ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study
Abstract
:1. Introduction
2. Results
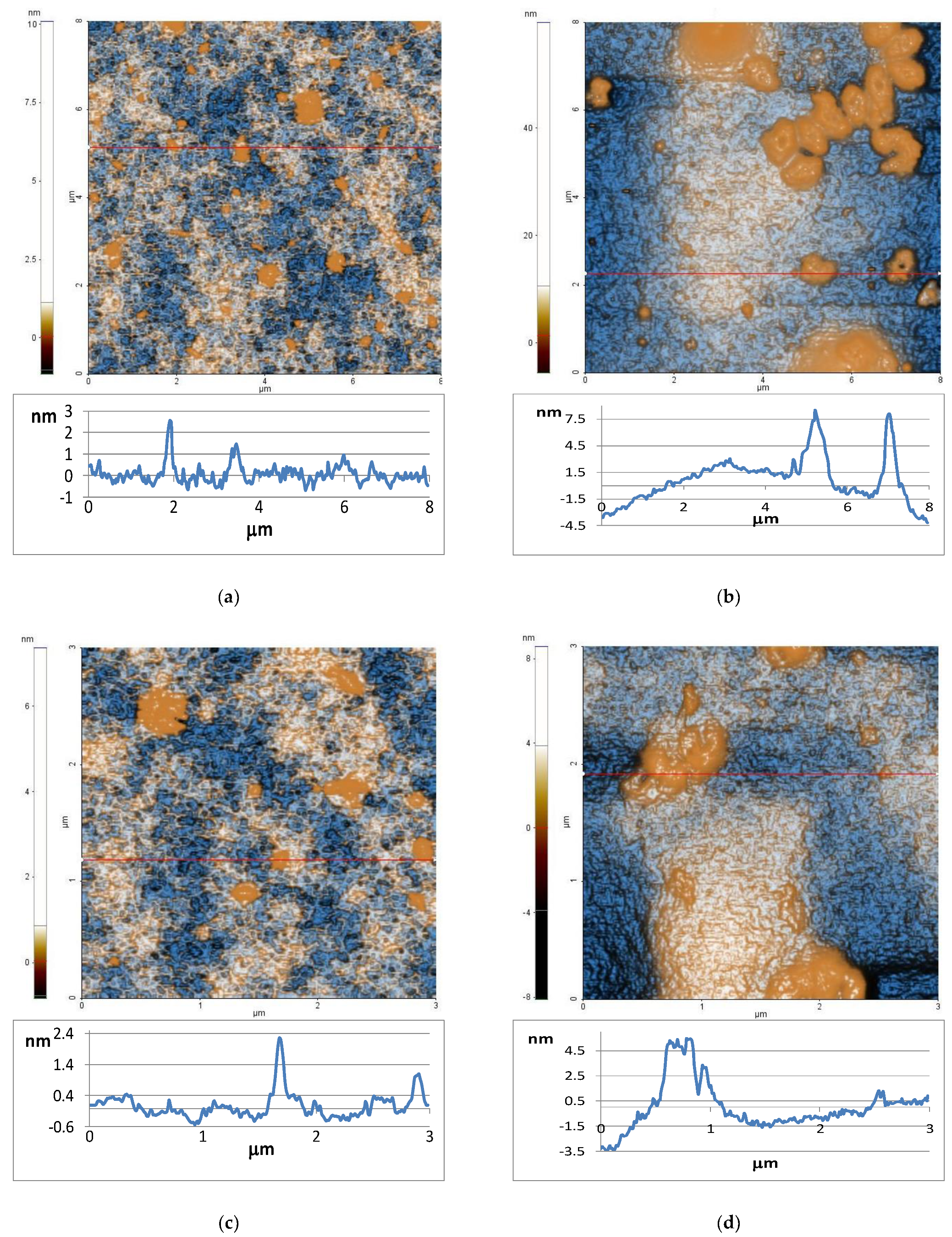
2.1. Atomic Force Microscopy
2.2. Rheology
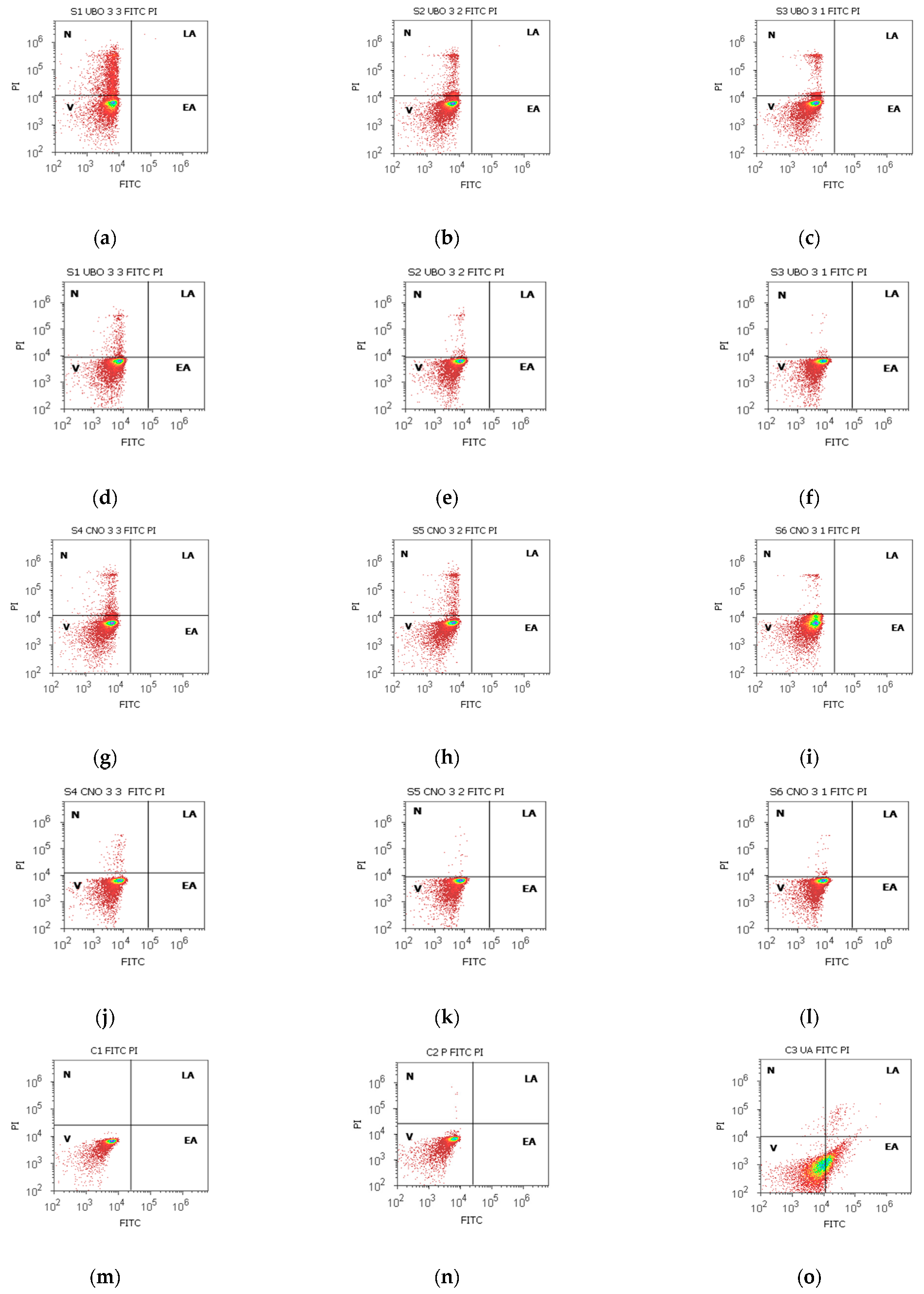
2.3. Annexin V-FITC Apoptosis Assay
2.4. Total ROS Activity Assay
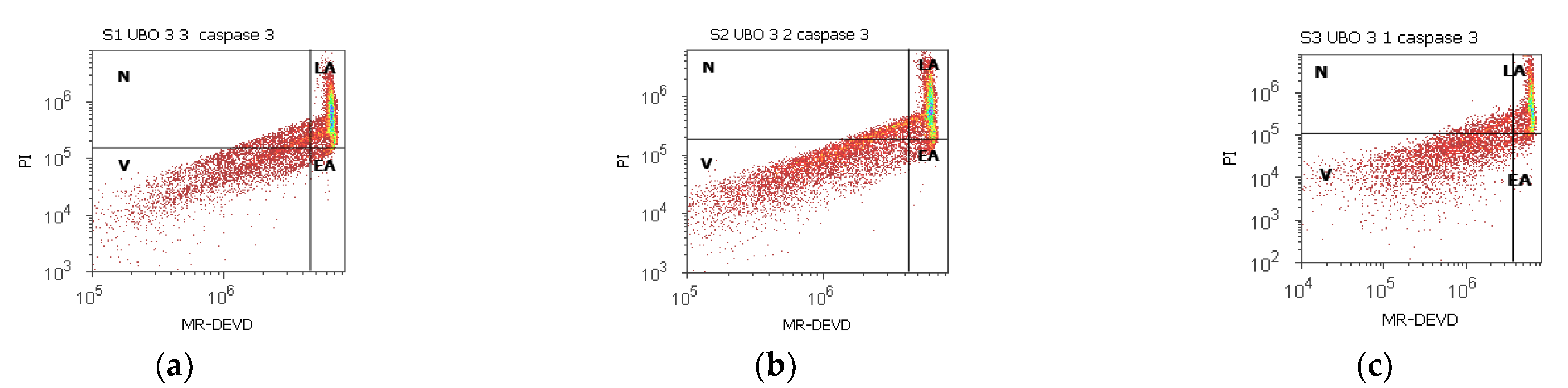
2.5. Enzymatic Activity of Caspase 3/7
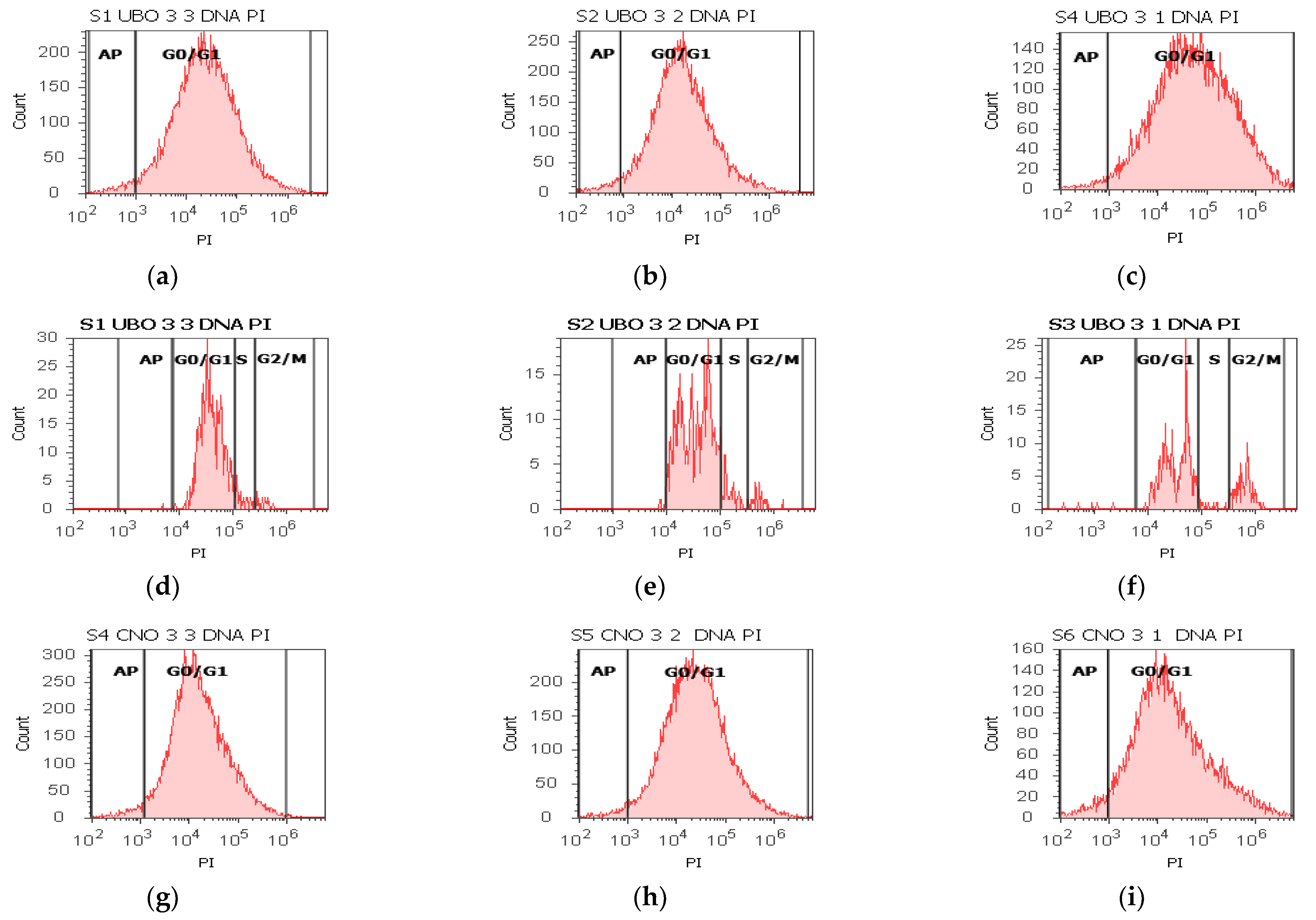
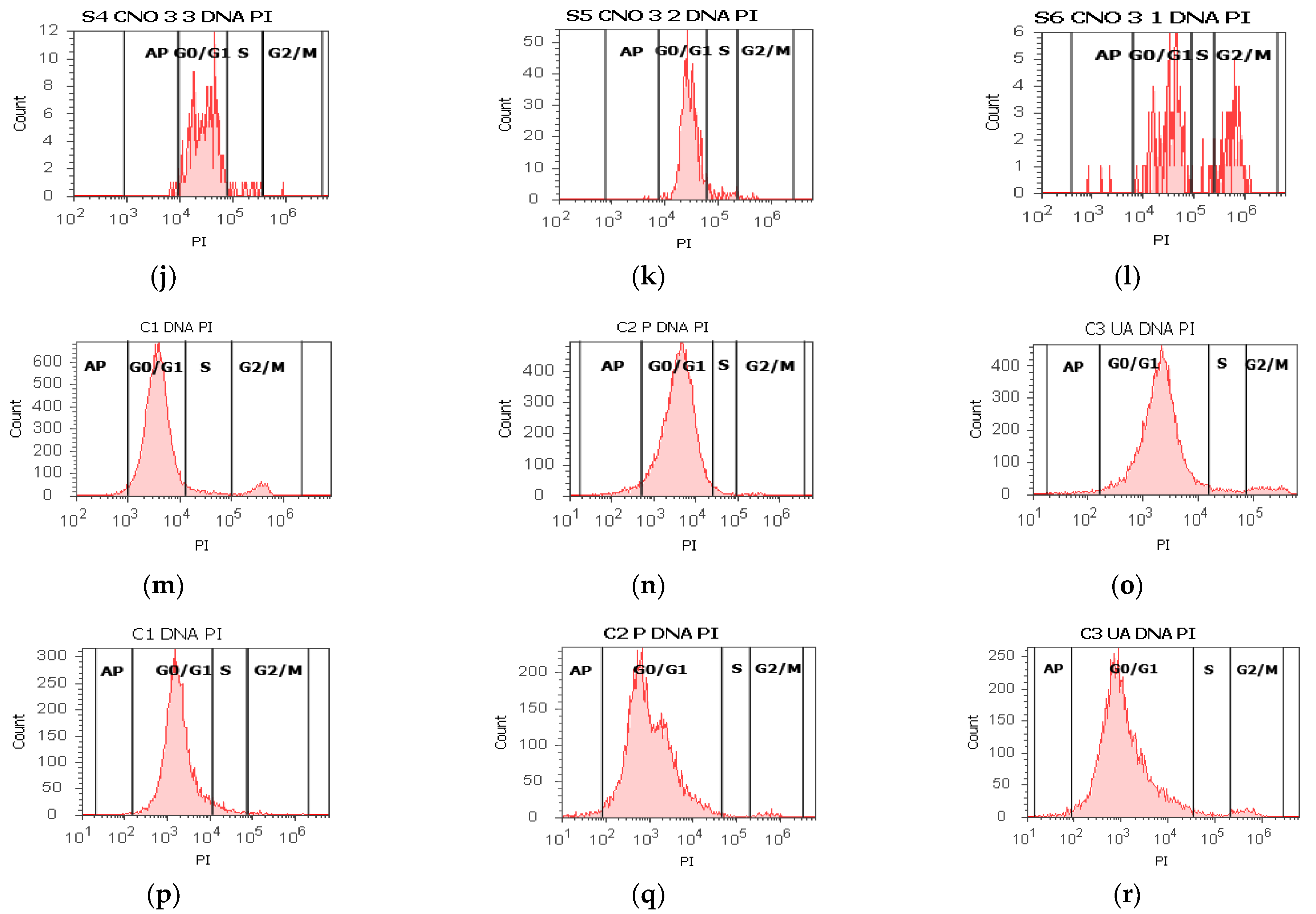
2.6. Cell Cycle Assay
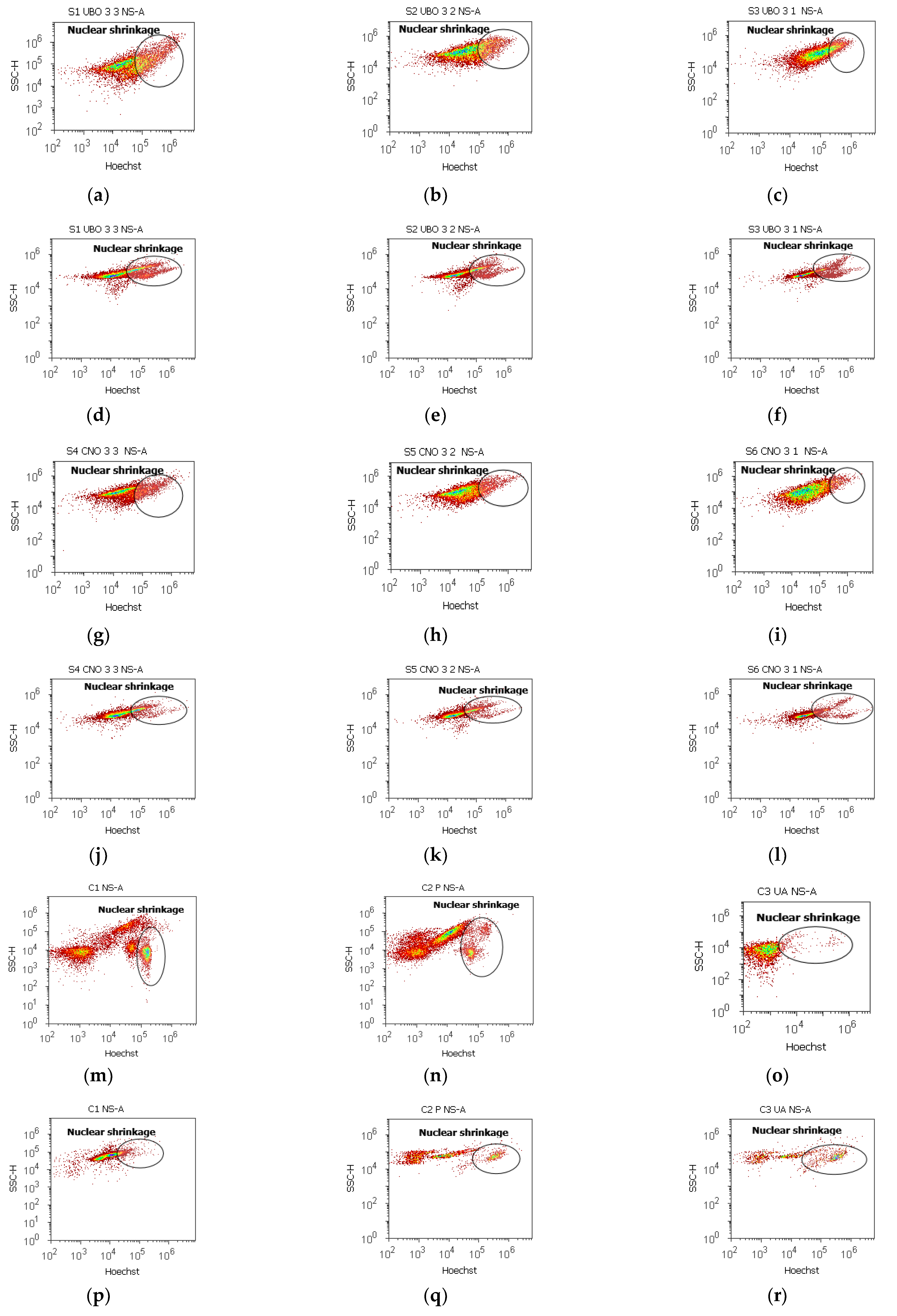
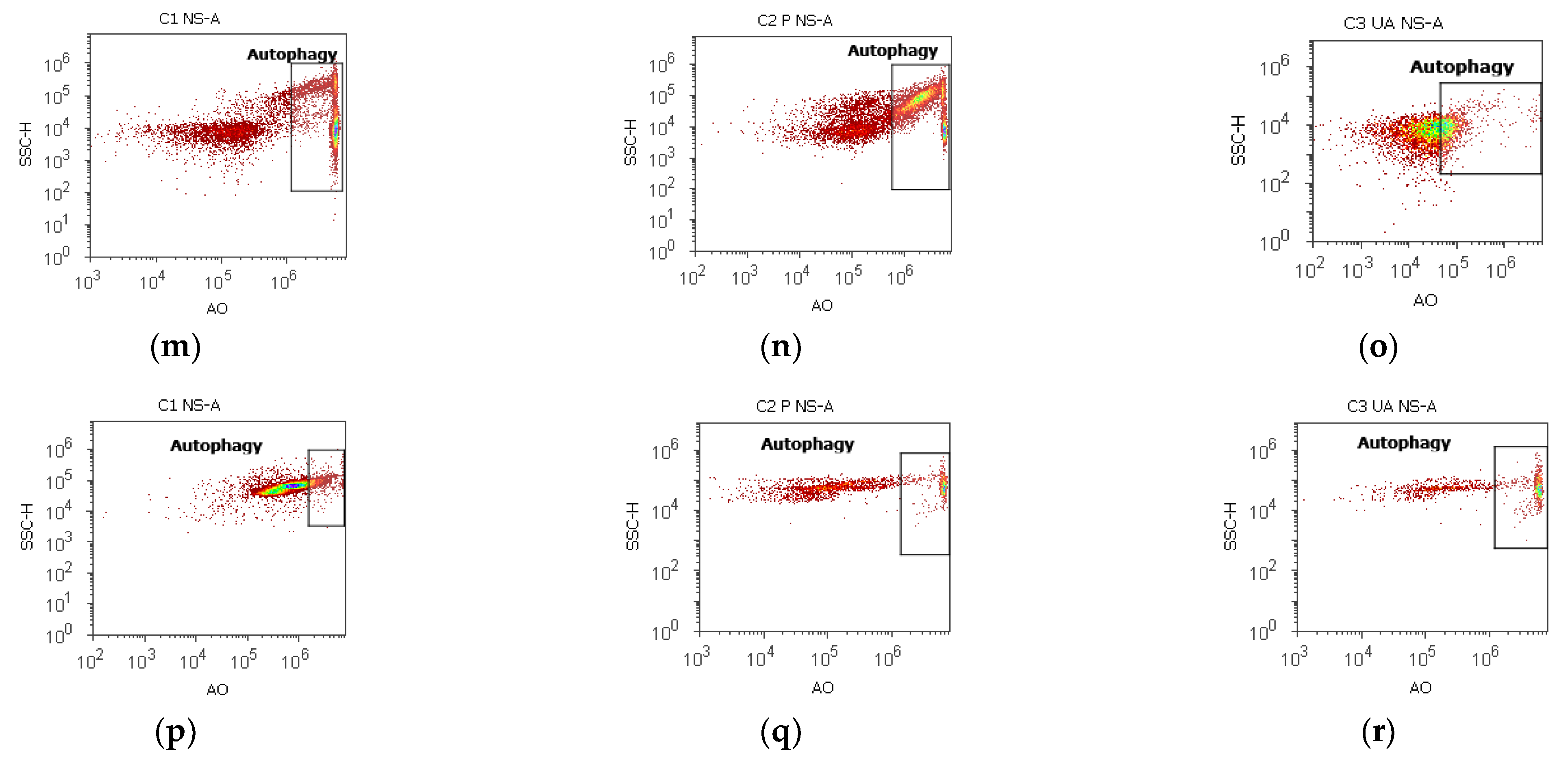
2.7. Nuclear Shrinkage and Autophagy
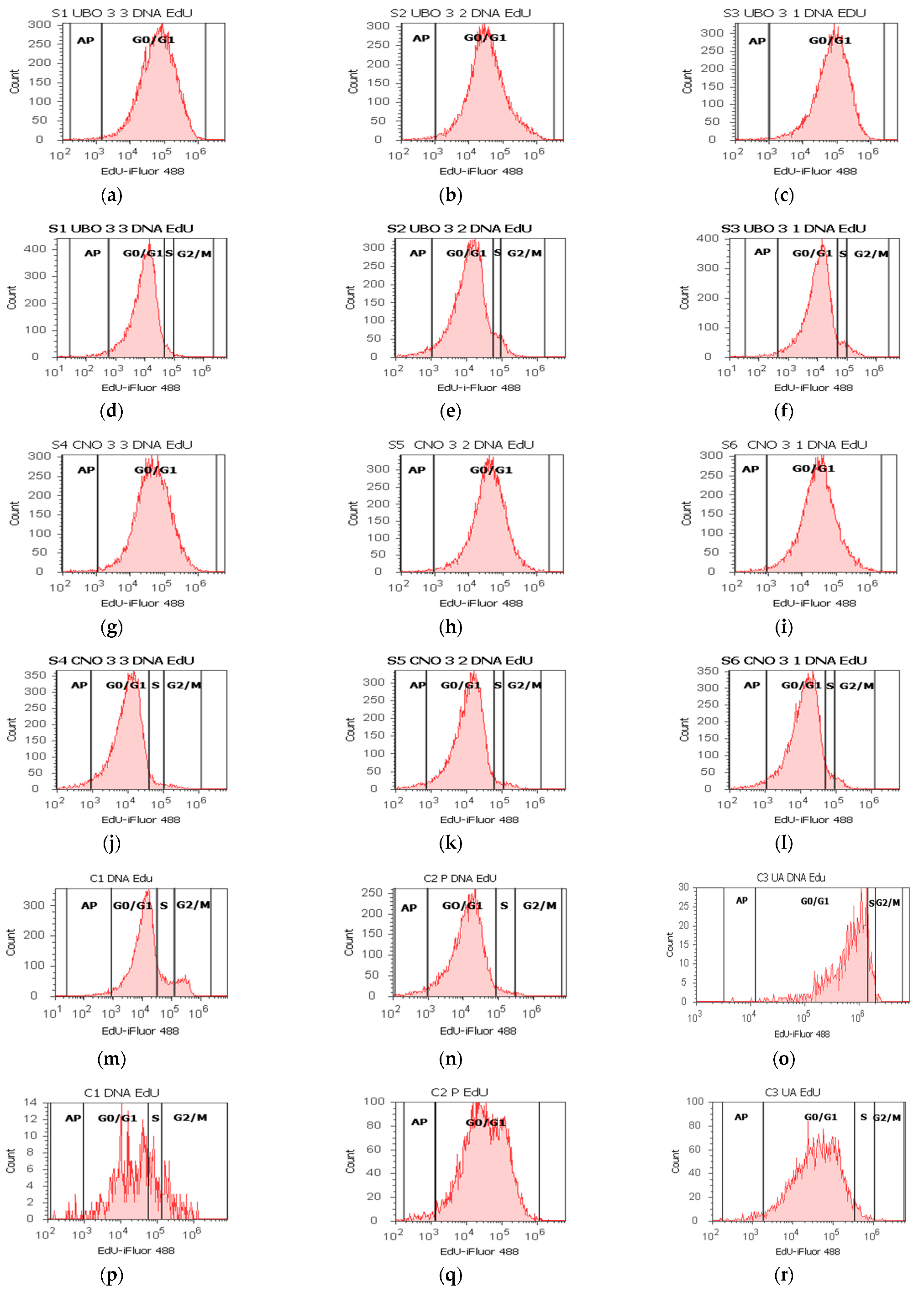
2.8. DNA Synthesis Assay
2.9. Principal Component Analysis
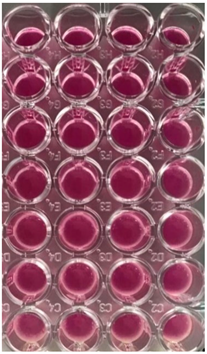
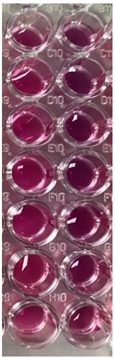
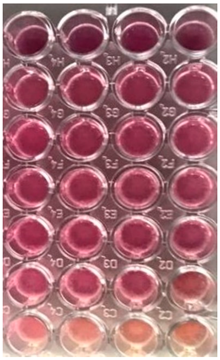
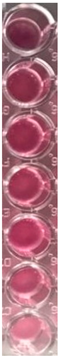
2.10. Antibacterial and Antifungal Activities
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Lichen Extract in Canola Oil
4.3. Physico-Chemical Properties of Usnea barbata (L.) F.H. Wigg Extract in Canola Oil
4.3.1. Atomic Force Microscopy
4.3.2. Rheology
4.4. In Vitro Cytotoxicity
4.4.1. Human Blood Cell Cultures
4.4.2. CLS-354 Cell Line, Cell Culture
4.4.3. Samples and Controls
4.4.4. Equipment
4.4.5. Data Analysis
4.5. Antibacterial and Antifungal Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Bikker, H.; Kempers, M.J.E.; van Trotsenburg, A.S.P.; Baas, F.; de Vijlder, J.J.M.; Vulsma, T.; Ris-Stalpers, C. Inactivating Mutations in the Gene for Thyroid Oxidase 2 (THOX2) and Congenital Hypothyroidism. N. Engl. J. Med. 2002, 347, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, K.H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007, 42, 256–262. [Google Scholar] [CrossRef]
- Krause, K.H.; Bedard, K. NOX enzymes in immuno-inflammatory pathologies. Semin. Immunopathol. 2008, 30, 193–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Nakashima, C.; Fujiwara-Tani, R.; Mori, S.; Kishi, S.; Ohmori, H.; Fujii, K.; Mori, T.; Miyagawa, Y.; Yamamoto, K.; Kirita, T.; et al. An Axis between the Long Non-Coding RNA HOXA11-AS and NQOs Enhances Metastatic Ability in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 10704. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Bandala, C.; Cá, N.; Garciadiego-cá, D.; Lara-padilla, E.; Ibá, G. Trends in Gliosis in Obesity, and the Role of Antioxidants as a Therapeutic Alternative. Antioxidants 2022, 11, 1972. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. Nutr. 2016, 66, 87–100. [Google Scholar]
- Castaneda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zambare, V.; Malek, L.; Gottardo, C.; Suntres, Z.; Christopher, L. Lichenochemicals: Extraction, purification, characterization, and application as potential anticancer agents. Expert Opin. Drug Discov. 2020, 15, 575–601. [Google Scholar] [CrossRef]
- Dar, T.U.H.; Dar, S.A.; Islam, S.U.; Mangral, Z.A.; Dar, R.; Singh, B.P.; Verma, P.; Haque, S. Lichens as a repository of bioactive compounds: An open window for green therapy against diverse cancers. Semin. Cancer Biol. 2021, 44, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Andania, M.M.; Ismed, F.; Taher, M.; Ichwan, S.J.A.; Bakhtiar, A.; Arbain, D. Cytotoxic activities of extracts and isolated compounds of some potential sumatran medicinal plants against MCF-7 and HSC-3 cell lines. J. Math. Fundam. Sci. 2019, 51, 225–242. [Google Scholar] [CrossRef] [Green Version]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsidones with Biological Interest. Planta Med. 2021, 88, 855–880. [Google Scholar] [CrossRef]
- Ivanovic, J.; Meyer, F.; Misic, D.; Asanin, J.; Jaeger, P.; Zizovic, I.; Eggers, R. Influence of different pre-treatment methods on isolation of extracts with strong antibacterial activity from lichen Usnea barbata using carbon dioxide as a solvent. J. Supercrit. Fluids 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Cordeiro, L.M.C.; Iacomini, M. Accumulation of potential pharmaceutically relevant lichen metabolites in lichens and cultured lichen symbionts. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444626158. [Google Scholar]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. fur Naturforsch. Sect. C J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef]
