The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation
Abstract
1. Introduction
2. Challenges in Renal Transplantation
2.1. Global Donor Kidney Shortage Crisis
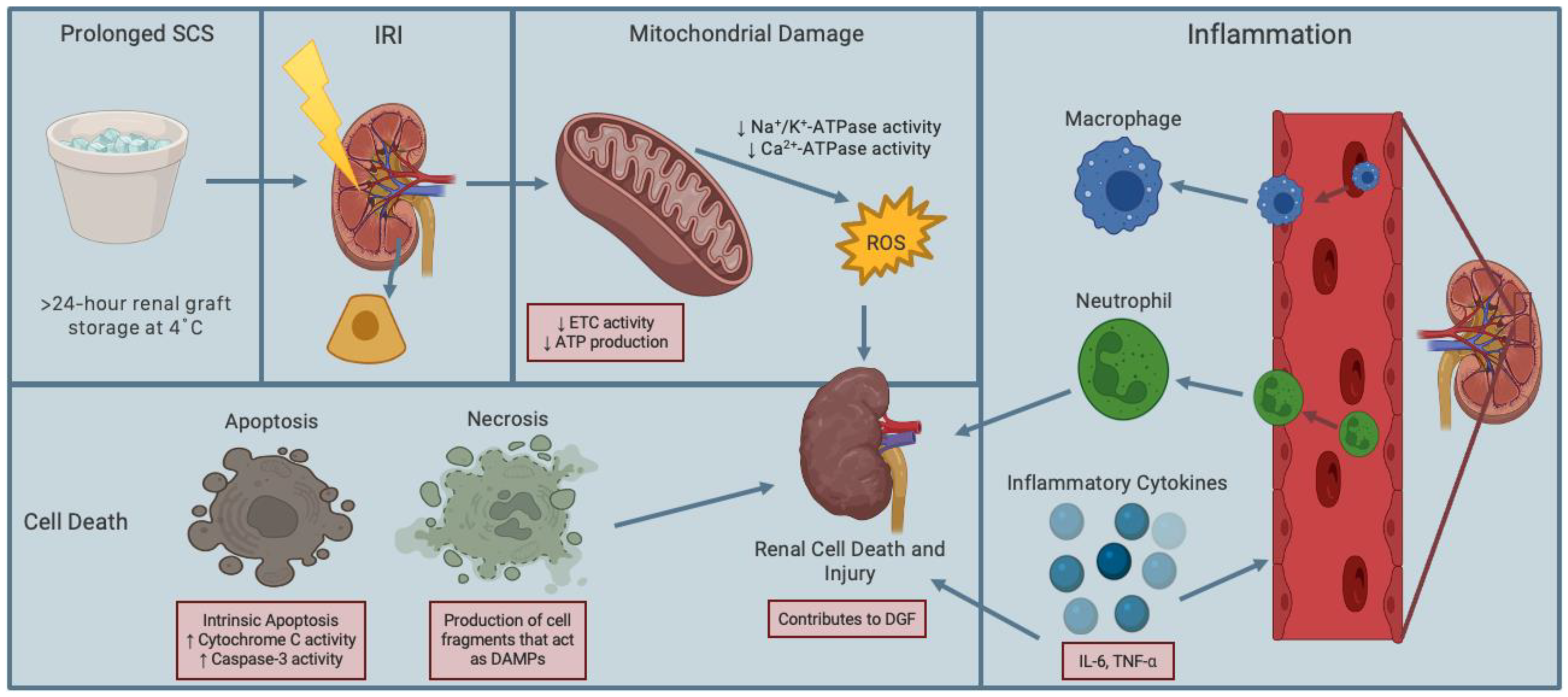
2.2. Renal Ischemia-Reperfusion Injury
3. Efforts to Mitigate Kidney Transplant-Induced Renal IRI and Improve Renal Graft Quality for Transplantation
3.1. Static Cold Storage
3.2. Hypothermic Machine Perfusion
4. Alternatives to Hypothermic Renal Graft Preservation
4.1. Normothermic Temperatures at 35–37 °C
4.2. Subnormothermic Temperatures at 20–22 °C
4.3. Subnormothermic Temperatures at 8–10 °C
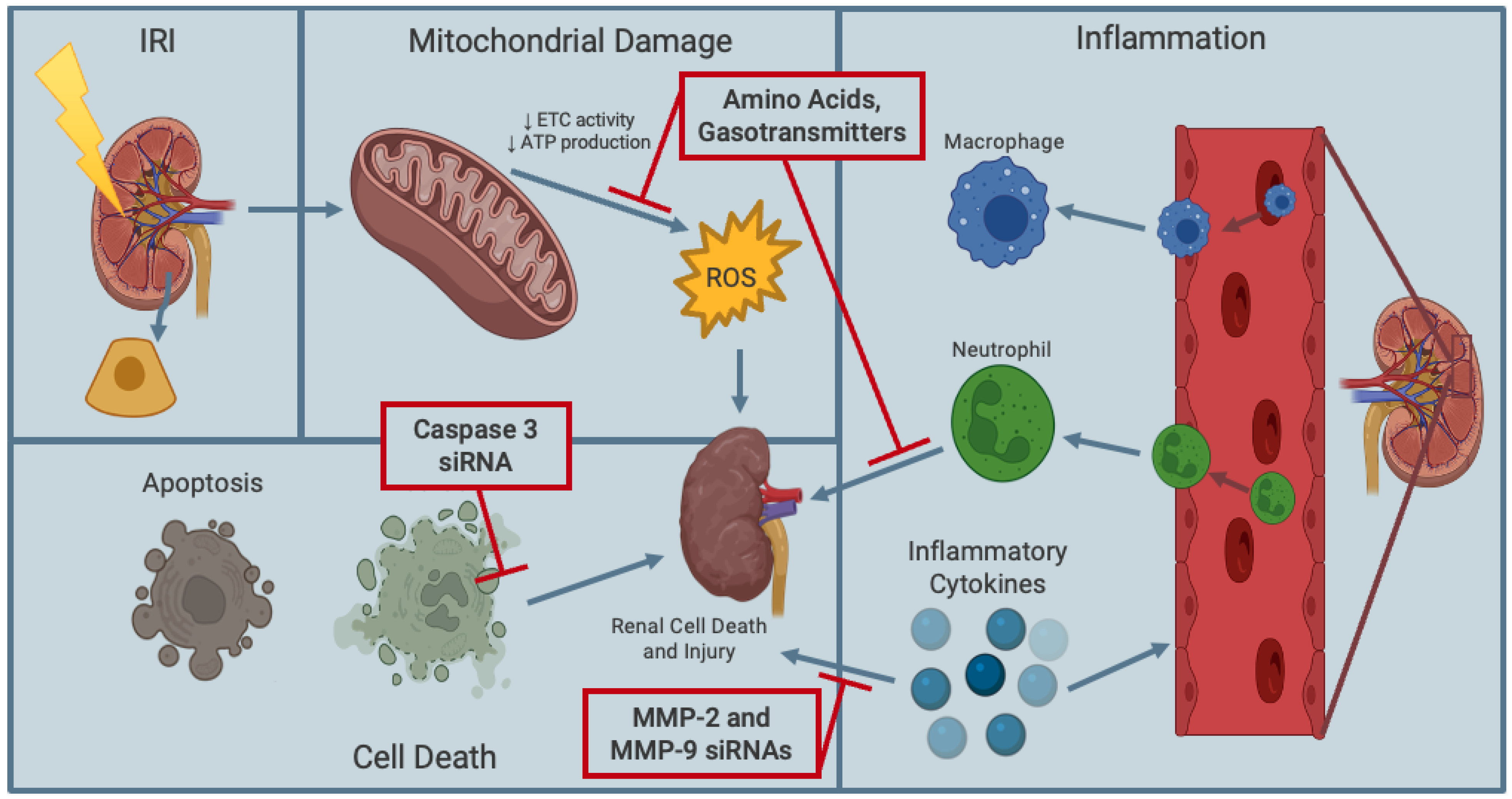
5. Supplementing Kidney Preservation Solutions with Pharmacological Additives at Various Temperatures to Mitigate Renal IRI
5.1. Amino Acids and Proteins
5.2. Signaling Pathway Inhibitors
5.3. Hemopure
5.4. Gasotransmitters
5.5. Hydrogen Sulfide as a Gasotransmitter
5.6. Sodium Thiosulfate—A Clinically Approved H2S Donor Drug
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.D.; Mahoney, J.E.; Knoll, G.A. Renal transplantation using non-heart-beating donors: A potential solution to the organ donor shortage in Canada. Can. J. Surg. 2004, 47, 1182–1183. [Google Scholar]
- Himmelfarb, J.; Vanholder, R.; Mehrota, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and advances in kidney preservation. BioMed Res. Int. 2018, 2018, 9206257. [Google Scholar] [CrossRef]
- van Eck van der Sluijs, A.; Vonk, S.; van Jaarsveld, B.C.; Bonenkamp, A.A.; Abrahams, A.C. Good practices for dialysis education, treatment, and eHealth: A scoping review. PLoS ONE 2021, 16, e0255734. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Akoh, J.A. Kidney donation after cardiac death. World J. Nephrol. 2012, 1, 79. [Google Scholar] [CrossRef]
- Resch, T.; Cardini, B.; Oberhuber, R.; Weissenbacher, A.; Dumfarth, J.; Krapf, C.; Boesmueller, C.; Oefner, D.; Grimm, M.; Schneeberger, S. Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring. Front. Immunol. 2020, 11, 631. [Google Scholar] [CrossRef]
- Min, Y.; Cheng, L.; Tu, C.; Li, H.; He, D.; Huang, D.; Chen, D.; Huang, X.; Chen, F.; Xiong, F. Clinical characteristics of deceased hemodialysis patients affected by COVID-19. Int. Urol. Nephrol. 2021, 53, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Grimshaw, J.M.; Taljaard, M.; Linklater, S.; Chassé, M.; Shemie, S.D.; Knoll, G.A. Design, implementation, and evaluation of a knowledge translation intervention to increase organ donation after cardiocirculatory death in Canada: A study protocol. Implement. Sci. 2014, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alejandro, R.; Caumartin, Y.; Marotta, P.J.; Ghent, C.; Levstik, M.A.; Quan, D.; Muirhead, N.; House, A.A.; McAlister, V.; Jevnikar, A.M.; et al. Kidney and liver transplants from donors after cardiac death: Initial experience at the London Health Sciences Centre. Can. J. Surg. 2010, 53, 93–102. [Google Scholar] [PubMed]
