Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay
Abstract
1. Introduction
2. Results and Discussion
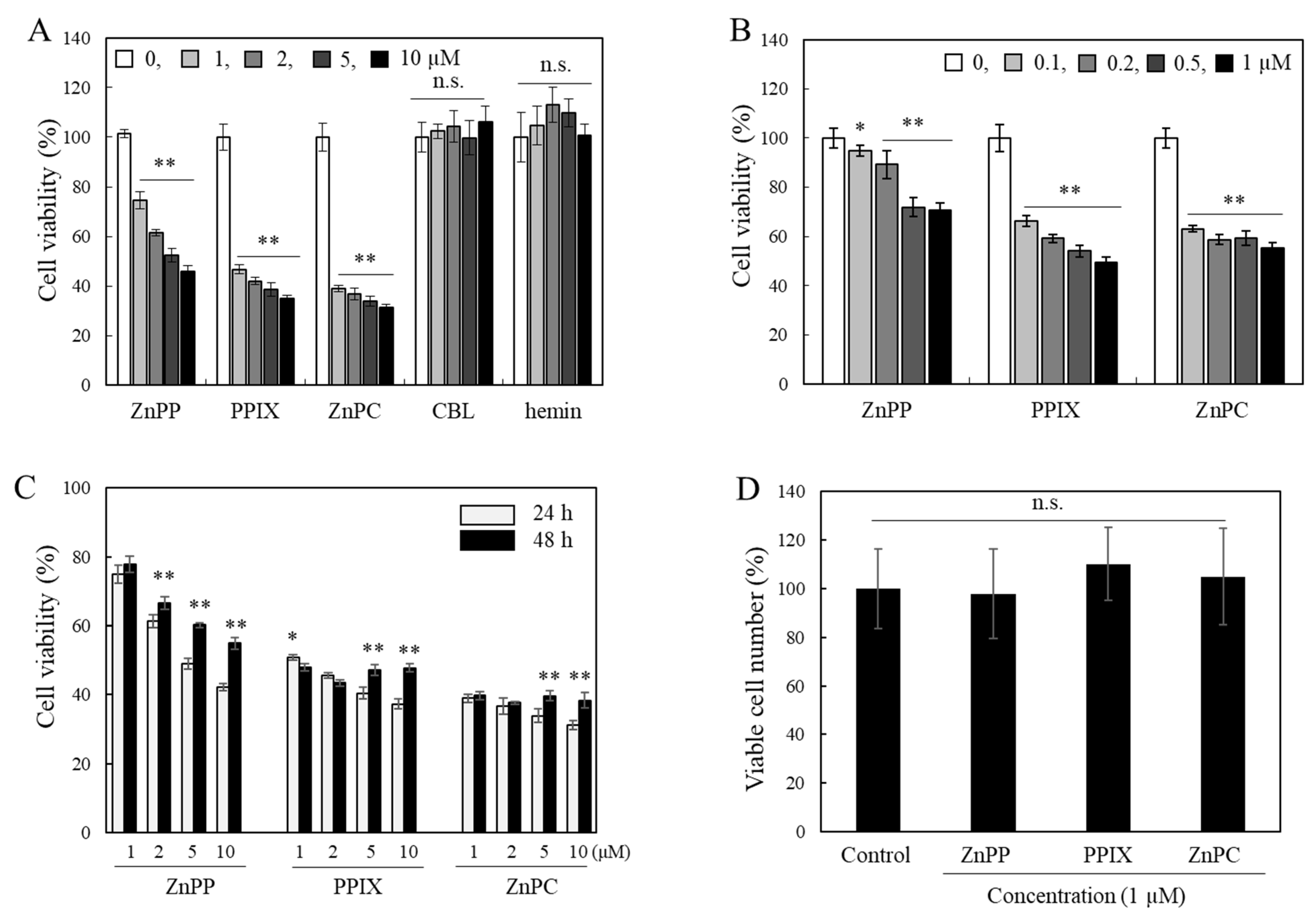
2.1. Effects of Porphyrin Derivatives on Cell Viability Measured by the MTT Assay
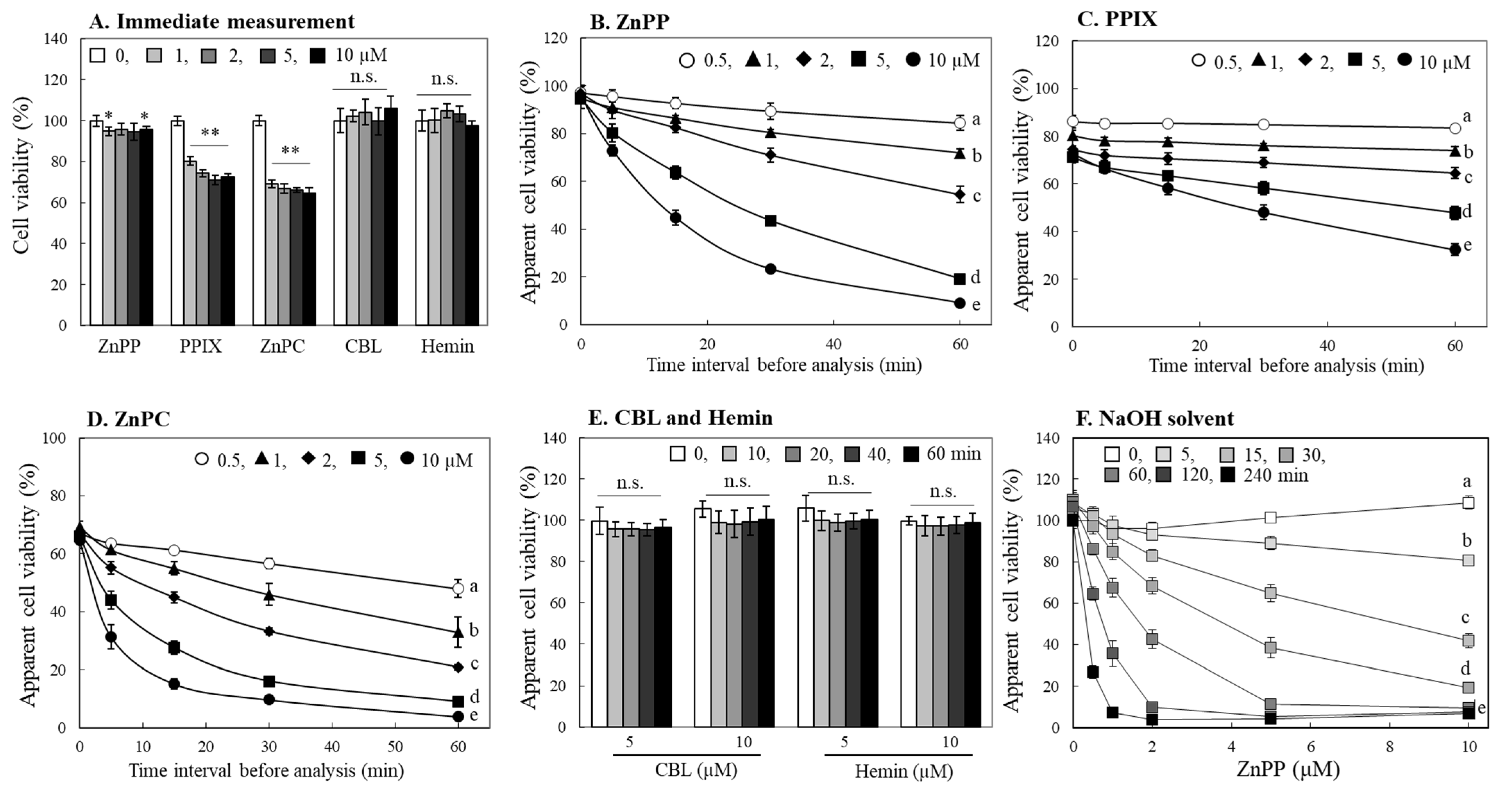
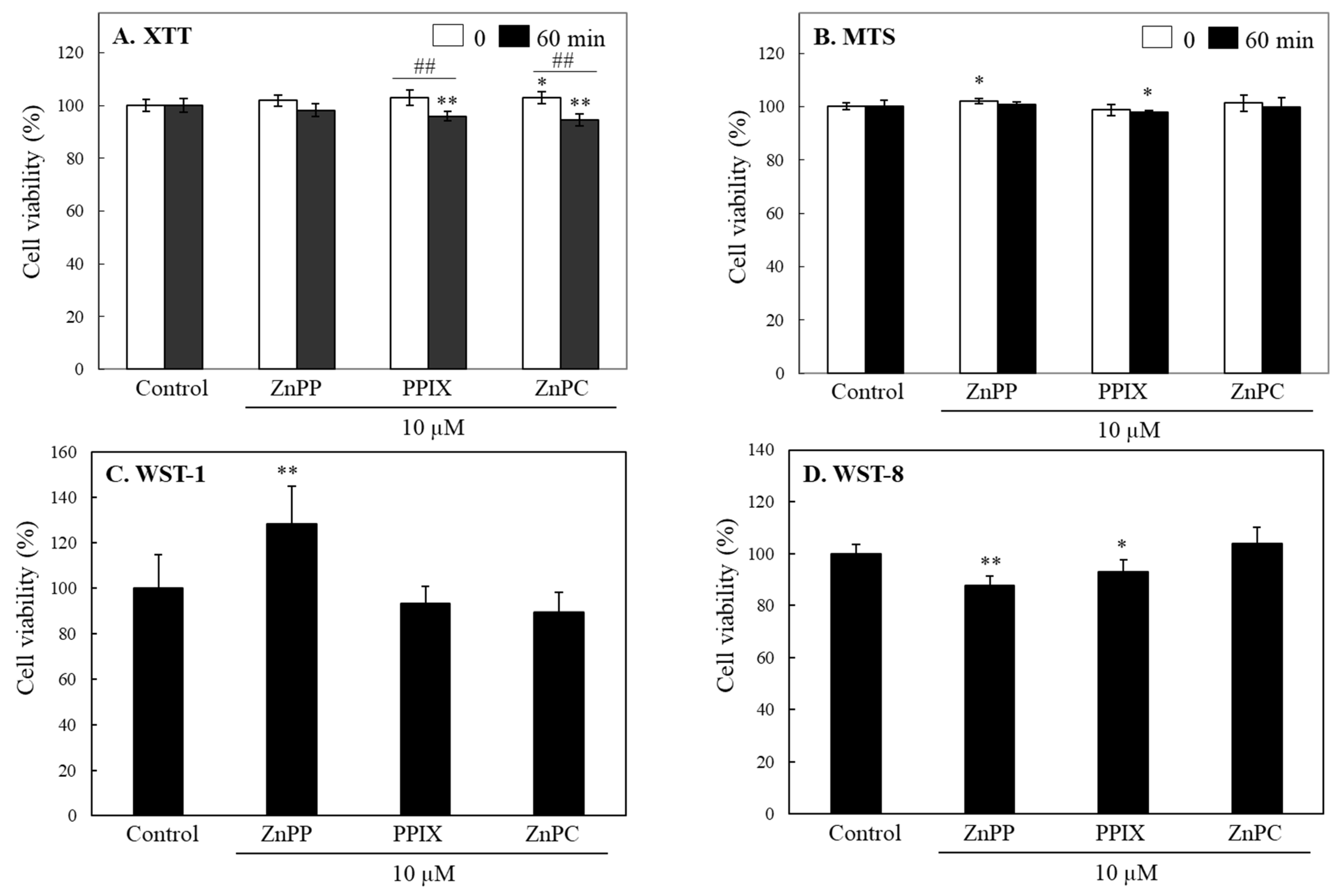
2.2. Effects of Porphyrin-Related Compounds in Different Tetrazolium Based-Assays
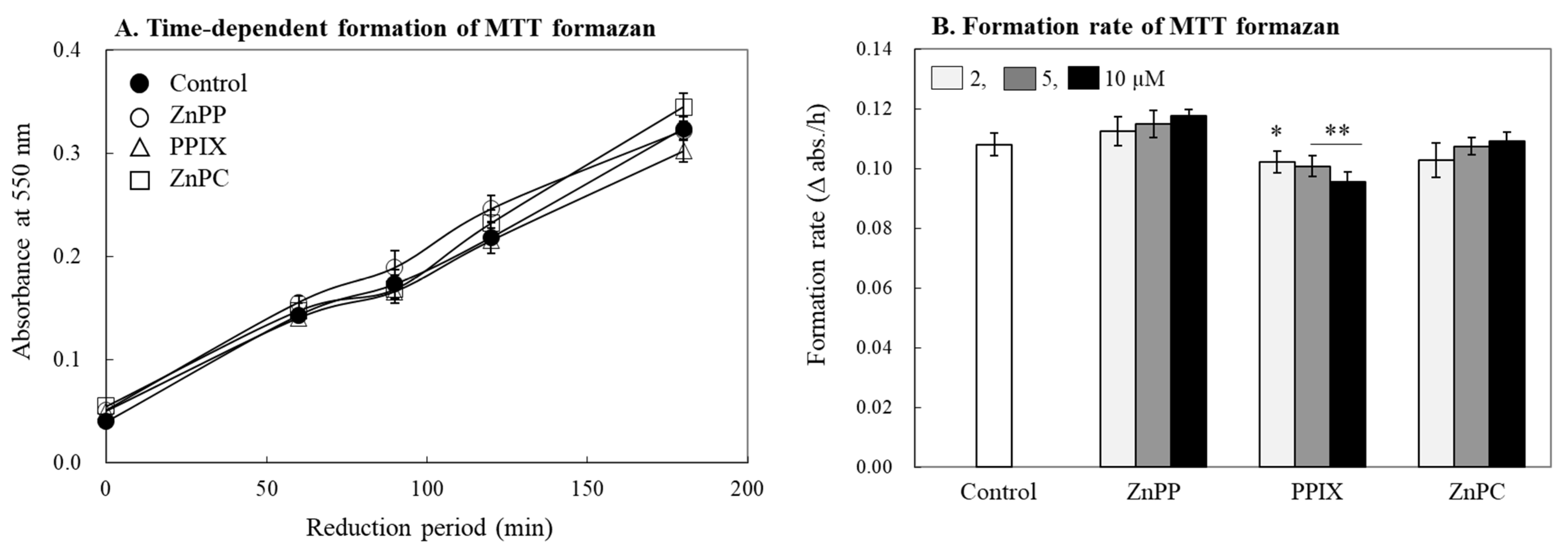
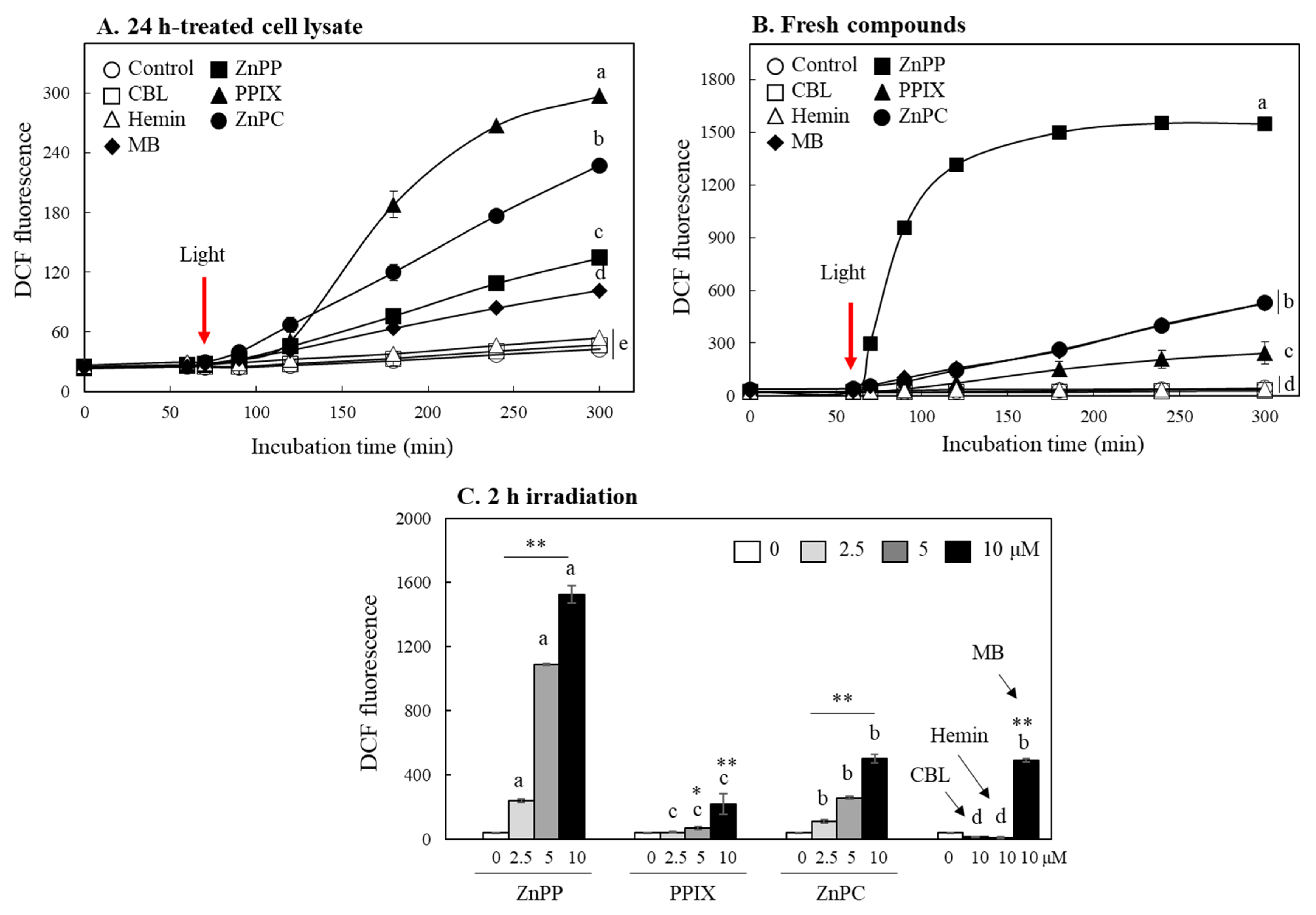
2.3. Effects on Formation of MTT Tetrazolium in Cells
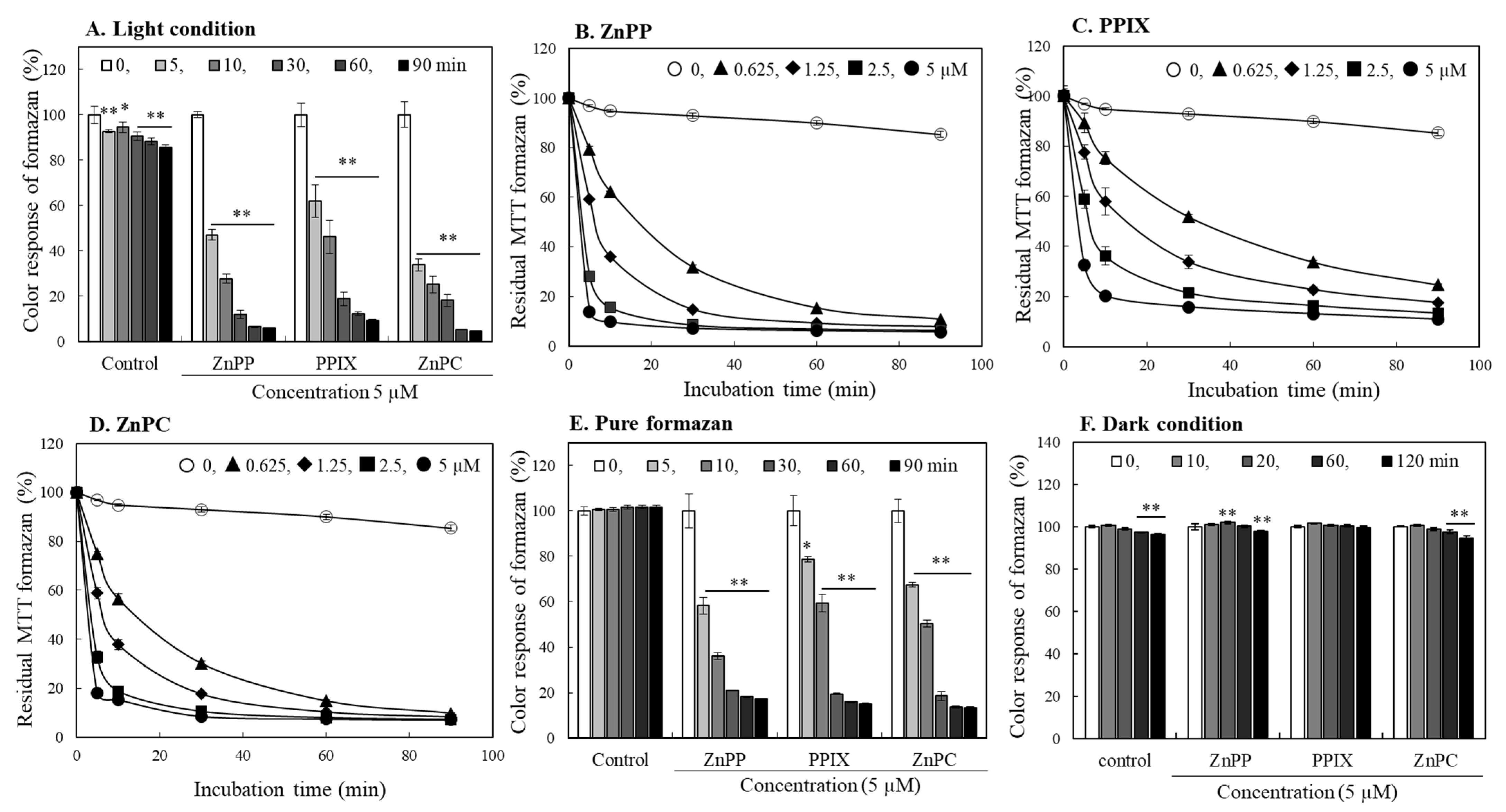
2.4. Effects of Porphyrins on Stability of MTT Formazan
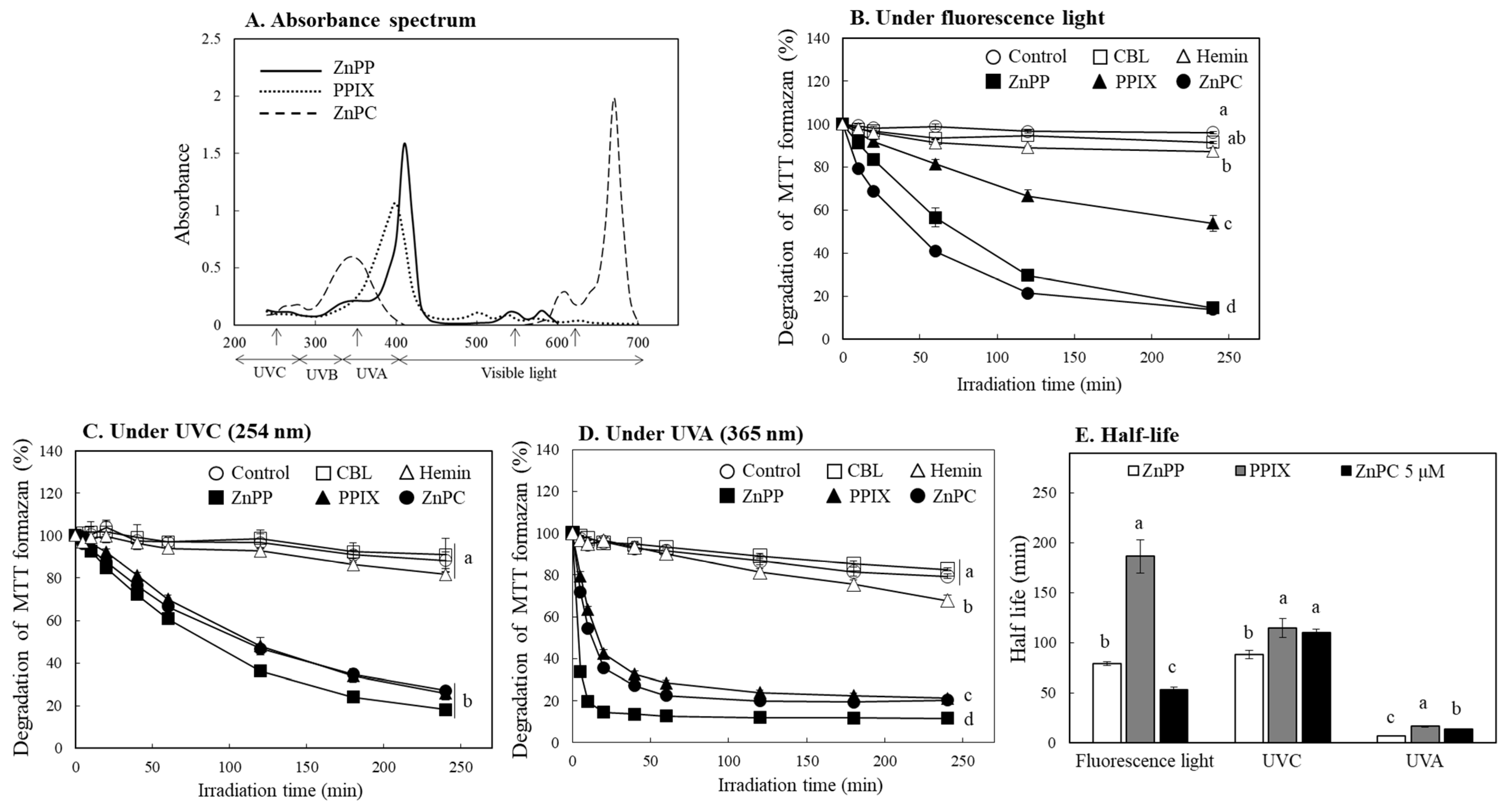
2.5. Evaluation of Photo-Reactive Property of Porphyrin Derivatives
2.6. Effects of Porphyrins on MTT Formazan Degradation under Different Light Sources
