Full-Length Transcriptome of Myotis pilosus as a Reference Resource and Mining of Auditory and Immune Related Genes
Abstract
1. Introduction
2. Results
2.1. PacBio Iso-Seq Sequencing Data Analysis
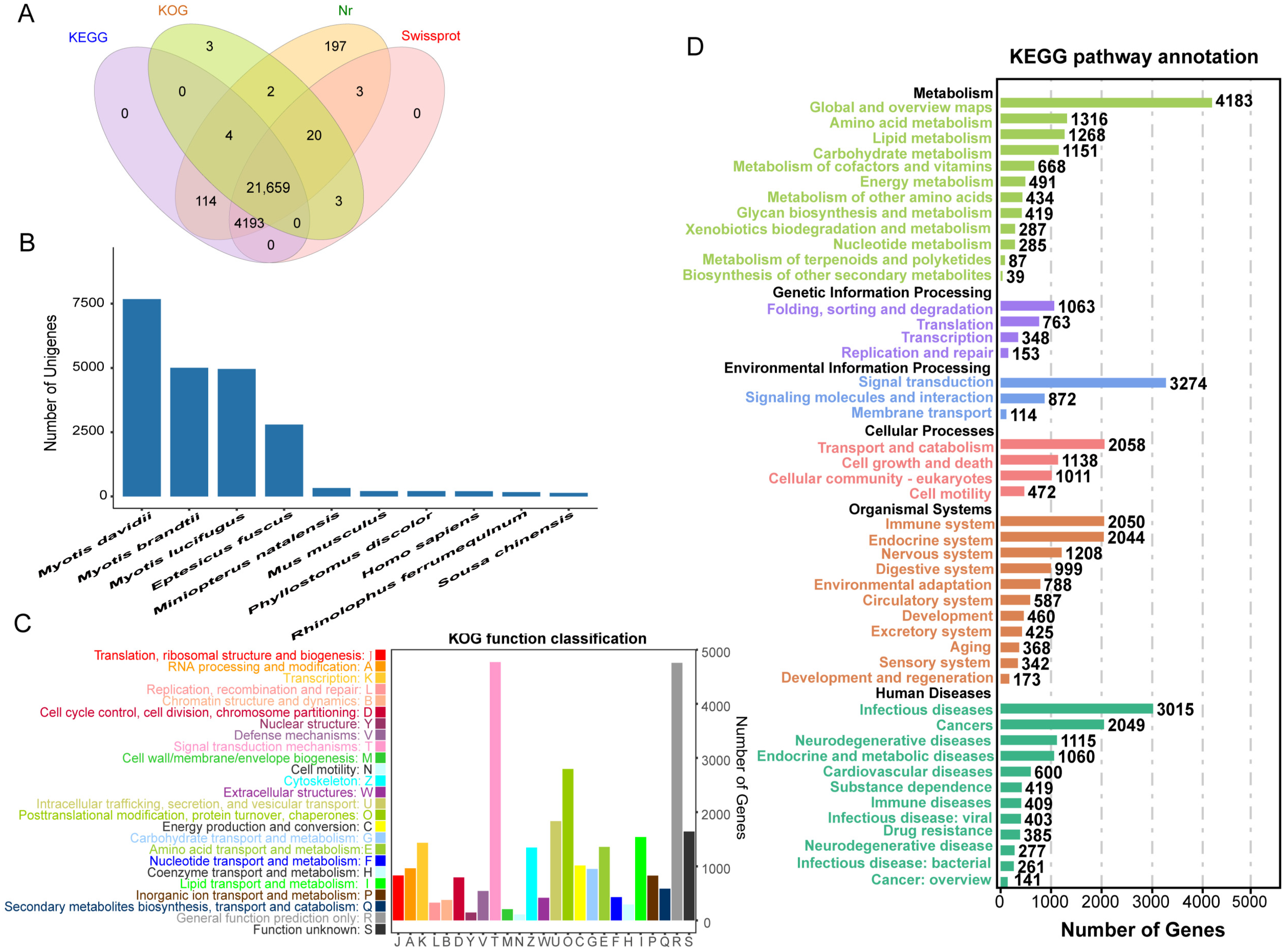
2.2. Functional Annotation
2.3. High-Level Annotation of Transcripts
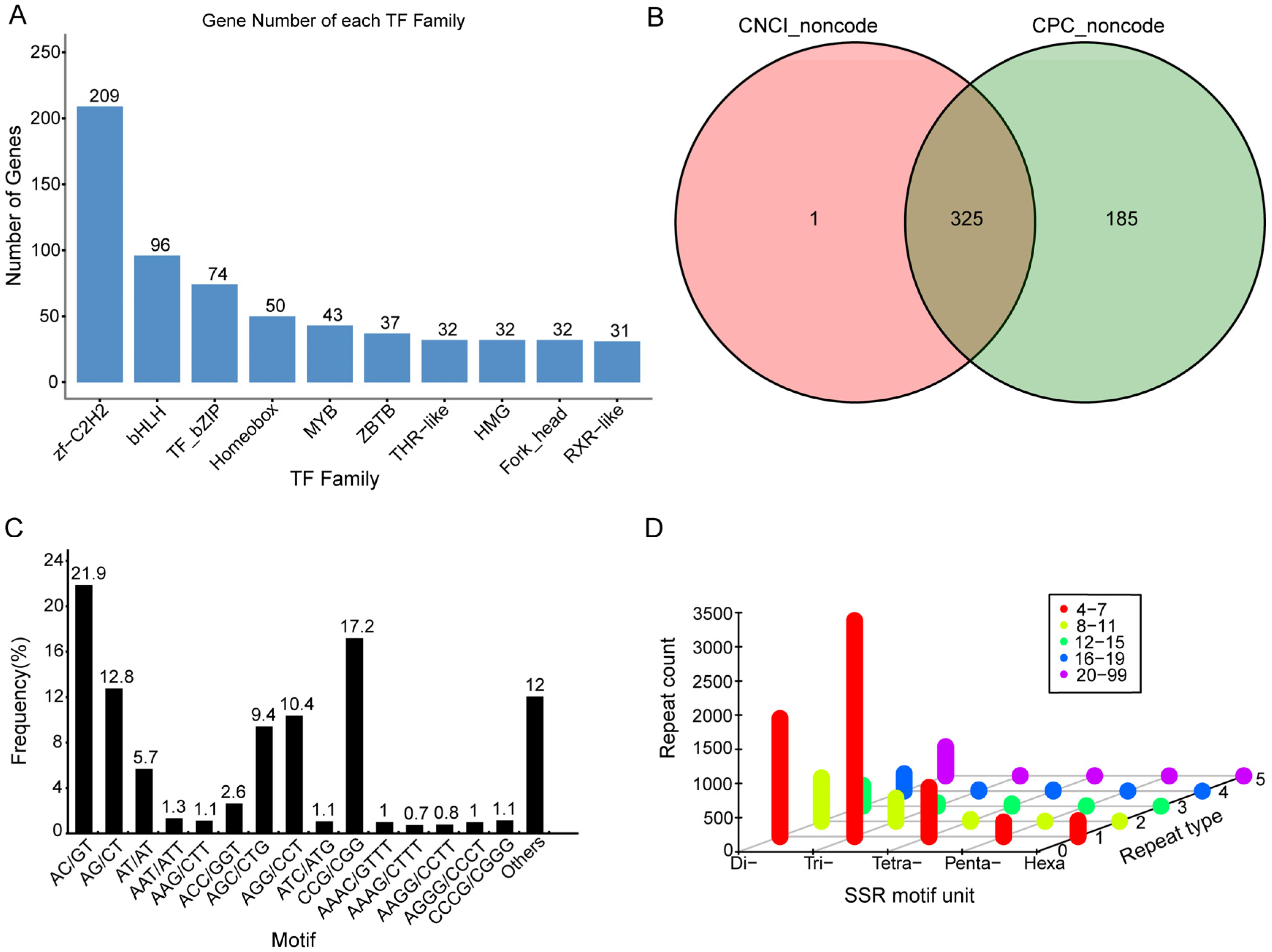
2.3.1. Transcription Factor (TF) Analysis
2.3.2. Identification of Long Non-Coding RNAs
2.3.3. SSR Prediction