- Canadian Institute for Health Information. CORR Annual Statistics. Organ Replacement in Canada. 2021. Available online: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics (accessed on 30 August 2022).
- Health Resources & Services Administration. Learn About Donation. Organ Donation Statistics. 2022. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 2 September 2022).
- de Vries, E.E.; Snoeijs, M.G.; van Heurn, E. Kidney donation from children after cardiac death. Crit Care Med. 2010, 38, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef]
- Batal, I.; De Serres, S.A.; Safa, K.; Bijol, V.; Ueno, T.; Onozato, M.L.; Iafrate, A.J.; Herter, J.M.; Lichtman, A.H.; Mayadas, T.N.; et al. Dendritic cells in kidney transplant biopsy samples are associated with T cell infiltration and poor allograft survival. J. Am. Soc. Nephrol. 2015, 26, 3102–3113. [Google Scholar] [CrossRef] [PubMed]
- Kosieradzki, M.; Rowiński, W. Ischemia/Reperfusion Injury in Kidney Transplantation: Mechanisms and Prevention. Transplant. Proc. 2008, 40, 3279–3288. [Google Scholar] [CrossRef]
- Salahudeen, A.K.; Huang, H.; Joshi, M.; Moore, N.A.; Jenkins, J.K. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis in human renal proximal tubular cells. Am J. Transplant. 2003, 3, 273–280. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Rychkov, D.; Sur, S.; Sirota, M. Molecular diversity of clinically stable human kidney allografts. JAMA Network Open 2021, 4, e2035048. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and reperfusion injury in kidney transplantation: Relevant mechanisms in injury and repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Mishabi, M.A.; Teymoori, E.; Nikbakht, D.; Sarraf, N.; Jabinian, F.; Khalilpour, A.; Askarkhah, A.; Rahmani, V. A systematic review of the relationship between acute tubular necrosis and kidney transplantation. J. Ren. Inj. Prev. 2021, 10, 18. [Google Scholar] [CrossRef]
- Hartzell, S.; Bin, S.; Cantarelli, C.; Haverly, M.; Manrique, J.; Angeletti, A.; Manna, G.L.; Murphy, B.; Zhang, W.; Levitsky, J.; et al. Kidney Failure Associates with T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front. Immunol. 2020, 11, 583702. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Patel, N.S.; Chatterjee, P.K.; Di Paola, R.; Mazzon, E.; Britti, D.; De Sarro, A.; Cuzzocrea, S.; Thiemermann, C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J. Pharmacol. Exp. Ther. 2005, 312, 1170–1178. [Google Scholar] [CrossRef]
- Burne, M.J.; Elghandour, A.; Haq, M.; Saba, S.R.; Norman, J.; Condon, T.; Bennett, F.; Rabb, H. IL-1 and TNF independent pathways mediate ICAM-1/VCAM-1 up-regulation in ischemia reperfusion injury. J. Leukoc. Biol. 2001, 70, 192–198. [Google Scholar] [CrossRef]