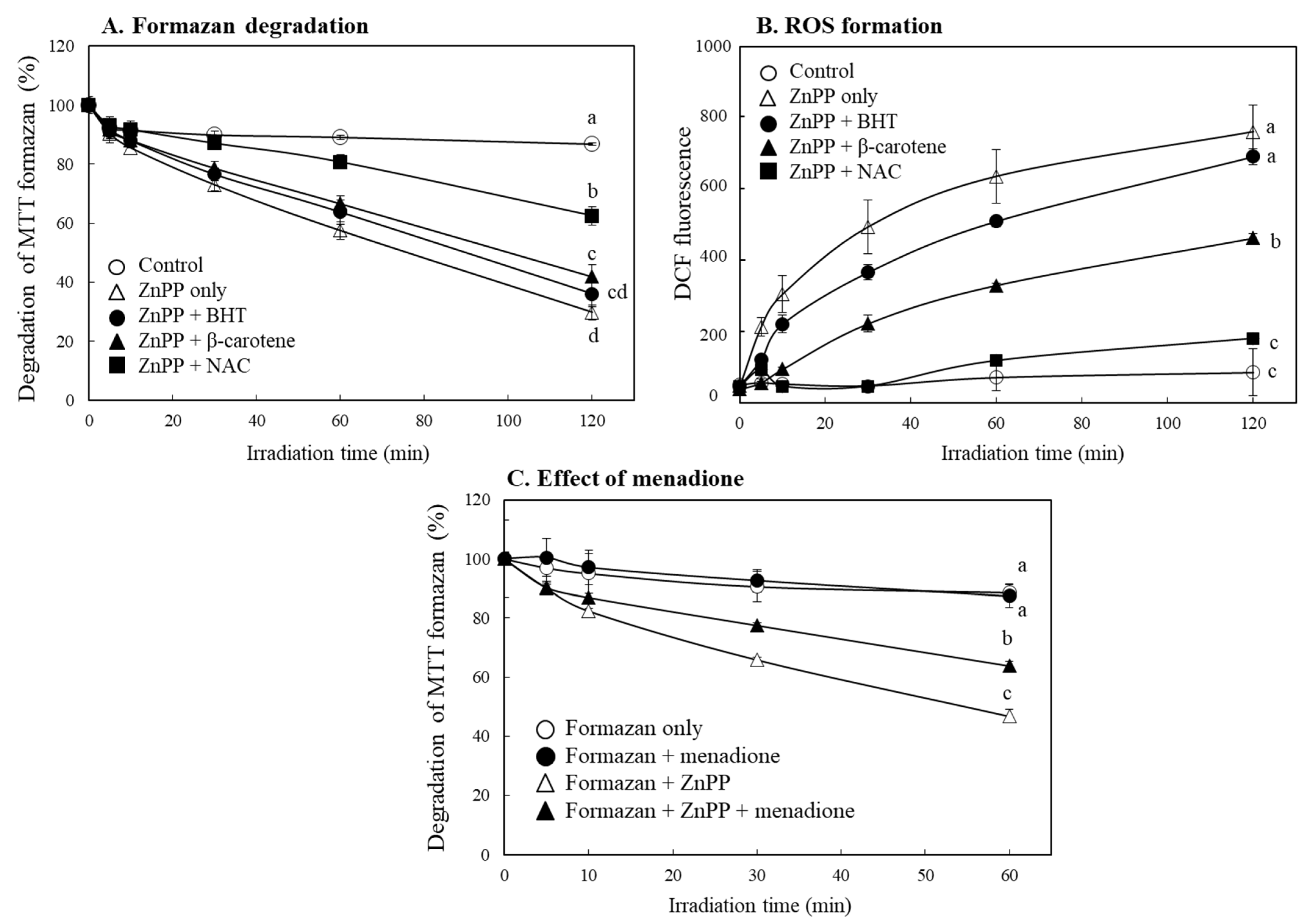
2.7. Effects of Different Antioxidants and Electron Coupling Agents
3. Methods and Materials
3.1. Chemicals and Cell Lines
3.2. Analysis of Cell Viability
3.3. Analysis of Effects on the MTT Formazan Formation
3.4. Analysis for Changes in Color Stability of Different Formazans by Porphyrin Compounds
3.5. Evaluation of Photosensitizing Properties of Porphyrins
3.6. Assessment of Various Factors Affecting MTT Formazan Decolorization by Porphyrins
3.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Method. 1986, 94, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble tetrazolium/formazan Assay for Cell Growth and Drug Sensitivity in Culture using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Barzowska, A.; Dąbrowski, J.M. Photodynamic