2.4. Alternative Splicing Events
2.5. Validation of the Full-Length Transcriptome of M. pilosus
2.6. Identification of M. pilosus Transcripts
2.6.1. Identification of Neural-Related Transcripts in M. pilosus
2.6.2. Identification of Metabolite-Related Transcripts in M. pilosus
2.6.3. Transcript Identification Associated with Auditory Perception in M. pilosus
2.6.4. Identification of Immune-Related Transcripts in M. pilosus
3. Discussion
4. Materials and Methods
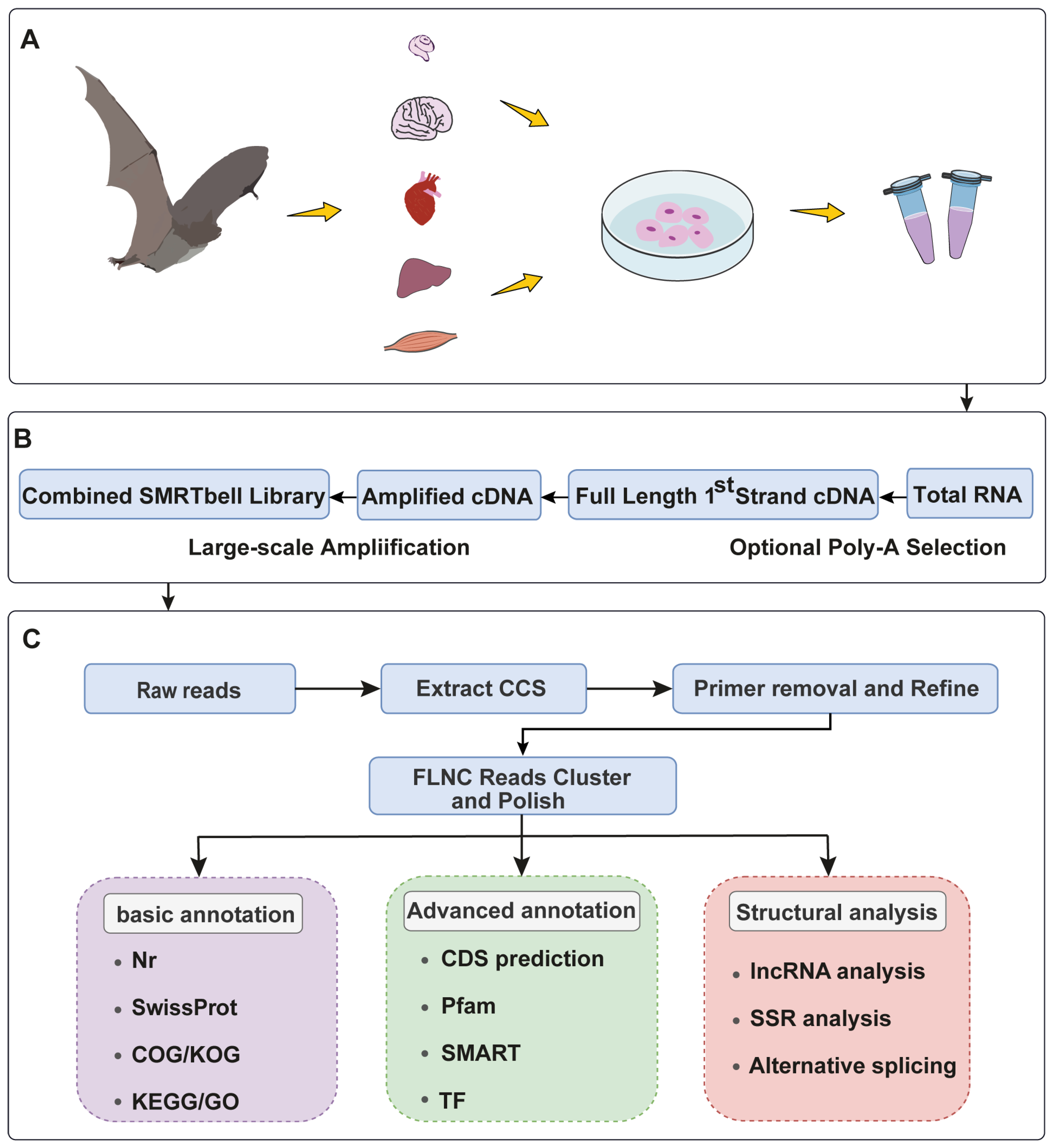
4.1. Sample Collection
4.2. Library Construction and SMRT Sequencing
4.3. Data Processing and Error Correction of PacBio Iso-Seq Reads
4.4. Functional Annotation of Transcripts
4.5. Open Reading Frame Prediction and Protein Domain Prediction
4.6. Transcription Factor (TF) Analysis and Potential Protein Function Analysis
4.7. Simple Sequence Repeats (SSR) Prediction
- (1)
- (n, m): n is the length of repeat units. m is the minimum number of repeat units.
