Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma
Abstract
1. Introduction
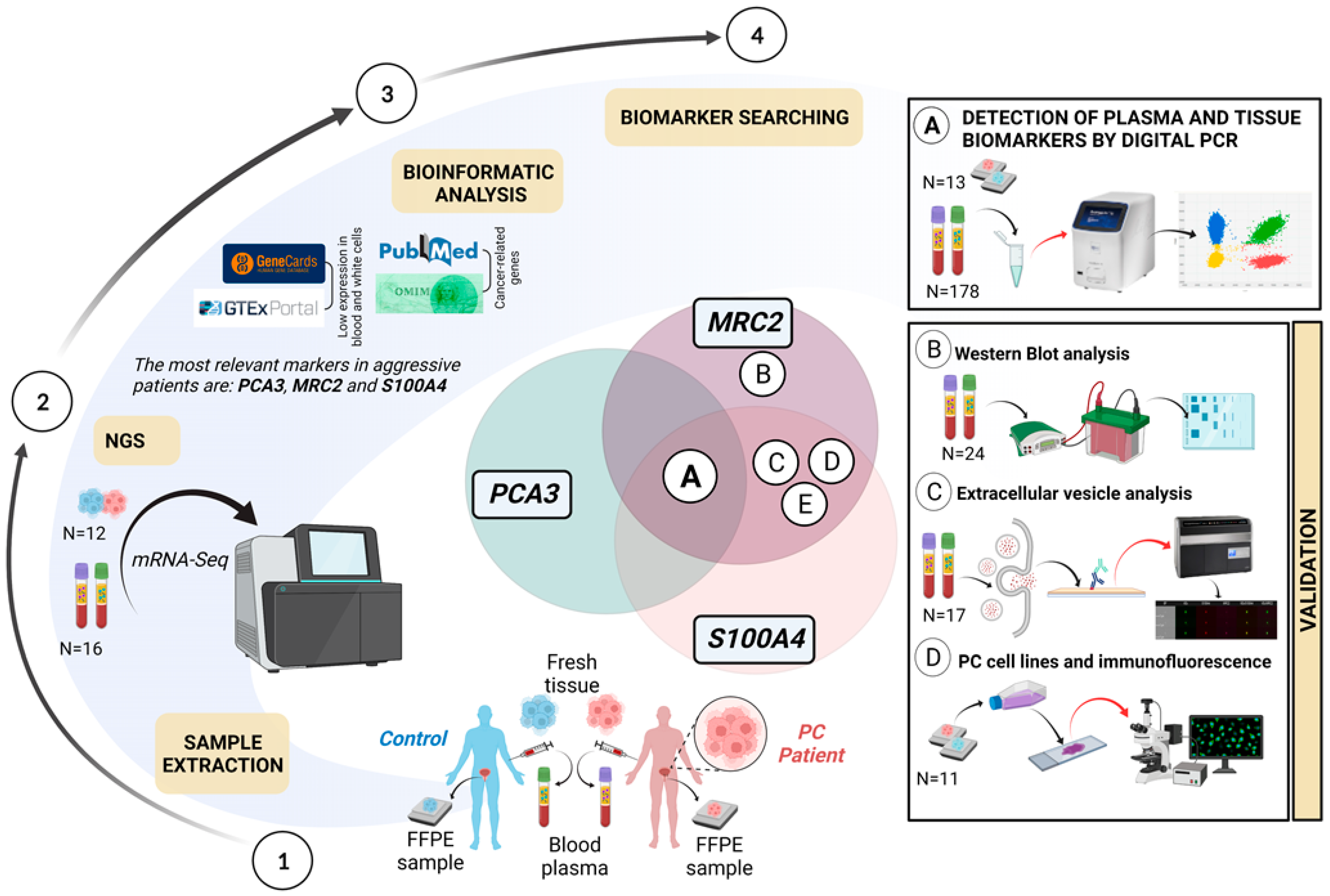
2. Results
2.1. Phase 1: Biomarker Searching
2.1.1. RNA Analysis and Gene Prioritization
2.1.2. Differential Gene Expression in CP Patients Versus Controls
2.2. Phase 2: Validation of the Biomarkers
2.2.1. Western blot Analysis
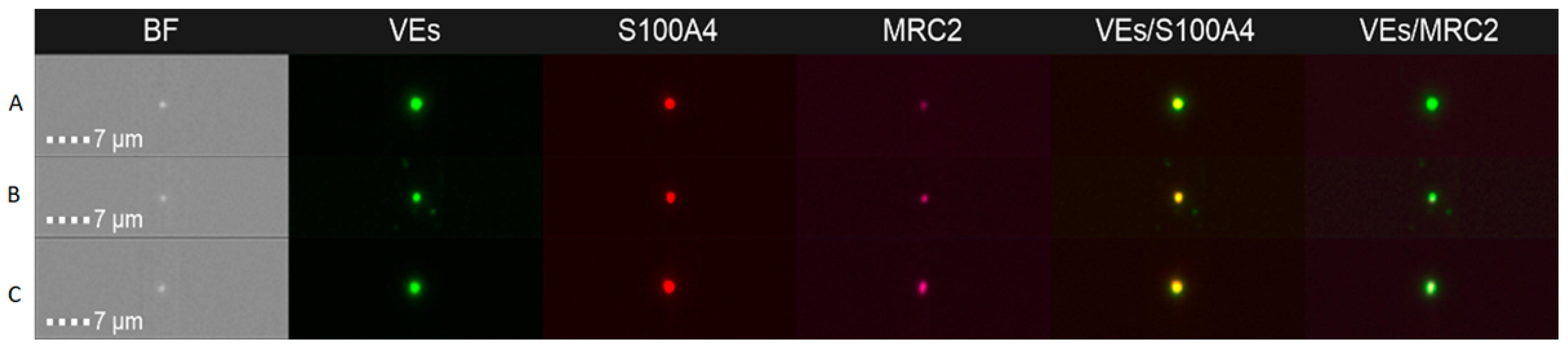
2.2.2. Extracellular Vesicles Analysis
2.2.3. Immunohistochemistry and Immunofluorescence Analysis
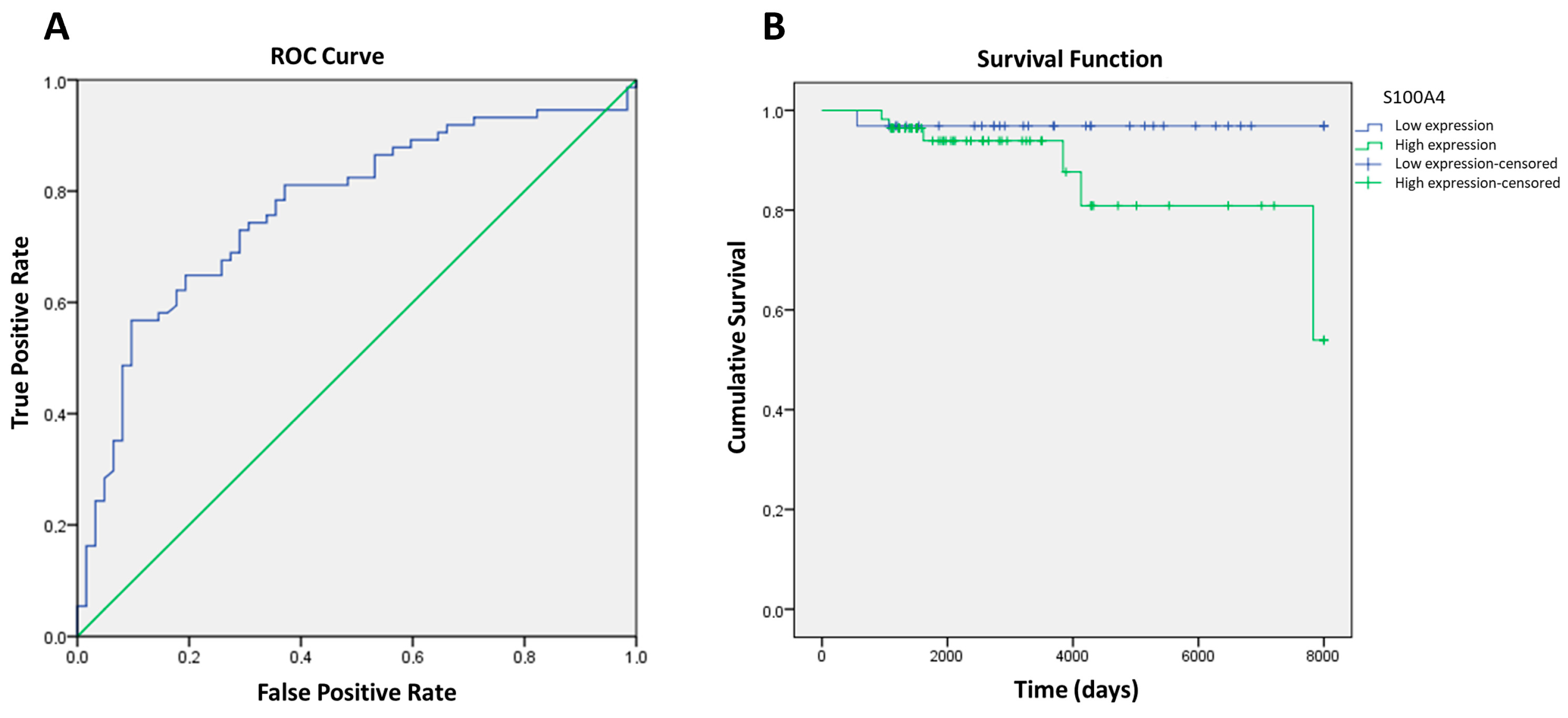
2.2.4. Sensitivity and Specificity and Biomarker Correlation with Overall Survival or Progression-Free Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Sample Collection
4.2. Methodology
4.2.1. Biomarker Searching
4.2.2. Validation of the Biomarkers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Gelmann, E.P.; Kvale, P.A.; Reding, D.J.; et al. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N. Engl. J. Med. 2009, 360, 1310–1319. [Google Scholar] [CrossRef]
- Etzioni, R.; Penson, D.F.; Legler, J.M.; di Tommaso, D.; Boer, R.; Gann, P.H.; Feuer, E.J. Overdiagnosis Due to Prostate-Specific Antigen Screening: Lessons from U.S. Prostate Cancer Incidence Trends. J. Natl. Cancer Inst. 2002, 94, 981–990. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Christidis, D.; Perera, M.; Hong, S.K.; Manning, T.; Vela, I.; Lawrentschuk, N. Prostate Cancer Biomarkers: Are We Hitting the Mark? Prostate Int. 2016, 4, 130–135. [Google Scholar] [CrossRef]
- Filella, X.; Foj, L. Emerging Biomarkers in the Detection and Prognosis of Prostate Cancer. Clin. Chem. Lab. Med. 2015, 53. [Google Scholar] [CrossRef]
- Leyten, G.H.J.M.; Hessels, D.; Jannink, S.A.; Smit, F.P.; de Jong, H.; Cornel, E.B.; de Reijke, T.M.; Vergunst, H.; Kil, P.; Knipscheer, B.C.; et al. Prospective Multicentre Evaluation of PCA3 and TMPRSS2-ERG Gene Fusions as Diagnostic and Prognostic Urinary Biomarkers for Prostate Cancer. Eur. Urol. 2014, 65, 534–542. [Google Scholar] [CrossRef]