Inactivation of Bacteria with Porphyrin Derivatives: Effect of Charge, Lipophilicity, ROS Generation, and Cellular Uptake on Their Biological Activity In Vitro. Int. J. Mol. Sci. 2020, 21, 8716. [Google Scholar] [CrossRef]
- Mauzerall, D. Spectra of molecular complexes of porphyrins in aqueous solution. Biochemistry 1965, 4, 1801–1810. [Google Scholar] [CrossRef]
- Mölzer, C.; Huber, H.; Steyrer, A.; Ziesel, G.; Ertl, A.; Plavotic, A.; Wallner, M.; Bulmer, A.C.; Wagner, K.-H. In vitro antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radic. Res. 2012, 46, 1369–1377. [Google Scholar] [CrossRef]
- Jadhav, A.; Ndisang, J.F. Treatment with heme arginate alleviates adipose tissue inflammation and improves insulin sensitivity and glucose metabolism in a rat model of Human primary aldosteronism. Free Radic. Biol. Med. 2012, 53, 2277–2286. [Google Scholar] [CrossRef]
- Wolf, P.; Rieger, E.; Kerl, H. Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid: An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas. J. Am. Acad. Dermatol. 1993, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H. New trends in photobiology: Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B 1992, 14, 275–292. [Google Scholar] [CrossRef] [PubMed]
- De Annunzio, S.R.; Costa, N.C.S.; Mezzina, R.D.; Graminha, M.A.S.; Fontana, C.R. Chlorin, Phthalocyanine, and Porphyrin Types Derivatives in Phototreatment of Cutaneous Manifestations: A Review. Int. J. Mol. Sci. 2019, 20, 3861. [Google Scholar] [CrossRef]
- Kim, S.J.; An, B.R.; Lee, G.-D.; Park, S.S. The syntheses of phthalocyanine hybrid derivatives and their properties. Appl. Chem. Eng. 2013, 24, 266–273. [Google Scholar]