- Jha, B.N.; Shrestha, M.; Pandey, D.P.; Bhattarai, T.; Bhattarai, H.D.; Paudel, B. Investigation of antioxidant, antimicrobial and toxicity activities of lichens from high altitude regions of Nepal. BMC Complement. Altern. Med. 2017, 17, 282. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, G.; Raphael, J.; Leavitt, S.D.; St Clair, L.L. Pharmaceutical Biology In vitro evaluation of the antibacterial activity of extracts from 34 species of North American lichens In vitro evaluation of the antibacterial activity of extracts from 34 species of North American lichens. John M. Pezzuto Pharm. Biol. 2014, 52, 1262–1266. [Google Scholar] [CrossRef]
- Kello, M.; Kuruc, T.; Petrova, K.; Goga, M.; Michalova, Z.; Coma, M.; Rucova, D.; Mojzis, J. Pro-apoptotic potential of Pseudevernia furfuracea (L.) Zopf extract and isolated physodic acid in acute lymphoblastic leukemia model in vitro. Pharmaceutics 2021, 13, 2173. [Google Scholar] [CrossRef]
- Tripathi, A.H.; Negi, N.; Gahtori, R.; Kumari, A.; Joshi, P.; Tewari, L.M.; Joshi, Y.; Bajpai, R.; Upreti, D.K.; Upadhyay, S.K. A Review of Anti-Cancer and Related Properties of Lichen-Extracts and Metabolites. Anticancer Agents Med. Chem. 2021, 22, 115–142. [Google Scholar] [CrossRef]
- Varol, M.; Tay, T.; Candan, M.; Türk, A.; Koparal, A.T. Evaluation of the sunscreen lichen substances usnic acid and atranorin. Biocell 2015, 39, 25–31. [Google Scholar]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Oroian, M.; Mironeasa, S.; Schröder, V.; et al. Advances in the Characterization of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Appl. Sci. 2022, 12, 4234. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-derived compounds and extracts as biologically active substances with anticancer and neuroprotective properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef]
- Dobrescu, D.; Tanasescu, M.; Mezdrea, A.; Ivan, C.; Ordosch, E.; Neagoe, F.; Rizeanu, A.; Trifu, L.; Enescu, V. Contributions to the complex study of some lichens-Usnea genus. Pharmacological studies on Usnea barbata and Usnea hirta species. Rom. J. Physiol. 1993, 30, 101–107. [Google Scholar] [PubMed]
- Tas, I.; Yildirim, A.B.; Ozkan, E.; Ozyigitoglu, G.C.; Yavuz, M.Z.; Turker, A.U. Evaluation of pharmaceutical potential and phytochemical analysis of selected traditional lichen species. Farmacia 2021, 69, 1101–1106. [Google Scholar] [CrossRef]
- Shrestha, G.; Clair, L.L.S. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Emsen, B.; Ozdemir, O.; Engin, T.; Togar, B.; Cavusoglu, S.; Turkez, H. Inhibition of growth of U87MG human glioblastoma cells by Usnea longissima ach. An. Acad. Bras. Cienc. 2019, 91, 1–14. [Google Scholar] [CrossRef]
- Noh, J.I.; Mun, S.K.; Lim, E.H.; Kim, H.; Chang, D.J.; Hur, J.S.; Yee, S.T. Induction of apoptosis in mda-mb-231 cells treated with the methanol extract of lichen physconia hokkaidensis. J. Fungi 2021, 7, 188. [Google Scholar] [CrossRef]
- Matvieieva, N.A.; Pasichnyk, L.A.; Zhytkevych, N.V.; Jacinto, P.G.; Pidgorskyi, V.S. Antimicrobial Activity of Extracts from Ecuadorian Lichens. Mikrobiol. Z. 2015, 77, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.M.; Kim, Y.J.; Gang, H.S.; Han, J.; Ha, H.H.; Kim, H. Antimicrobial Activity of Divaricatic Acid Isolated from the Lichen Evernia mesomorpha against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 3068. [Google Scholar] [CrossRef] [Green Version]