- Qin, Z.; Yao, J.; Xu, L.; Xu, Z.; Ge, Y.; Zhou, L.; Zhao, F.; Jia, R. Diagnosis Accuracy of PCA3 Level in Patients with Prostate Cancer: A Systematic Review with Meta-Analysis. Int. Braz. J. Urol. 2020, 46, 691–704. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.; Yu, J.; Yang, G.; Yi, H.; Xu, B. Plasma Messenger RNAs Identified Through Bioinformatics Analysis Are Novel, Non-Invasive Prostate Cancer Biomarkers. OncoTargets Ther. 2020, 13, 541–548. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Li, F.; Xiao, D.; Hou, Y.; Zhu, S.; Liu, D.; Ye, X.; Ye, M.; Yang, J.; et al. Frequent Alterations in Cytoskeleton Remodelling Genes in Primary and Metastatic Lung Adenocarcinomas. Nat. Commun. 2015, 6, 10131. [Google Scholar] [CrossRef]
- Cao, C.-M.; Yang, F.-X.; Wang, P.-L.; Yang, Q.-X.; Sun, X.-R. Clinicopathologic Significance of S100A4 Expression in Osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 833–839. [Google Scholar] [PubMed]
- Kim, B.; Jung, S.; Kim, H.; Kwon, J.-O.; Song, M.-K.; Kim, M.K.; Kim, H.J.; Kim, H.-H. The Role of S100A4 for Bone Metastasis in Prostate Cancer Cells. BMC Cancer 2021, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Garcia-Marques, F.; Zhang, C.A.; Lee, J.J.; Nolley, R.; Shen, M.; Hsu, E.-C.; Aslan, M.; Koul, K.; Pitteri, S.J.; et al. Discovery of CASP8 as a Potential Biomarker for High-Risk Prostate Cancer through a High-Multiplex Immunoassay. Sci. Rep. 2021, 11, 7612. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.; Rezaeian, I.; Singireddy, S.; Cavallo-Medved, D.; Porter, L.A.; Rueda, L. Transcriptomics Signature from Next-Generation Sequencing Data Reveals New Transcriptomic Biomarkers Related to Prostate Cancer. Cancer Inform. 2019, 18, 117693511983552. [Google Scholar] [CrossRef]