- (2)
- Interruptions: the distance between two SSRs less than 100 bp is considered as one SSR
4.8. Characterization of Long Non-Coding RNAs and Alternative Splicing Detection
4.9. Validating the Accuracy of Sequencing Result
4.10. Identification of Immune-Related Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurme, E.; Gurarie, E.; Greif, S.; Herrera, M.L.; Flores-Martínez, J.J.; Wilkinson, G.S.; Yovel, Y. Acoustic evaluation of behavioral states predicted from GPS tracking: A case study of a marine fishing bat. Mov. Ecol. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cowled, C.; Shi, Z.; Huang, Z.; Bishop-Lilly, K.A.; Fang, X.; Wynne, J.W.; Xiong, Z.; Baker, M.L.; Zhao, W.; et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 2013, 339, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Song, S.; Li, A.; Zhang, Y.; Li, Z.; Xiao, Y.; Jiang, T.; Feng, J.; Lin, A. The roles of morphological traits, resource variation and resource partitioning associated with the dietary niche expansion in the fish-eating bat Myotis pilosus. Mol. Ecol. 2019, 28, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Tang, X.; Zhang, J.; Liao, Z.; Wang, G.; Cheng, R.; Zhang, Z.; Zhao, H.; Wang, J.; Tan, Z.; et al. An inhibitor of leukotriene-A4 hydrolase from bat salivary glands facilitates virus infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2110647119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chu, Y.; Jiang, T.; Li, J.; Feng, L.; Wu, H.; Wang, H.; Feng, J. Comparative analysis of the daily brain transcriptomes of Asian particolored bat. Sci. Rep. 2022, 12, 3876. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Lei, M.; Liu, Y.; Zhang, S. Comparative inner ear transcriptome analysis between the Rickett’s big-footed bats (Myotis ricketti) and the greater short-nosed fruit bats (Cynopterus sphinx). BMC Genom. 2013, 14, 916. [Google Scholar] [CrossRef]
- Ahn, M.; Anderson, D.E.; Zhang, Q.; Tan, C.W.; Lim, B.L.; Luko, K.; Wen, M.; Chia, W.N.; Mani, S.; Wang, L.C.; et al. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019, 4, 789–799. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Aierken, R.; Kang, Q.; Wang, X.; Zeng, Q.; Fan, Z.; Zhen, Y.; Zhao, L. Integrated Full-Length Transcriptome and RNA-Seq to Identify Immune System Genes from the Skin of Sperm Whale (Physeter macrocephalus). Genes 2021, 12, 233. [Google Scholar] [CrossRef]
- Ramberg, S.; Høyheim, B.; Østbye, T.K.; Andreassen, R. A De Novo Full-Length mRNA Transcriptome Generated from Hybrid-Corrected PacBio Long-Reads Improves the Transcript Annotation and Identifies Thousands of Novel Splice Variants in Atlantic Salmon. Front. Genet. 2021, 12, 656334. [Google Scholar] [CrossRef]
- Ma, L.; Sun, H.; Mao, X. Transcriptome sequencing of cochleae from constant-frequency and frequency-modulated echolocating bats. Sci. Data 2020, 7, 341. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Huang, X.; Sun, K.; Feng, J. Comparative cochlear transcriptomics of echolocating bats provides new insights into different nervous activities of CF bat species. Sci. Rep. 2018, 8, 15934. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Kulcsar, K.A.; Elliott, O.; Khiabanian, H.; Nagle, E.R.; Jones, M.E.; Amman, B.R.; Sanchez-Lockhart, M.; Towner, J.S.; Palacios, G.; et al. De novo transcriptome reconstruction and annotation of the Egyptian rousette bat. BMC Genom. 2015, 16, 1033. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Chu, Y.; Feng, J.; Sun, K. Assessing evidence for adaptive evolution in two hearing-related genes important for high-frequency hearing in echolocating mammals. G3 2021, 11, jkab069. [Google Scholar] [CrossRef]