- Duckers, H.J.; Boehm, M.; True, A.L.; Yet, S.F.; San, H.; Park, J.L.; Webb, R.C.; Lee, M.E.; Nabel, G.J.; Nabel, E.G. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001, 7, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993S–1026S. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Talorete, T.P.; Bouaziz, M.; Sayadi, S.; Isoda, H. Influence of medium type and serum on MTT reduction by flavonoids in the absence of cells. Cytotechnology 2006, 52, 189–198. [Google Scholar] [CrossRef]
- Park, K.A.; Choi, H.A.; Kim, M.R.; Choi, Y.M.; Kim, H.J.; Hong, J. Changes in color response of MTT formazan by zinc protoporphyrin. Korean J. Food Sci. Technol. 2011, 43, 754–759. [Google Scholar] [CrossRef]
- Stapelfeldt, K.; Ehrke, E.; Steinmeier, J.; Rastedt, W.; Dringen, R. Menadione-mediated WST1 reduction assay for the determination of metabolic activity of cultured neural cells. Anal. Biochem. 2017, 538, 42–52. [Google Scholar] [CrossRef]
- Edmondson, J.M.; Armstrong, L.S.; Martinez, A.O. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J. Tissue Cult. Method. 1998, 11, 15–17. [Google Scholar] [CrossRef]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture: Methods and Protocols; Langdon, S.P., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2004; Volume 88, pp. 165–169. [Google Scholar]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Nguyen, V.N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Antioxidant properties of BHT estimated by ABTS assay in systems differing in pH or metal ion or water concentration. Eur. Food Res. Technol. 