- Lage-Vickers, S.; Bizzotto, J.; Valacco, M.P.; Sanchis, P.; Nemirovsky, S.; Labanca, E.; Scorticati, C.; Mazza, O.; Mitrofanova, A.; Navone, N.; et al. The Expression of YWHAZ and NDRG1 Predicts Aggressive Outcome in Human Prostate Cancer. Commun. Biol. 2021, 4, 103. [Google Scholar] [CrossRef]
- Hamzeh, O.; Alkhateeb, A.; Zheng, J.Z.; Kandalam, S.; Leung, C.; Atikukke, G.; Cavallo-Medved, D.; Palanisamy, N.; Rueda, L. A Hierarchical Machine Learning Model to Discover Gleason Grade-Specific Biomarkers in Prostate Cancer. Diagnostics 2019, 9, 219. [Google Scholar] [CrossRef]
- Wang, J.; Chang, S.; Li, G.; Sun, Y. Application of Liquid Biopsy in Precision Medicine: Opportunities and Challenges. Front Med. 2017, 11, 522–527. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, S.; Fang, C.; Hu, X.; Zhang, Y.-S.; Zhang, L.-L.; Zeng, X.-T. Nanomaterials-Based Urinary Extracellular Vesicles Isolation and Detection for Non-Invasive Auxiliary Diagnosis of Prostate Cancer. Front Med. (Lausanne) 2022, 8, 800889. [Google Scholar] [CrossRef]
- Friedemann, M.; Horn, F.; Gutewort, K.; Tautz, L.; Jandeck, C.; Bechmann, N.; Sukocheva, O.; Wirth, M.P.; Fuessel, S.; Menschikowski, M. Increased Sensitivity of Detection of RASSF1A and GSTP1 DNA Fragments in Serum of Prostate Cancer Patients: Optimisation of Diagnostics Using OBBPA-DdPCR. Cancers 2021, 13, 4459. [Google Scholar] [CrossRef]
- Hatano, K.; Nonomura, N. Genomic Profiling of Prostate Cancer: An Updated Review. World J. Mens Health 2021, 39, 368–379. [Google Scholar] [CrossRef]
- Bae, J.; Yang, S.-H.; Kim, A.; Kim, H.G. RNA-Based Biomarkers for the Diagnosis, Prognosis, and Therapeutic Response Monitoring of Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 105.e1–105.e10. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Mahon, K.L.; Spielman, C.; Gurney, H.; Mallesara, G.; Stockler, M.R.; Bastick, P.; Briscoe, K.; Marx, G.; Swarbrick, A.; et al. Phase 2 Study of Circulating MicroRNA Biomarkers in Castration-Resistant Prostate Cancer. Br. J. Cancer 2017, 116, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Arance, E.; Ramírez, V.; Rubio-Roldan, A.; Ocaña-Peinado, F.M.; Romero-Cachinero, C.; Jódar-Reyes, A.B.; Vazquez-Alonso, F.; Martinez-Gonzalez, L.J.; Alvarez-Cubero, M.J. Determination of Exosome Mitochondrial DNA as a Biomarker of Renal Cancer Aggressiveness. Cancers 2021, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.; Liu, L.Y.; Nyalwidhe, J.O.; Semmes, O.J.; Vesprini, D.; Downes, M.R.; Boutros, P.C.; Liu, S.K.; Kislinger, T. Proteomic Discovery of Non-Invasive Biomarkers of Localized Prostate Cancer Using Mass Spectrometry. Nat. Rev. Urol. 2021, 18, 707–724. [Google Scholar] [CrossRef]
- Ganaie, A.A.; Mansini, A.P.; Hussain, T.; Rao, A.; Siddique, H.R.; Shabaneh, A.; Ferrari, M.G.; Murugan, P.; Klingelhöfer, J.; Wang, J.; et al. Anti-S100A4 Antibody Therapy Is Efficient in Treating Aggressive Prostate Cancer and Reversing Immunosuppression: Serum and Biopsy S100A4 as a Clinical Predictor. Mol. Cancer Ther. 2020, 19, 2598–2611. [Google Scholar] [CrossRef]
- Boerrigter, E.; Benoist, G.E.; van Oort, I.M.; Verhaegh, G.W.; de Haan, A.F.J.; van Hooij, O.; Groen, L.; Smit, F.; Oving, I.M.; de Mol, P.; et al. RNA Biomarkers as a Response Measure for Survival in Patients with Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 6279. [Google Scholar] [CrossRef]
- Caley, M.P.; King, H.; Shah, N.; Wang, K.; Rodriguez-Teja, M.; Gronau, J.H.; Waxman, J.; Sturge, J. Tumor-Associated Endo180 Requires Stromal-Derived LOX to Promote Metastatic Prostate Cancer Cell Migration on Human ECM Surfaces. Clin. Exp. Metastasis 2016, 33, 151–165. [Google Scholar] [CrossRef]
- Maestroni, U.; Cavalieri, D.M.; Campobasso, D.; Guarino, G.; Ziglioli, F. PSA-IgM and IXip in the Diagnosis and Management of Prostate Cancer: Clinical Relevance and Future Potential. A Review. Acta Biomed. 2022, 92, e2021344. [Google Scholar] [CrossRef]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Springer: Singapore, 2021; pp. 27–56. [Google Scholar]