- Wan, H.; Jia, X.; Zou, P.; Zhang, Z.; Wang, Y. The Single-molecule long-read sequencing of Scylla paramamosain. Sci. Rep. 2019, 9, 12401. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tseng, E.; Regulski, M.; Clark, T.A.; Hon, T.; Jiao, Y.; Lu, Z.; Olson, A.; Stein, J.C.; Ware, D. Unveiling the complexity of the maize transcriptome by single-molecule long-read sequencing. Nat. Commun. 2016, 7, 11708. [Google Scholar] [CrossRef] [PubMed]
- Hackl, T.; Hedrich, R.; Schultz, J.; Förster, F. proovread: Large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics 2014, 30, 3004–3011. [Google Scholar] [CrossRef]
- Gracheva, E.O.; Cordero-Morales, J.F.; González-Carcacía, J.A.; Ingolia, N.T.; Manno, C.; Aranguren, C.I.; Weissman, J.S.; Julius, D. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 2011, 476, 88–91. [Google Scholar] [CrossRef]
- Chen, W.; Mao, X. Extensive alternative splicing triggered by mitonuclear mismatch in naturally introgressed Rhinolophus bats. Ecol. Evol. 2021, 11, 12003–12010. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Huang, S.; Wang, G. Full-length transcriptome sequencing of Heliocidaris crassispina using PacBio single-molecule real-time sequencing. Fish Shellfish Immunol. 2022, 120, 507–514. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Sun, K.; Huang, X.; Jin, L.; Feng, J. Evolutionary Basis of High-Frequency Hearing in the Cochleae of Echolocators Revealed by Comparative Genomics. Genome Biol. Evol. 2020, 12, 3740–3753. [Google Scholar] [CrossRef]
- Ranum, P.T.; Goodwin, A.T.; Yoshimura, H.; Kolbe, D.L.; Walls, W.D.; Koh, J.Y.; He, D.Z.Z.; Smith, R.J.H. Insights into the Biology of Hearing and Deafness Revealed by Single-Cell RNA Sequencing. Cell Rep. 2019, 26, 3160–3171.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, J.; Rossiter, S.J.; Jones, G.; Cotton, J.A.; Zhang, S. The hearing gene Prestin reunites echolocating bats. Proc. Natl. Acad. Sci. USA 2008, 105, 13959–13964. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Délicat, A.; Paweska, J.T.; Gonzalez, J.P.; Swanepoel, R. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; Hayman, D.T.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.; Mills, J.N.; Timonin, M.E.; Willis, C.K.; Cunningham, A.A.; et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc. Biol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef]
- Gordon, S.P.; Tseng, E.; Salamov, A.; Zhang, J.; Meng, X.; Zhao, Z.; Kang, D.; Underwood, J.; Grigoriev, I.V.; Figueroa, M.; et al. Widespread Polycistronic Transcripts in Fungi Revealed by Single-Molecule mRNA Sequencing. PLoS ONE 2015, 10, e0132628. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Shimizu, K.; Adachi, J.; Muraoka, Y. ANGLE: A sequencing errors resistant program for predicting protein coding regions in unfinished cDNA. J. Bioinform. Comput. Biol. 2006, 4, 649–664. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Harata-Lee, Y.; Denton, M.D.; Feng, Q.; Rathjen, J.R.; Qu, Z.; Adelson, D.L. Long read reference genome-free reconstruction of a full-length transcriptome from Astragalus membranaceus reveals transcript variants involved in bioactive compound biosynthesis. Cell Discov. 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Alamancos, G.P.; Pagès, A.; Trincado, J.L.; Bellora, N.; Eyras, E. Leveraging transcript quantification for fast computation of alternative splicing profiles. RNA 2015, 21, 1521–1531. [Google Scholar] [CrossRef]
