound Repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cauvi, G.; Witherden, D.A.; Havran, W.L. A Keratinocyte-Responsive Gamma Delta TCR Is Necessary for Dendritic Epidermal T Cell Activation by Damaged Keratinocytes and Maintenance in the Epidermis. J. Immunol. 2004, 172, 3573–3579. [Google Scholar] [CrossRef]
- Jameson, J.M.; Cauvi, G.; Sharp, L.L.; Witherden, D.A.; Havran, W.L. Gammadelta T Cell-Induced Hyaluronan Production by Epithelial Cells Regulates Inflammation. J. Exp. Med. 2005, 201, 1269–1279. [Google Scholar] [CrossRef]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167, 1323–1338.e14. [Google Scholar] [CrossRef]
- Witherden, D.A.; Watanabe, M.; Garijo, O.; Rieder, S.E.; Sarkisyan, G.; Cronin, S.J.F.; Verdino, P.; Wilson, I.A.; Kumanogoh, A.; Kikutani, H.; et al. The CD100 Receptor Interacts with Its Plexin B2 Ligand to Regulate Epidermal Γδ T Cell Function. Immunity 2012, 37, 314–325. [Google Scholar] [CrossRef]
- Havran, W.L.; Jameson, J.M. Epidermal T Cells and Wound Healing. J. Immunol. 2010, 184, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Panduro, M.; Benoist, C.; Mathis, D. Tissue Tregs. Annu. Rev. Immunol. 2016, 34, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R.; et al. Regulatory T Cell-Derived Interleukin-10 Limits Inflammation at Environmental Interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.-A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129.e11. [Google Scholar] [CrossRef]
- Sanchez Rodriguez, R.; Pauli, M.L.; Neuhaus, I.M.; Yu, S.S.; Arron, S.T.; Harris, H.W.; Yang, S.H.-Y.; Anthony, B.A.; Sverdrup, F.M.; Krow-Lucal, E.; et al. Memory Regulatory T Cells Reside in Human Skin. J. Clin. Investig. 2014, 124, 1027–1036. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Minutti, C.M.; Knipper, J.A. Immune- and Non-Immune-Mediated Roles of Regulatory T-Cells during Wound Healing. Immunology 2019, 157, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Nosbaum, A.; Prevel, N.; Truong, H.A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Boothby, I.C.; Cohen, J.N.; Rosenblum, M.D. Regulatory T Cells in Skin Injury: At the Crossroads of Tolerance and Tissue Repair. Sci. Immunol. 2020, 5, eaaz9631. [Google Scholar] [CrossRef] [PubMed]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.C.; John, S.; Taams, L.S. CD4+CD25+Foxp3+ Regulatory T Cells Induce Alternative Activation of Human Monocytes/Macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e6. [Google Scholar] [CrossRef]
- Headland, S.E.; Norling, L.V. The Resolution of Inflammation: Principles and Challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Bratton, D.L.; Henson, P.M. Neutrophil Clearance: When the Party Is over, Clean-up Begins. Trends Immunol. 2011, 32, 350–357. [Google Scholar] [CrossRef]
- Jun, J.-I.; Kim, K.-H.; Lau, L.F. The Matricellular Protein CCN1 Mediates Neutrophil Efferocytosis in Cutaneous Wound Healing. Nat. Commun. 2015, 6, 7386. [Google Scholar] [CrossRef]
- Ji, J.; Fan, J. Neutrophil in Reverse Migration: Role in Sepsis. Front. Immunol. 2021, 12, 656039. [Google Scholar] [CrossRef]