- Meldrum, K.K.; Meldrum, D.R.; Meng, X.; Ao, L.; Harken, A.H. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am. J. Physiol. Heart. Circ. Physiol. 2002, 282, H540–H546. [Google Scholar] [CrossRef]
- Mannon, R.B. Delayed graft function: The AKI of kidney transplantation. Nephron 2018, 140, 94–98. [Google Scholar] [CrossRef]
- Wang, W.; Xie, D.; Hu, X.; Yin, H.; Liu, H.; Zhang, X. Effect of Hypothermic Machine Perfusion on the Preservation of Kidneys Donated After Cardiac Death: A Single-Center, Randomized, Controlled Trial. Artif. Organs 2017, 41, 753–758. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 2016, 311, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Hoff, U.; Park, J.K.; Qun, Y.; Schneider, W.; Luft, F.C.; Haller, H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001, 60, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, M.; Franchin, M.; Soldini, G.; Ietto, G.; Chiappa, C.; Maritan, E.; Villa, F.; Carcano, G.; Dionigi, R. Impact of static cold storage VS hypothermic machine preservation on ischemic kidney graft: Inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. Int. J. Surg. 2013, 11 (Suppl. S1), S110–S114. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transplant. 2020, 20, 1192–1195. [Google Scholar] [CrossRef]
- Burlage, L.C.; Tessier, S.N.; Etra, J.W.; Uygun, K.; Brandacher, G. Advances in machine perfusion, organ preservation, and cryobiology: Potential impact on vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 561–567. [Google Scholar] [CrossRef]
- van Smaalen, T.C.; Hoogland, E.R.; van Heurn, L.W. Machine perfusion viability testing. Curr. Opin. Organ Transplant. 2013, 18, 168–173. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschläger, J.; Rauen, U.; Paul, A.; Minor, T. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl. Int. 2014, 27, 1097–1106. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Patel, S.V.B.; Sun, Q.; Jiang, L.; Richard-Mohamed, M.; Ruthirakanthan, A.; Aquil, S.; Al-Ogaili, R.; Juriasingani, S.; Sener, A.; et al. Renal protection against ischemia reperfusion injury: Hemoglobin-based oxygen carrier-201 versus blood as an oxygen carrier in ex vivo subnormothermic machine perfusion. Transplantation 2020, 104, 482–489. [Google Scholar] [CrossRef]
- Juriasingani, S.; Ruthirakanthan, A.; Richard-Mohamed, M.; Akbari, M.; Aquil, S.; Patel, S.; Al-Ogaili, R.; Whiteman, M.; Luke, P.; Sener, A. Subnormothermic perfusion with h2s donor ap39 improves dcd porcine renal graft outcomes in an ex vivo model of kidney preservation and reperfusion. Biomolecules 2021, 11, 446. [Google Scholar] [CrossRef]
- Michel, S.G.; La Muraglia, G.M.; Madariaga, M.L.L.; Titus, J.S.; Selig, M.K.; Farkash, E.A.; Allan, J.S.; Anderson, L.M.; Madsen, J.C. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann. Transplant. 2015, 20, 461–468. [Google Scholar] [CrossRef]