- Ru, B.; Han, N.; He, G.; Brayer, K.; Zhang, S.; Wang, Z. Molecular cloning and evolutionary analysis of GJB6 in mammals. Biochem. Genet. 2012, 50, 213–226. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Fan, R.Y.Y.; Luk, H.K.H.; Zhu, L.; Fung, J.; Li, K.S.M.; Wong, E.Y.M.; Ahmed, S.S.; Chan, J.F.W.; Kok, R.K.H.; et al. Replication of MERS and SARS coronaviruses in bat cells offers insights to their ancestral origins. Emerg. Microbes Infect. 2018, 7, 209. [Google Scholar] [CrossRef]
- Kawai, K.; Nikaido, M.; Harada, M.; Matsumura, S.; Lin, L.K.; Wu, Y.; Hasegawa, M.; Okada, N. The status of the Japanese and East Asian bats of the genus Myotis (Vespertilionidae) based on mitochondrial sequences. Mol. Phylogenet. Evol. 2003, 28, 297–307. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, J.; Lin, B.; Zhang, J.; Zhang, S. Differential expression and functional constraint of PRL-2 in hibernating bat. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 375–381. [Google Scholar] [CrossRef]



| Sample | M. pilosus |
|---|---|
| Subreads number | 17,570,601 |
| Mean length (bp) | 2622 |
| CCS number | 752,249 |
| Mean CCS read length (bp) | 2978 |
| Number of transcripts | 29,456 |
| Number of non-redundant transcripts | 26,717 |
| Mean length (bp) | 3018.91 |
| Isoform | Structure |
|---|---|
| Isoform0026163 | NACHT |
| Isoform0007843 | NACHT |
| Isoform0007306 | NA |
| Isoform0006981 | RIG-I_C-RD |
| Isoform0008035 | RIG-I_C-RD |
| Isoform0015581 | RIG-I_C-RD |
| Isoform0017682 | IFNGR1 |
| Isoform0019310 | IFNGR1 |
| Isoform0022891 | IRF-3 |
| Isoform0023355 | IRF-3 |
| Isoform0006241 | IRF-3 |
| Isoform0011995 | IRF-3 |
| Isoform0018495 | IRF-3 |
| Isoform0019133 | IRF-3 |
| Isoform0019959 | IRF-3 |
| Isoform0022054 | CD20 |
| Isoform0022520 | CD20 |
| Isoform0024768 | CD20 |
| Isoform0010536 | CD20 |
| Isoform0015085 | CD20 |
| Isoform0019840 | CD20 |
| Isoform0021250 | CD20 |
| Isoform0021512 | CD20 |
| Isoform0025649 | Cd27 binding protein (Siva) |
| Isoform0000889 | TGF_beta_GS |
| Isoform0026593 | TGF_beta_GS |
| Isoform0001938 | TGFb_propeptide |
| Isoform0001938 | TGF_beta |
| Isoform0003427 | TGF_beta_GS |
| Isoform0009838 | TGF_beta_GS |
| Isoform0012071 | TGF_beta_GS |
| Isoform0011693 | TGF_beta |
| Isoform0014684 | TGFb_propeptide |
| Isoform0014684 | TGF_beta |
| Isoform0012071 | TGF_beta_GS |
| Isoform0014741 | TGFb_propeptide |
| Isoform0014741 | TGF_beta |
| Isoform0016251 | TGFb_propeptide |
| Isoform0016251 | TGF_beta |
| Isoform0017557 | TGFb_propeptide |
| Isoform0017557 | TGF_beta |
| Isoform0010407 | TGF_beta_GS |
| Isoform0011693 | TGFb_propeptide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Bao, M.; Xu, N.; Sun, R.; Dai, W.; Sun, K.; Wang, H.; Feng, J. Full-Length Transcriptome of Myotis pilosus as a Reference Resource and Mining of Auditory and Immune Related Genes. Int. J. Mol. Sci. 2023, 24, 62. https://doi.org/10.3390/ijms24010062
Wang X, Bao M, Xu N, Sun R, Dai W, Sun K, Wang H, Feng J. Full-Length Transcriptome of Myotis pilosus as a Reference Resource and Mining of Auditory and Immune Related Genes. International Journal of Molecular Sciences. 2023; 24(1):62. https://doi.org/10.3390/ijms24010062
Chicago/Turabian StyleWang, Xue, Mingyue Bao, Ningning Xu, Ruyi Sun, Wentao Dai, Keping Sun, Hui Wang, and Jiang Feng. 2023. "Full-Length Transcriptome of Myotis pilosus as a Reference Resource and Mining of Auditory and Immune Related Genes" International Journal of Molecular Sciences 24, no. 1: 62. https://doi.org/10.3390/ijms24010062
APA StyleWang, X., Bao, M., Xu, N., Sun, R., Dai, W., Sun, K., Wang, H., & Feng, J. (2023). Full-Length Transcriptome of Myotis pilosus as a Reference Resource and Mining of Auditory and Immune Related Genes. International Journal of Molecular Sciences, 24(1), 62. https://doi.org/10.3390/ijms24010062