- Fitriani, L.; Afifah; Ismed, F.; Bakhtiar, A. Hydrogel formulation of usnic acid and antibacterial activity test against propionibacterium acne. Sci. Pharm. 2019, 87, 1. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Baláž, M.; Daneu, N.; Elečko, J.; Tkáčiková, Ľ.; Marcinčinová, M.; Bačkor, M. Biological activity of selected lichens and lichen-based Ag nanoparticles prepared by a green solid-state mechanochemical approach. Mater. Sci. Eng. C 2021, 119, 111640. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.; et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F.H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H. Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Mitu, M.A.; Musuc, A.M.; Petrescu, S.; et al. Design, Characterization, and Anticancer and Antimicrobial Activities of Mucoadhesive Oral Patches Loaded with Usnea barbata (L.) F.H. Wigg Ethanol Extract F-UBE-HPMC. Antioxidants 2022, 11, 1801. [Google Scholar] [CrossRef] [PubMed]
- Tadić, V.; Žugić, A.; Đorđević, S.; Žižović, I.; Homšek, I.; Mišić, D.; Nešić, I. The RP-HPLC method for analysis of usnic acid as potential marker of herbal drugs-based formulations containing Usnea barbata. J. Serbian Chem. Soc. 2022, 71, 45. [Google Scholar]
- Pavithra, G.M.; Vinayaka, K.S.; Rakesh, K.N.; Junaid, S.; Dileep, N.; Kekuda, P.T.R.; Siddiqua, S.; Naik, A.S. Antimicrobial and antioxidant activities of a macrolichen Usnea pictoides G. Awasthi (Parmeliaceae). J. Appl. Pharm. Sci. 2013, 3, 154–160. [Google Scholar]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Antioxidant and antibacterial activities of lichen Usnea ghattensis in vitro. Biotechnol. Lett. 2005, 27, 991–995. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- Bate, P.N.N.; Orock, A.E.; Nyongbela, K.D.; Babiaka, S.B.; Kukwah, A.; Ngemenya, M.N. In vitro activity against multi-drug resistant bacteria and cytotoxicity of lichens collected from Mount Cameroon. J. King Saud Univ. Sci. 2020, 32, 614–619. [Google Scholar] [CrossRef]
- Londoñe-Bailon, P.; Sánchez-Robinet, C.; Alvarez-Guzman, G. In vitro antibacterial, antioxidant and cytotoxic activity of methanol-acetone extracts from Antarctic lichens (Usnea antarctica and Usnea aurantiaco-atra). Polar Sci. 2019, 22, 100477. [Google Scholar] [CrossRef]
- Madamombe, I.T.; Afolayan, A.J. Evaluation of antimicrobial activity of extracts from South African Usnea barbata. Pharm. Biol. 2003, 41, 199–202. [Google Scholar] [CrossRef]
- Dieu, A.; Mambu, L.; Champavier, Y.; Chaleix, V.; Sol, V.; Gloaguen, V.; Millot, M. Antibacterial activity of the lichens Usnea Florida and Flavoparmelia caperata (Parmeliaceae). Nat. Prod. Res. 2020, 34, 3358–3362. [Google Scholar] [CrossRef]
- Yadav, H.; Nayaka, S.; Dwivedi, M. Analytics on antimicrobial activity of lichen extract. J. Pure Appl. Microbiol. 2021, 15, 701–708. [Google Scholar] [CrossRef]
- Çelikler Kasimoğullari, S.; Oran, S.; Ari, F.; Ulukaya, E.; Aztopal, N.; Sarimahmut, M.; Öztürk, Ş. Genotoxic, cytotoxic, and apoptotic effects of crude extract of Usnea filipendula Stirt. in vitro. Turkish J. Biol. 2014, 38, 940–947. [Google Scholar] [CrossRef]
- Tang, J.Y.; Wu, K.H.; Wang, Y.Y.; Farooqi, A.A.; Huang, H.W.; Yuan, S.S.F.; Jian, R.I.; Tsao, L.Y.; Chen, P.A.; Chang, F.R.; et al. Methanol extract of usnea barbata induces cell killing, apoptosis, and dna damage against oral cancer cells through oxidative stress. Antioxidants 2020, 9, 694. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the cytotoxic activity of the Usnea barbata (L.) F.H. Wigg dry extract. Molecules 2020, 25, 1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In vitro anticancer activity and oxidative stress biomarkers status determined by Usnea barbata (L.) F.H. wigg. dry extracts. Antioxidants 2021, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.R.; Nayaka, S.; Joshi, Y.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Gómez-Serranillos, M.P.; Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A. Parmeliaceae family: Phytochemistry, pharmacological potential and phylogenetic features. RSC Adv. 2014, 4, 59017–59047. [Google Scholar] [CrossRef]
- Sepahvand, A.; Studzińska-Sroka, E.; Ramak, P.; Karimian, V. Usnea sp.: Antimicrobial potential, bioactive compounds, ethnopharmacological uses and other pharmacological properties; a review article. J. Ethnopharmacol. 2021, 268, 113656. [Google Scholar] [CrossRef] [PubMed]