- Sage, A.T.; Richard-Greenblatt, M.; Zhong, K.; Bai, X.H.; Snow, M.B.; Babits, M.; Ali, A.; Baciu, C.; Yeung, J.C.; Liu, M.; et al. Prediction of donor related lung injury in clinical lung transplantation using a validated ex vivo lung perfusion inflammation score. J. Heart Lung Transplant. 2021, 40, 687–695. [Google Scholar] [CrossRef]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef]
- Gwangwa, M.V.; Joubert, A.M.; Visagie, M.H. Effects of glutamine deprivation on oxidative stress and cell survival in breast cell lines. Biol. Res. 2019, 52, 15. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Kolwicz Jr, S.C.; Abell, L.; Roe, N.D.; Kim, M.; Tian, R. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef]
- Silva, M.A.; Richards, D.A.; Bramhall, S.R.; Adams, D.H.; Mirza, D.F.; Murphy, N. A study of the metabolites of ischemia-reperfusion injury and selected amino acids in the liver using microdialysis during transplantation. Transplantation 2005, 79, 828–835. [Google Scholar] [CrossRef]
- Erkasap, S.; Ates, E. L-Arginine-enriched preservation solution decreases ischemia/reperfusion injury in canine kidneys after long-term cold storage. Nephrol. Dial. Transplant. 2000, 15, 1224–1227. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Foley, J.D.; Murphy, C.J.; McAnulty, J.F. The effect of trophic factor supplementation on cold ischemia-induced early apoptotic changes. Transplantation 2007, 83, 91–94. [Google Scholar] [CrossRef]
- Ćelić, T.; Omrčen, H.; Španjol, J.; Bobinac, D. Mechanisms of Bone Morphogenetic Protein-7 Protective Effects Against Cold Ischemia-Induced Renal Injury in Rats. Transplant. Proc. 2018, 50, 3822–3830. [Google Scholar] [CrossRef]
- Ćelić, T.; Spanjol, J.; Grskovic, A.; Markic, D.; Prebilic, I.; Fuckar, Z.; Bobinac, D. Bone morphogenetic protein-7 reduces cold ischemic injury in rat kidney. Transplant. Proc. 2011, 43, 2505–2509. [Google Scholar] [CrossRef]
- Yang, B.; Hosgood, S.A.; Nicholson, M.L. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation 2011, 91, 501–507. [Google Scholar] [CrossRef]
- Moser, M.A.; Arcand, S.; Lin, H.B.; Wojnarowicz, C.; Sawicka, J.; Banerjee, T.; Luo, Y.; Beck, G.R.; Luke, P.P.; Sawicki, G. Protection of the Transplant Kidney from Preservation Injury by Inhibition of Matrix Metalloproteinases. PLoS ONE 2016, 11, e0157508. [Google Scholar] [CrossRef]
- Sawicki, G.; Leon, H.; Sawicka, J.; Sariahmetoglu, M.; Schulze, C.J.; Scott, P.G.; Szczesna-Cordary, D.; Schulz, R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: A new intracellular target for matrix metalloproteinase-2. Circulation 2005, 112, 544–552. [Google Scholar] [CrossRef]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.; Sawicki, G.; Schulz, R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef]
- Ashenden, M.J.; Schumacher, Y.O.; Sharpe, K.; Varlet-Marie, E.; Audran, M. Effects of Hemopure on maximal oxygen uptake and endurance performance in healthy humans. Int. J. Sports Med. 2007, 28, 381–385. [Google Scholar] [CrossRef]