- Salameh, A.; Lee, A.K.; Cardó-Vila, M.; Nunes, D.N.; Efstathiou, E.; Staquicini, F.I.; Dobroff, A.S.; Marchiò, S.; Navone, N.M.; Hosoya, H.; et al. PRUNE2 Is a Human Prostate Cancer Suppressor Regulated by the Intronic Long Noncoding RNA PCA3. Proc. Natl. Acad. Sci. USA 2015, 112, 8403–8408. [Google Scholar] [CrossRef]
- Melander, M.C.; Jürgensen, H.J.; Madsen, D.H.; Engelholm, L.H.; Behrendt, N. The Collagen Receptor UPARAP/Endo180 in Tissue Degradation and Cancer. Int. J. Oncol. 2015, 47, 1177–1188. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Zhang, M.; Li, Y.; Zhang, S. S100A4 in Cancer Progression and Metastasis: A Systematic Review. Oncotarget 2017, 8, 73219–73239. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Caley, M.P.; Purshouse, K.; Fonseca, A.-V.; Rodriguez-Teja, M.; Kogianni, G.; Woodley, L.; Odendaal, J.; Elliott, K.; Waxman, J.; et al. Endo180 Modulation by Bisphosphonates and Diagnostic Accuracy in Metastatic Breast Cancer. Br. J. Cancer 2013, 108, 163–169. [Google Scholar] [CrossRef] [PubMed]
| Clinical Values | n (0) | n (1) | AUC | 95% CI | PPV | NPV | Sensitivity | Specificity | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| MRC2 & S100A4 | ISUP grade | 20 | 31 | 0.648 | 0.491–0.804 | 74.07% | 54.16% | 64.52% | 65.00% | 0.039 |
| PSA | 26 | 25 | 0.6 | 0.443–0.557 | 100.00% | 56.52% | 20.00% | 100.00% | 0.016 | |
| Metastasis | 12 | 34 | 0.669 | 0.494–0.845 | 86.95% | 39.13% | 58.82% | 75.00% | 0.044 | |
| PCA3 & S100A4 | ISUP | 22 | 37 | 0.561 | 0.410–0.713 | 69.23% | 42.42% | 48.65% | 63.64% | 0.358 |
| PSA | 27 | 29 | 0.601 | 0.452–0.750 | 80.00% | 54.33% | 27.58% | 92.59% | 0.049 | |
| Metastasis | 16 | 38 | 0.681 | 0.519–0.842 | 82.35% | 50.00% | 73.68% | 62.50% | 0.012 |
| Biomarker | AUC | 95% CI | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| MRC2 | 0.622 | 0.574–0.670 | 59.26 | 100 | 100 | 24.36 |