- Bazarnova, Y.; Politaeva, N.; Lyskova, N. Research for the lichen Usnea barbata metabolites. Z. fur Naturforsch. Sect. C J. Biosci. 2018, 73, 291–296. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Kleszcz, R.; Baer-Dubowska, W. (+)-Usnic acid modulates the Nrf2-ARE pathway in FaDu hypopharyngeal carcinoma cells. Mol. Cell. Biochem. 2021, 476, 2539–2549. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, X.; Zhou, B.; Zhang, C.; Tu, J.; Chen, X.; Wang, J.; Gao, H.; Qin, G.; Pan, W. Usnic acid induces cycle arrest, apoptosis, and autophagy in gastric cancer cells in vitro and in vivo. Med. Sci. Monit. 2018, 24, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Yu, H.; Kim, Y.M. Bactericidal activity of usnic acid-chitosan nanoparticles against persister cells of biofilm-forming pathogenic bacteria. Mar. Drugs 2020, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. And the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.S.; Sudha, S.S. Evaluation of the antibacterial properties of some Lichen species against human pathogens. Int. J. Adv. Res. Biol. Sci. 2015, 2, 177–181. [Google Scholar]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef]
- Wendakoon, C.; Gagnon, D. Evaluation of Selected Medicinal Plants Extracted in Different Ethanol Concentrations for Antibacterial Activity against Human Pathogens. J. Med. Act. Plants 2012, 1, 60–68. [Google Scholar]
- Bézivin, C.; Tomasi, S.; Lohezic-Le Devehat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of old man’s beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiouni, S.; Fayed, M.A.A.; Tarabees, R.; El-Sayed, M.; Elkhatam, A.; Töllner, K.R.; Hessel, M.; Geisberger, T.; Huber, C.; Eisenreich, W.; et al. Characterization of sunflower oil extracts from the lichen Usnea barbata. Metabolites 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Häckl, K.; Kunz, W. Some aspects of green solvents. C. R. Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [Green Version]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, E.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2022, 13, e0314421. [Google Scholar] [CrossRef] [PubMed]
- Jardón-Romero, E.A.; Lara-Carrillo, E.; González-Pedroza, M.G.; Sánchez-Mendieta, V.; Salmerón-Valdés, E.N.; Toral-Rizo, V.H.; Olea-Mejía, O.F.; López-González, S.; Morales-Luckie, R.A. Antimicrobial Activity of Biogenic Silver Nanoparticles from Syzygium aromaticum against the Five Most Common Microorganisms in the Oral Cavity. Antibiotics 2022, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Rafey, A.; Amin, A.; Kamran, M.; Haroon, U.; Farooq, K.; Foubert, K.; Pieters, L. Analysis of plant origin antibiotics against oral bacterial infections using in vitro and in silico techniques and characterization of active constituents. Antibiotics 2021, 10, 1504. [Google Scholar] [CrossRef]
- Thiyahuddin, N.M.; Lamping, E.; Rich, A.M.; Cannon, R.D. Yeast species in the oral cavities of older people: A comparison between people living in their own homes and those in rest homes. J. Fungi 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, N.; Esparza, Y.; Wu, J. Exfoliating nanomaterials in canola protein derived adhesive improves strength and water resistance. RSC Adv. 2017, 7, 6743–6752. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, H.; Toker, O.S.; Dogan, M. Effect of oil type and fatty acid composition on dynamic and steady shear rheology of vegetable Oils. J. Oleo Sci. 2012, 61, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.; Pollard, M.; Erni, P.; Marti, I.; Padar, S. Rheological approaches to food systems. C. R. Phys. 2009, 10, 740–750. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb. Protoc. 2016, 2016, 953–957. [Google Scholar] [CrossRef]
- Lecoeur, H. Nuclear apoptosis detection by flow cytometry: Influence of endogenous endonucleases. Exp. Cell Res. 2002, 277, 1–14. [Google Scholar] [CrossRef]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Murugan, S.; Amaravadi, R.K. Methods for Studying Autophagy Within the Tumor Microenvironment. Adv. Exp. Med. Biol. 2016, 899, 145–166. [Google Scholar] [PubMed] [Green Version]