- Chen, W.Y.J.; Rogers, A.A. Recent Insights into the Causes of Chronic Leg Ulceration in Venous Diseases and Implications on Other Types of Chronic Wounds. Wound Repair Regen. 2007, 15, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, W.; Lu, Z.; Sheng, Z.; Yao, Y. The Growing Spectrum of Anti-Inflammatory Interleukins and Their Potential Roles in the Development of Sepsis. J. Interf. Cytokine Res. 2015, 35, 242–251. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual Regulation of Inflammation: A Duet by Transforming Growth Factor-Beta and Interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, A.E.; Granowitz, E.V.; Shapiro, L.; Vannier, E.; Lonnemann, G.; Angel, J.B.; Kennedy, J.S.; Rabson, A.R.; Wolff, S.M.; Dinarello, C.A. A Randomized, Controlled Trial of IL-10 in Humans. Inhibition of Inflammatory Cytokine Production and Immune Responses. J. Immunol. 1995, 154, 5492–5499. [Google Scholar] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Palolahti, M.; Lauharanta, J.; Stephens, R.W.; Kuusela, P.; Vaheri, A. Proteolytic Activity in Leg Ulcer Exudate. Exp. Dermatol. 1993, 2, 29–37. [Google Scholar] [CrossRef]
- Harris, I.R.; Yee, K.C.; Walters, C.E.; Cunliffe, W.J.; Kearney, J.N.; Wood, E.J.; Ingham, E. Cytokine and Protease Levels in Healing and Non-Healing Chronic Venous Leg Ulcers. Exp. Dermatol. 1995, 4, 342–349. [Google Scholar] [CrossRef]
- Barrick, B.; Campbell, E.J.; Owen, C.A. Leukocyte Proteinases in Wound Healing: Roles in Physiologic and Pathologic Processes. Wound Repair Regen. 1999, 7, 410–422. [Google Scholar] [CrossRef]
- Saarialho-Kere, U.K. Patterns of Matrix Metalloproteinase and TIMP Expression in Chronic Ulcers. Arch. Dermatol. Res. 1998, 290 (Suppl. S1), S47–S54. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Mast, B.A.; Schultz, G.S. Interactions of Cytokines, Growth Factors, and Proteases in Acute and Chronic Wounds. Wound Repair Regen. 1996, 4, 411–420. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as Protagonists and Targets in Chronic Inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Thamm, O.C.; Koenen, P.; Bader, N.; Schneider, A.; Wutzler, S.; Neugebauer, E.A.; Spanholtz, T.A. Acute and Chronic Wound Fluids Influence Keratinocyte Function Differently. Int. Wound J. 2015, 12, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Pastar, I.; Vukelic, S.; Mahoney, M.G.; Brennan, D.; Krzyzanowska, A.; Golinko, M.; Brem, H.; Tomic-Canic, M. Deregulation of Keratinocyte Differentiation and Activation: A Hallmark of Venous Ulcers. J. Cell Mol. Med. 2008, 12, 2675–2690. [Google Scholar] [CrossRef] [PubMed]
- Bucalo, B.; Eaglstein, W.H.; Falanga, V. Inhibition of Cell Proliferation by Chronic Wound Fluid. Wound Repair Regen. 1993, 1, 181–186. [Google Scholar] [CrossRef]
- Cha, J.; Kwak, T.; Butmarc, J.; Kim, T.-A.; Yufit, T.; Carson, P.; Kim, S.-J.; Falanga, V. Fibroblasts from Non-Healing Human Chronic Wounds Show Decreased Expression of Βig-H3, a TGF-β Inducible Protein. J. Dermatol Sci. 2008, 50, 15–23. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef]
- Olingy, C.E.; San Emeterio, C.L.; Ogle, M.E.; Krieger, J.R.; Bruce, A.C.; Pfau, D.D.; Jordan, B.T.; Peirce, S.M.; Botchwey, E.A. Non-Classical Monocytes Are Biased Progenitors of Wound Healing Macrophages during Soft Tissue Injury. Sci. Rep. 2017, 7, 447. [Google Scholar] [CrossRef]