- Aburawi, M.M.; Fontan, F.M.; Karimian, N.; Eymard, C.; Cronin, S.; Pendexter, C.; Nagpal, S.; Banik, P.; Ozer, S.; Mahboub, P.; et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am. J. Transplant. 2019, 19, 2814–2824. [Google Scholar] [CrossRef]
- Mahboub, P.; Aburawi, M.; Karimian, N.; Lin, F.; Karabacak, M.; Fontan, F.; Tessier, S.N.; Markmann, J.; Yeh, H.; Uygun, K. The efficacy of HBOC-201 in ex situ gradual rewarming kidney perfusion in a rat model. Artif. Organs 2020, 44, 81–90. [Google Scholar] [CrossRef]
- Juriasingani, S.; Jackson, A.; Zhang, M.Y.; Ruthirakanthan, A.; Dugbartey, G.J.; Sogutdelen, E.; Levine, M.; Mandurah, M.; Whiteman, M.; Luke, P.; et al. Evaluating the Effects of Subnormothermic Perfusion with AP39 in a Novel Blood-Free Model of Ex Vivo Kidney Preservation and Reperfusion. Int. J. Mol. Sci. 2021, 22, 7180. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar]
- Wang, R. (Ed.) Signal Transduction and the Gasotransmitters: NO, CO, and H2S in Biology and Medicine; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Mir, J.M.; Maurya, R.C. A gentle introduction to gasotransmitters with special reference to nitric oxide: Biological and chemical implications. Rev. Inorg. Chem. 2018, 38, 193–220. [Google Scholar]
- Sener, A.; Tran, K.C.; Deng, J.P.; Garcia, B.; Lan, Z.; Liu, W.; Sun, T.; Arp, J.; Salna, M.; Acott, P.; et al. Carbon monoxide releasing molecules inhibit cell death resulting from renal transplantation related stress. J. Urol. 2013, 190, 772–778. [Google Scholar]
- Dugbartey, G.J.; Alornyo, K.K.; Luke, P.W.; Sener, A. Application of carbon monoxide in kidney and heart transplantation: A novel pharmacological strategy for broader use of suboptimal renal and cardiac grafts. Pharmacol. Res. 2021, 173, 105883. [Google Scholar]
- Untereiner, A.A.; Wu, L.; Wang, R. The role of carbon monoxide as a gasotransmitter in cardiovascular and metabolic regulation. In Gasotransmitters: Physiology and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–70. [Google Scholar]
- Hosgood, S.A.; Bagul, A.; Kaushik, M.; Rimoldi, J.; Gadepalli, R.S.; Nicholson, M.L. Application of nitric oxide and carbon monoxide in a model of renal preservation. British J. Surg. 2008, 95, 1060–1067. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25. [Google Scholar]
- Hosgood, S.A.; Nicholson, M.L. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br. J. Surg. 2010, 97, 202–209. [Google Scholar] [CrossRef]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox. Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Sener, A. Hydrogen sulfide metabolite, sodium thiosulfate: Clinical applications and underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 6452. [Google Scholar]