- Way, L.S.; Gray, C.; Reilly, G.; Scutt, A. An improved method for the measurement of tenocyte proliferation in situ. In IFMBE Proceedings; IFMBE: Berlin, Germany, 2010; Volume 31, pp. 926–929. [Google Scholar]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “Sub-G1” peaks on DNA content histograms. Cytom. Part A 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Hui, C.W.; Wu, W.C.; Leung, S.O.; Kenter, A.L. Interleukins 4 and 21 Protect Anti-IgM Induced Cell Death in Ramos B Cells: Implication for Autoimmune Diseases. Front. Immunol. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, A.; Ramu, S.; Gong, Y.; Cooper, M.A.; Blaskovich, M.A.T. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrob. Agents Chemother. 2019, 63, e01760-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Phe, K.; Dao, D.; Palmer, H.R.; Tama, V.H. In vitro ceftriaxone susceptibility in methicillin-susceptible staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 1370. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, S.A.; Cruz-Córdova, A.; Rodea, G.E.; Cázares-Domínguez, V.; Escalona, G.; Arellano-Galindo, J.; Hernández-Castro, R.; Reyes-López, A.; Xicohtencatl-Cortes, J. Phenotypic characterization of multidrug-resistant Pseudomonas aeruginosa strains isolated from pediatric patients associated to biofilm formation. Microbiol. Res. 2015, 172, 68–78. [Google Scholar] [CrossRef]
- Bitacura, J.G. The Use of Baker’s Yeast in the Resazurin Reduction Test: A Simple, Low-Cost Method for Determining Cell Viability in Proliferation and Cytotoxicity Assays. J. Microbiol. Biol. Educ. 2018, 19, jmbe-19-87. [Google Scholar] [CrossRef] [PubMed]
- Madushan, R.; Vidanarachchi, J.K.; Prasanna, P.H.P.; Werellagama, S.; Priyashantha, H. Use of natural plant extracts as a novel microbiological quality indicator in raw milk: An alternative for resazurin dye reduction method. LWT 2021, 144, 111221. [Google Scholar] [CrossRef]
- Cheikhyoussef, N.; Cheikhyoussef, A. Vegetable oils as green solvents in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Solvents for the Pharmaceutical Industry; Inamuddin, Boddula, R., Ahamed, M.I., Abdullah, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–11. ISBN 9780128218853. [Google Scholar]
- Nandasiri, R.; Eskin, N.A.M.; Eck, P.; Thiyam-Höllander, U. Application of green technology on extraction of phenolic compounds in oilseeds (Canola). In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Tańska, M.; Mikołajczak, N.; Konopka, I. Comparison of the effect of sinapic and ferulic acids derivatives (4-vinylsyringol vs. 4-vinylguaiacol) as antioxidants of rapeseed, flaxseed, and extra virgin olive oils. Food Chem. 2018, 240, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef] [PubMed]
- McDowell, D.; Elliott, C.T.; Koidis, A. Characterization and comparison of UK, Irish, and French cold pressed rapeseed oils with refined rapeseed oils and extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 201600327. [Google Scholar] [CrossRef]
- Sobota, A.; Wirkijowska, A.; Zarzycki, P. Application of vegetable concentrates and powders in coloured pasta production. Int. J. Food Sci. Technol. 2020, 55, 2677–2687. [Google Scholar] [CrossRef]
- Siger, A.; Kachlicki, P.; Czubiński, J.; Polcyn, D.; Dwiecki, K.; Nogala-Kalucka, M. Isolation and purification of plastochromanol-8 for HPLC quantitative determinations. Eur. J. Lipid Sci. Technol. 2014, 116, 413–422. [Google Scholar] [CrossRef]
- Siger, A.; Kaczmarek, A.; Rudzińska, M. Antioxidant activity and phytochemical content of cold-pressed rapeseed oil obtained from roasted seeds. Eur. J. Lipid Sci. Technol. 2015, 117, 1225–1237. [Google Scholar] [CrossRef]
- Kraljić, K.; Škevin, D.; Barišić, L.; Kovačević, M.; Obranović, M.; Jurčević, I. Changes in 4-vinylsyringol and other phenolics during rapeseed oil refining. Food Chem. 2015, 187, 236–242. [Google Scholar] [CrossRef]