| PCA3 | 0.644 | 0.584–0.703 | 63.63 | 90.90 | 97.47 | 31.25 |
| S100A4 | 0.735 | 0.668–0.803 | 87.5 | 63.63 | 56.76 | 90.32 |
| MRC2-PCA3 | 0.759 | 0.692–0.826 | 69.66 | 94.28 | 96.88 | 55.00 |
| MRC2-S100A4 | 0.747 | 0.673–0.0820 | 89.19 | 66.21 | 56.90 | 92.45 |
| PCA3-S100A4 | 0.745 | 0.675–0.815 | 92.31 | 60.27 | 55.38 | 93.62 |
| MRC2-PCA3-S100A4 | 0.761 | 0.688–0.835 | 93.75 | 65.67 | 56.60 | 95.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Cubero, M.J.; Arance, E.; de Santiago, E.; Sanchez, P.; Sepúlveda, M.R.; Marrero, R.; Lorente, J.A.; Gonzalez-Cabezuelo, J.M.; Cuenca-Lopez, S.; Cozar, J.M.; et al. Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma. Int. J. Mol. Sci. 2023, 24, 547. https://doi.org/10.3390/ijms24010547
Alvarez-Cubero MJ, Arance E, de Santiago E, Sanchez P, Sepúlveda MR, Marrero R, Lorente JA, Gonzalez-Cabezuelo JM, Cuenca-Lopez S, Cozar JM, et al. Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma. International Journal of Molecular Sciences. 2023; 24(1):547. https://doi.org/10.3390/ijms24010547
Chicago/Turabian StyleAlvarez-Cubero, Maria Jesus, Elena Arance, Esperanza de Santiago, Pilar Sanchez, Maria Rosario Sepúlveda, Raquel Marrero, Jose Antonio Lorente, Jose Maria Gonzalez-Cabezuelo, Sergio Cuenca-Lopez, Jose Manuel Cozar, and et al. 2023. "Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma" International Journal of Molecular Sciences 24, no. 1: 547. https://doi.org/10.3390/ijms24010547
APA StyleAlvarez-Cubero, M. J., Arance, E., de Santiago, E., Sanchez, P., Sepúlveda, M. R., Marrero, R., Lorente, J. A., Gonzalez-Cabezuelo, J. M., Cuenca-Lopez, S., Cozar, J. M., Vazquez-Alonso, F., & Martinez-Gonzalez, L. J. (2023). Follow-Up Biomarkers in the Evolution of Prostate Cancer, Levels of S100A4 as a Detector in Plasma. International Journal of Molecular Sciences, 24(1), 547. https://doi.org/10.3390/ijms24010547