- Lobb, I.; Mok, A.; Lan, Z.; Liu, W.; Garcia, B.; Sener, A. Supplemental hydrogen sulfide protects transplant kidney function and prolongs recipient survival after prolonged cold ischemia-reperfusion injury by mitigating renal apoptosis and inflammation. BJU Int. 2012, 110, E1187–E1195. [Google Scholar] [CrossRef]
- Lobb, I.; Davidson, M.; Carter, D.; Liu, W.; Haig, A.; Gunaratnam, L.; Sener, A. Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J. Urol. 2015, 194, 1806–1815. [Google Scholar] [CrossRef]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, N.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Lobb, I.; Zhu, J.; Liu, W.; Haig, A.; Lan, Z.; Sener, A. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can. Urol. Assoc. J. 2014, 8, E413–E418. [Google Scholar] [CrossRef] [PubMed]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J.; Juriasingani, S.; Zhang, M.Y.; Sener, A. H2S donor molecules against cold ischemia-reperfusion injury in preclinical models of solid organ transplantation. Pharmacol. Res. 2021, 172, 105842. [Google Scholar] [CrossRef] [PubMed]
- Hendry-Hofer, T.B.; Ng, P.C.; Witeof, A.E.; Mahon, S.B.; Brenner, M.; Boss, G.R.; Bebarta, V.S. A Review on Ingested Cyanide: Risks, Clinical Presentation, Diagnostics, and Treatment Challenges. J. Med. Toxicol. 2019, 15, 128–133. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, L.; Pang, H.; Fang, Y.; Yan, H.; Fang, W. Sodium thiosulfate: An emerging treatment for calciphylaxis in dialysis patients. Case Rep. Nephrol. Dial. 2015, 5, 77–82. [Google Scholar] [CrossRef]
- Farese, S.; Stauffer, E.; Kalicki, R.; Hildebrandt, T.; Frey, B.M.; Frey, F.J.; Uehlinger, D.E.; Pasch, A. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin. J. Am. Soc. Nephrol. 2011, 6, 1447–1455. [Google Scholar] [CrossRef]
- Laplace, N.; Kepenekian, V.; Friggeri, A.; Vassal, O.; Ranchon, F.; Rioufol, C.; Gertych, W.; Villeneuve, L.; Glehen, O.; Bakrin, N. Sodium thiosulfate protects from renal impairment following hyperthermic intraperitoneal chemotherapy (HIPEC) with Cisplatin. Int. J. Hyperthermia. 2020, 37, 897–902. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Taka, M.; Dugbartey, G.J.; Sener, A. The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 567. https://doi.org/10.3390/ijms24010567
Abou Taka M, Dugbartey GJ, Sener A. The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation. International Journal of Molecular Sciences. 2023; 24(1):567. https://doi.org/10.3390/ijms24010567
Chicago/Turabian StyleAbou Taka, Maria, George J. Dugbartey, and Alp Sener. 2023. "The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation" International Journal of Molecular Sciences 24, no. 1: 567. https://doi.org/10.3390/ijms24010567
APA StyleAbou Taka, M., Dugbartey, G. J., & Sener, A. (2023). The Optimization of Renal Graft Preservation Temperature to Mitigate Cold Ischemia-Reperfusion Injury in Kidney Transplantation. International Journal of Molecular Sciences, 24(1), 567. https://doi.org/10.3390/ijms24010567