- Wakamatsu, D.; Morimura, S.; Imai, T.; Yueqin, T.; Maeda, H.; Kida, K. Production of canola oil showing radical scavenging activity based on a high canolol concentration. Nippon Shokuhin Kagaku Kogaku Kaishi 2008, 55, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Siger, A.; Gawrysiak-Witulska, M.; Bartkowiak-Broda, I. Antioxidant (Tocopherol and Canolol) Content in Rapeseed Oil Obtained from Roasted Yellow-Seeded Brassica napus. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Nandasiri, R.; Eskin, N.A.M. Canolol and its derivatives: A novel bioactive with antioxidant and anticancer properties. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2022; Volume 100, pp. 109–129. ISBN 9780323990820. [Google Scholar]
- Kraljić, K.; Brkan, V.; Škevin, D.; Srček, V.G.; Radošević, K. Canolol Dimer, a Biologically Active Phenolic Compound of Edible Rapeseed Oil. Lipids 2019, 54, 189–200. [Google Scholar] [CrossRef]
- Baig, A.; Zubair, M.; Sumrra, S.H.; Nazar, M.F.; Zafar, M.N.; Jabeen, K.; Hassan, M.B.; Rashid, U. Heating effect on quality characteristics of mixed canola cooking oils. BMC Chem. 2022, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuevas, L.; Castellano, G.; Raikos, V. Natural antioxidants from herbs and spices improve the oxidative stability and frying performance of vegetable oils. Int. J. Food Sci. Technol. 2017, 52, 2422–2428. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Rambu, D.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Antioxidant, Cytotoxic, and Rheological Properties of Canola Oil Extract of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Boroski, M.; Aguiar, A.C.; Rotta, E.M.; Bonafe, E.G.; Valderrama, P.; Souza, N.E.; Visentainer, J.V. Antioxidant activity of herbs and extracted phenolics from oregano in canola oil. Int. Food Res. J. 2018, 25, 2444–2452. [Google Scholar]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, 1–22. [Google Scholar] [CrossRef]
- Malkin, A.Y. Oil as an Object of Rheology (Review). Pet. Chem. 2019, 59, 1092–1107. [Google Scholar] [CrossRef]
- Aho, J.; Hvidt, S.; Baldursdottir, S. Rheology in Pharmaceutical Sciences. In Analytical Techniques in the Pharmaceutical Sciences; Springer Science+Business Media: Berlin, Germany, 2016; pp. 719–750. ISBN 9781493940295. [Google Scholar]
- Rao, M.R.P.; Sapate, S.; Sonawane, A. Pharmacotechnical Evaluation by SeDeM Expert System to Develop Orodispersible Tablets. AAPS PharmSciTech 2022, 23, 133. [Google Scholar] [CrossRef]
- J Mastropietro, D. Rheology in Pharmaceutical Formulations-A Perspective. J. Dev. Drugs 2013, 2, 108. [Google Scholar] [CrossRef] [Green Version]
- Hasan, W.; Khan, M.N. Rheological characterization of vegetable oil blends: Effect of shear rate, temperature, and short-term heating. J. Food Process Eng. 2020, 43, e13396. [Google Scholar] [CrossRef]
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Effect of heating and cooling on rheological parameters of edible vegetable oils. J. Food Eng. 2005, 67, 401–405. [Google Scholar] [CrossRef]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; dos Santos, J.P.A.; da Rocha, R.F.; de Pasquali, M.A.B.; Rybarczyk-Filho, J.L.; Araújo, A.A.S.; Moreira, J.C.F.; Gelain, D.P. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. In Vitro 2012, 26, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessadottir, M.; Egilsson, M.; Einarsdottir, E.; Magnusdottir, I.H.; Ogmundsdottir, M.H.; Omarsdottir, S.; Ogmundsdottir, H.M. Proton-Shuttling Lichen Compound Usnic Acid Affects Mitochondrial and Lysosomal Function in Cancer Cells. PLoS ONE 2012, 7, e51296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsapras, P.; Nezis, I.P. Caspase involvement in autophagy. Cell Death Differ. 2017, 24, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W.; et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010, 1, e18. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Gao, W.; Loughran, P.; Shapiro, R.; Fan, J.; Billiar, T.R.; Scott, M.J. Caspase 1 activation is protective against hepatocyte cell death by up-regulating beclin 1 protein and mitochondrial autophagy in the setting of redox stress. J. Biol. Chem. 2013, 288, 15947–15958. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Hou, W.; Goldstein, L.A.; Stolz, D.B.; Watkins, S.C.; Rabinowich, H. A complex between Atg7 and caspase-9: A novel mechanism of cross-regulation between autophagy and apoptosis. J. Biol. Chem. 2014, 289, 6485–6497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fader, C.M.; Colombo, M.I. Autophagy and multivesicular bodies: Two closely related partners. Cell Death Differ. 2009, 16, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoletopoulou, V.; Papandreou, M.E.; Tavernarakis, N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015, 22, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Dyshlovoy, S.A.; Hauschild, J.; Amann, K.; Tabakmakher, K.M.; Venz, S.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; et al. Marine alkaloid Monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget 2015, 6, 17328–17341. [Google Scholar] [CrossRef] [PubMed]
- Matthaus, B.; Özcan, M.M.; Juhaimi, F. Al Some rape/canola seed oils: Fatty acid composition and tocopherols. Z. fur Naturforsch. Sect. C J. Biosci. 2016, 71, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Waraho, T.; McClements, D.J.; Decker, E.A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011, 129, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, P.; Ring, J.; Muschett, V.; Beranek, A.; Buettner, S.; Carmona-Gutierrez, D.; Eisenberg, T.; Khoury, C.; Rechberger, G.; Kohlwein, S.D.; et al. Fatty acids trigger mitochondrion-dependent necrosis. Cell Death Dis. 2010, 11, 2908–2914. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Sun, Y.; Du, J.; Li, X.; Xun, Z.; Li, Y.C. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G453–G462. [Google Scholar] [CrossRef] [PubMed]
- Prem, P.N.; Kurian, G.A. High-Fat Diet Increased Oxidative Stress and Mitochondrial Dysfunction Induced by Renal Ischemia-Reperfusion Injury in Rat. Front. Physiol. 2021, 12, 715693. [Google Scholar] [CrossRef]
- Dodson, M.; Wani, W.Y.; Redmann, M.; Benavides, G.A.; Johnson, M.S.; Ouyang, X.; Cofield, S.S.; Mitra, K.; Darley-Usmar, V.; Zhang, J. Regulation of autophagy, mitochondrial dynamics, and cellular bioenergetics by 4-hydroxynonenal in primary neurons. Autophagy 2017, 13, 1828–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ahmadi, A.A.; Ayuob, N.N.; Ali, S.S.; Al-Robai, A.A.; Abo-Khatwa, N.A. Effect of (+)-Usnic Acid as a Fat Burner on the Rat Hepatocyte; Correlated Histological and Biochemical in vivo Study. J. Anim. Vet. Adv. 2012, 11, 1368–1377. [Google Scholar]
- Correcché, E.R.; Enriz, R.D.; Piovano, M.; Garbarino, J.; Gómez-Lechón, M.J. Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens. ATLA Altern. Lab. Anim. 2004, 32, 605–615. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Tang, M.; Li, L.; Lei, Y.; Cheng, P.; Guo, W.; Zheng, Y.; Wang, W.; Luo, N.; et al. The role of ROS and subsequent DNA-damage response in PUMAinduced apoptosis of ovarian cancer cells. Oncotarget 2017, 8, 23492–23506. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, R.C.; Kutzner, B.C.; Truong, M.; Sanchez-Dardon, J.; McLean, J.R.N. Analysis of radiation-induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry 2002, 48, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. In Biochemical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 66, pp. 1527–1535. [Google Scholar]