2011, 232, 837–842. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Costa, D.; Fernandes, E.; Santos, J.L.; Pinto, D.C.; Silva, A.M.; Lima, J.L. New Noncellular Fluorescence Microplate Screening Assay for Scavenging Activity Against Singlet Oxygen. Anal. Bioanal. Chem. 2007, 387, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Vitamins E and C, β-Carotene, and Other Carotenoids as Antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, O.; Nakamura, K.; Hamada, S.; Kobayasi, Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Juzenas, P. Singlet oxygen in photosensitization. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 29–50. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.H.; Kim, M.-R.; Jung, Y.N.; Kang, S.; Hong, J. Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay. Int. J. Mol. Sci. 2023, 24, 562. https://doi.org/10.3390/ijms24010562
Choi BH, Kim M-R, Jung YN, Kang S, Hong J. Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay. International Journal of Molecular Sciences. 2023; 24(1):562. https://doi.org/10.3390/ijms24010562
Chicago/Turabian StyleChoi, Bo Hee, Mi-Ri Kim, Yu Na Jung, Smee Kang, and Jungil Hong. 2023. "Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay" International Journal of Molecular Sciences 24, no. 1: 562. https://doi.org/10.3390/ijms24010562
APA StyleChoi, B. H., Kim, M.-R., Jung, Y. N., Kang, S., & Hong, J. (2023). Interfering with Color Response by Porphyrin-Related Compounds in the MTT Tetrazolium-Based Colorimetric Assay. International Journal of Molecular Sciences, 24(1), 562. https://doi.org/10.3390/ijms24010562