- Dario, M.D.; Colombo, E.; Govi, C.; Feo, D.D.; Messina, M.J.; Romeo, M.; Sangalli, F.; Moiola, L.; Rodegher, M.; Martino, G.; et al. Myeloid Cells as Target of Fingolimod Action in Multiple Sclerosis. Neurol.-Neuroimmunol. Neuroinflamm. 2015, 2, e157. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.S.J.; Sham, A.; Chee, S.M.L.; Chan, C.; Raghunath, M. Combination of Ciclopirox Olamine and Sphingosine-1-Phosphate as Granulation Enhancer in Diabetic Wounds. Wound Repair Regen. 2016, 24, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Awojoodu, A.O.; Ogle, M.E.; Sefcik, L.S.; Bowers, D.T.; Martin, K.; Brayman, K.L.; Lynch, K.R.; Peirce-Cottler, S.M.; Botchwey, E. Sphingosine 1-Phosphate Receptor 3 Regulates Recruitment of Anti-Inflammatory Monocytes to Microvessels during Implant Arteriogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 13785–13790. [Google Scholar] [CrossRef]
- Sun, G. Pro-Regenerative Hydrogel Restores Scarless Skin during Cutaneous Wound Healing. Adv. Healthc. Mater. 2017, 6, 1700659. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.; VandeVord, P.; Van Dyke, M. Keratin Biomaterials Augment Anti-Inflammatory Macrophage Phenotype in Vitro. Acta Biomater. 2018, 66, 213–223. [Google Scholar] [CrossRef]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.-N. Antimicrobial Wound Dressing Film Utilizing Cellulose Nanocrystal as Drug Delivery System for Curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Berce, C.; Muresan, M.-S.; Soritau, O.; Petrushev, B.; Tefas, L.; Rigo, I.; Ungureanu, G.; Catoi, C.; Irimie, A.; Tomuleasa, C. Cutaneous Wound Healing Using Polymeric Surgical Dressings Based on Chitosan, Sodium Hyaluronate and Resveratrol. A Preclinical Experimental Study. Colloids Surf. B Biointerfaces 2018, 163, 155–166. [Google Scholar] [CrossRef]
- Kasiewicz, L.N.; Whitehead, K.A. Silencing TNFα with Lipidoid Nanoparticles Downregulates Both TNFα and MCP-1 in an in Vitro Co-Culture Model of Diabetic Foot Ulcers. Acta Biomater. 2016, 32, 120–128. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.H.; Kim, S.H.; Jung, Y. Skin Regeneration with Self-Assembled Peptide Hydrogels Conjugated with Substance P in a Diabetic Rat Model. Tissue Eng. Part A 2018, 24, 21–33. [Google Scholar] [CrossRef]
| Phenotype | Receptors | Functions |
|---|---|---|
| M1 (classically activated or proinflammatory) | CD68 CD86 CD80 | |
| M2a (alternatively activated or wound healing) | CD163 CD206 CD209Ym1 | |
| M2b (regulatory or type 2) | CD86 | |
| M2c (pro-resolving or deactivated) | CD86 CD163 CD206 |
|
| M2d (tumor-associated macrophages) | - |
|
| Repair Event | Functions |
|---|---|
| Hemostasis |
|
| Inflammation | |
| Re-epithelialization |
|
| Granulation tissue formation |
|
| Angiogenesis |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2023, 24, 641. https://doi.org/10.3390/ijms24010641
Soliman AM, Barreda DR. Acute Inflammation in Tissue Healing. International Journal of Molecular Sciences. 2023; 24(1):641. https://doi.org/10.3390/ijms24010641
Chicago/Turabian StyleSoliman, Amro M., and Daniel R. Barreda. 2023. "Acute Inflammation in Tissue Healing" International Journal of Molecular Sciences 24, no. 1: 641. https://doi.org/10.3390/ijms24010641
APA StyleSoliman, A. M., & Barreda, D. R. (2023). Acute Inflammation in Tissue Healing. International Journal of Molecular Sciences, 24(1), 641. https://doi.org/10.3390/ijms24010641