- Zong, W.X.; Ditsworth, D.; Bauer, D.E.; Wang, Z.Q.; Thompson, C.B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004, 18, 1272–1282. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.H.; Xu, M. DNA fragmentation in apoptosis. Cell Res. 2000, 10, 205–211. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Surova, O.; Zhivotovsky, B. Various modes of cell death induced by DNA damage. Oncogene 2013, 32, 3789–3797. [Google Scholar] [CrossRef] [Green Version]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [Green Version]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A.; Firsov, A.M.; Kotova, E.A.; Rokitskaya, T.I.; Khailova, L.S.; Popova, L.B.; Chernyak, B.V.; Antonenko, Y.N. Usnic acid as calcium ionophore and mast cells stimulator. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183303. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.A.; Da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Usnic Acid Activity on Oxidative and Nitrosative Stress of Azole-Resistant Candida albicans Biofilm. Planta Med. 2017, 83, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic acid and Usnea barbata (L.) F.H. wigg. dry extracts promote apoptosis and DNA damage in human blood cells through enhancing ROS levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Costache, T.; Gherghel, D.; Vochita, G.; Mihai, C.T.C.T.; Rotinberg, P.; Schroder, V.; Badea, F.C.F.C.; Badea, V.; et al. Studies on Preparation and UHPLC Analysis of the Usnea barbata (L.) F.H. Wigg Dry acetone extract. Rev. de Chim. 2019, 70, 3775–3777. [Google Scholar]
- Utaipan, T.; Boonyanuphong, P.; Chuprajob, T.; Suksamrarn, A.; Chunglok, W. A trienone analog of curcumin, 1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and caspase-mediated apoptosis in human oral squamous cell carcinoma cells in vitro. Appl. Biol. Chem. 2020, 63, 7. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Fathi, F.; Ghobeh, M.; Tabarzad, M. Anti-Microbial Peptides: Strategies of Design and Development and Their Promising Wound-Healing Activities. Mol. Biol. Rep. 2022, 8, 9001–9012. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, K.D.; Quello, K.; Deford, R.J.; Beckerman, J.L. A rapid method to quantify fungicide sensitivity in the brown rot pathogen Monilinia fructicola. Plant Dis. 2009, 93, 328–331. [Google Scholar] [CrossRef] [PubMed]







| CTR (mg/mL) | TRF (mg/mL) | P407 (mg/mL) | UBO/CNO (mg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 30.230 ± 0.630 | 122.330 ± 0.850 | 10.050 ± 0.180 | 50.133 ± 1.305 | 303.300 ± 15.275 | |||
| 1.511 ± 0.043 | 6.117 ± 0.042 | 0.500 ± 0.009 | 2.506 ± 0.065 | 15.167 ± 0.764 | |||
| 0.755 ± 0.022 | 4.893 ± 0.034 | 0.250 ± 0.004 | 1.253 ± 0.032 | 7.583 ± 0.382 | |||
| 0.377 ± 0.011 | 3.914 ± 0.027 | 0.125 ± 0.002 | 0.626 ± 0.016 | 3.790 ± 0.193 | |||
| 0.188 ± 0.005 | 3.131 ± 0.021 | 0.061 ± 0.001 | 0.315 ± 0.008 | 1.895 ± 0.097 | |||
| 0.094 ± 0.002 | 2.505 ± 0.017 | 0.031 ± 0.001 | 0.157 ± 0.004 | 0.947 ± 0.048 | |||
| 0.047 ± 0.002 | 2.004 ± 0.014 | 0.015 ± 0.001 | 0.078 ± 0.002 | 0.473 ± 0.024 | |||
| 0.023 ± 0.001 | 1.603 ± 0.011 | 0.007 ± 0.001 | 0.039 ± 0.001 | 0.237 ± 0.012 | |||
| S. aureus | P. aeruginosa | ||||||
| UBO | CNO | P407 | CTR | UBO | CNO | P407 | CTR |
 |  |  |  |  | 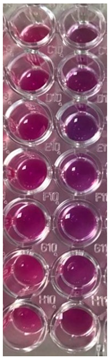 | ||
 * * | |||||||
 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Musuc, A.M.; Matei, E.; Karampelas, O.; Ozon, E.A.; Cozaru, G.C.; Schröder, V.; Bucur, L.; Aricov, L.; Anastasescu, M.; et al. ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 14836. https://doi.org/10.3390/ijms232314836
Popovici V, Musuc AM, Matei E, Karampelas O, Ozon EA, Cozaru GC, Schröder V, Bucur L, Aricov L, Anastasescu M, et al. ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(23):14836. https://doi.org/10.3390/ijms232314836
Chicago/Turabian StylePopovici, Violeta, Adina Magdalena Musuc, Elena Matei, Oana Karampelas, Emma Adriana Ozon, Georgeta Camelia Cozaru, Verginica Schröder, Laura Bucur, Ludmila Aricov, Mihai Anastasescu, and et al. 2022. "ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study" International Journal of Molecular Sciences 23, no. 23: 14836. https://doi.org/10.3390/ijms232314836
APA StylePopovici, V., Musuc, A. M., Matei, E., Karampelas, O., Ozon, E. A., Cozaru, G. C., Schröder, V., Bucur, L., Aricov, L., Anastasescu, M., Așchie, M., Badea, V., Lupuliasa, D., & Gîrd, C. E. (2022). ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study. International Journal of Molecular Sciences, 23(23), 14836. https://doi.org/10.3390/ijms232314836

















