Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury
Abstract
1. Introduction
2. Results
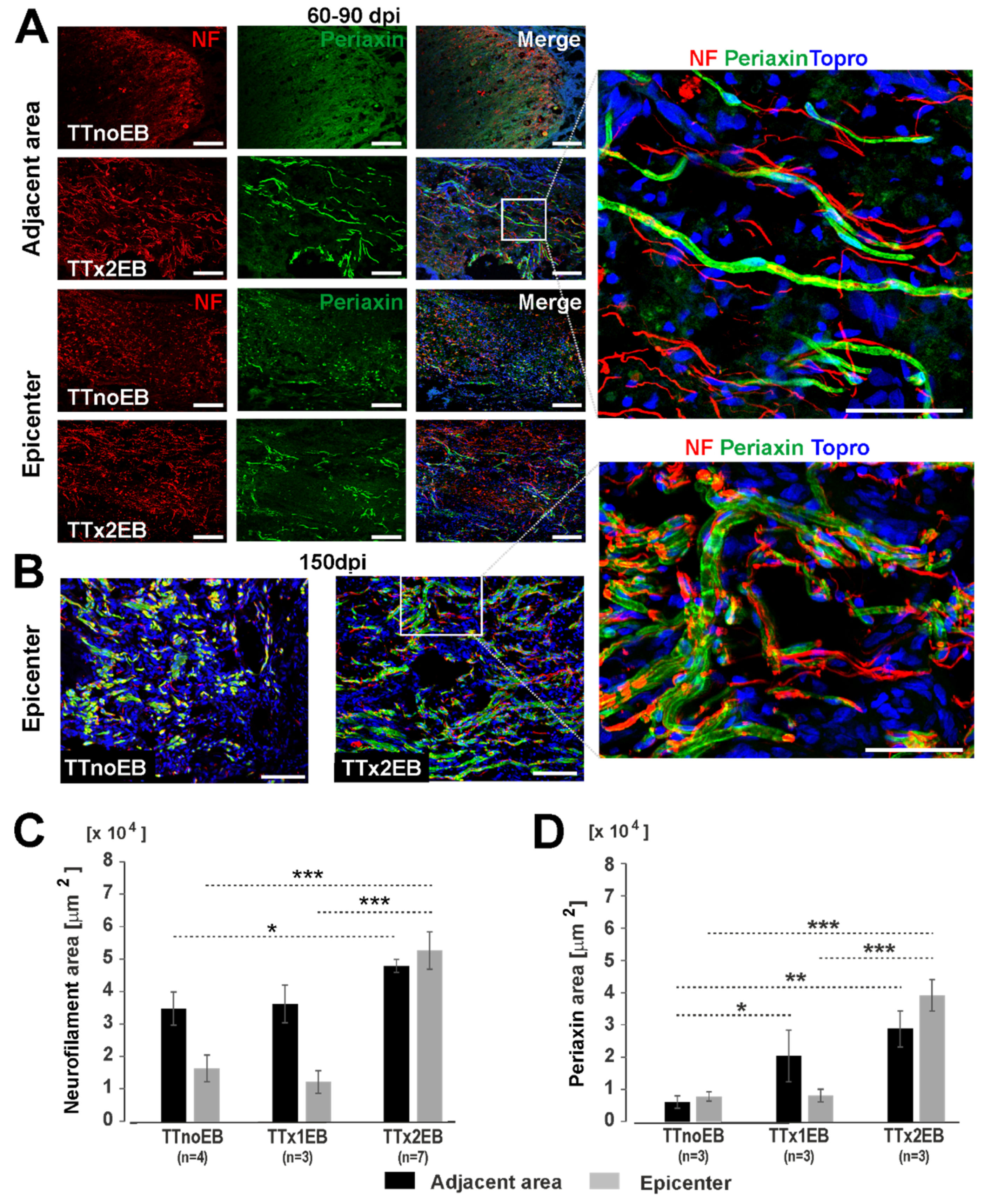
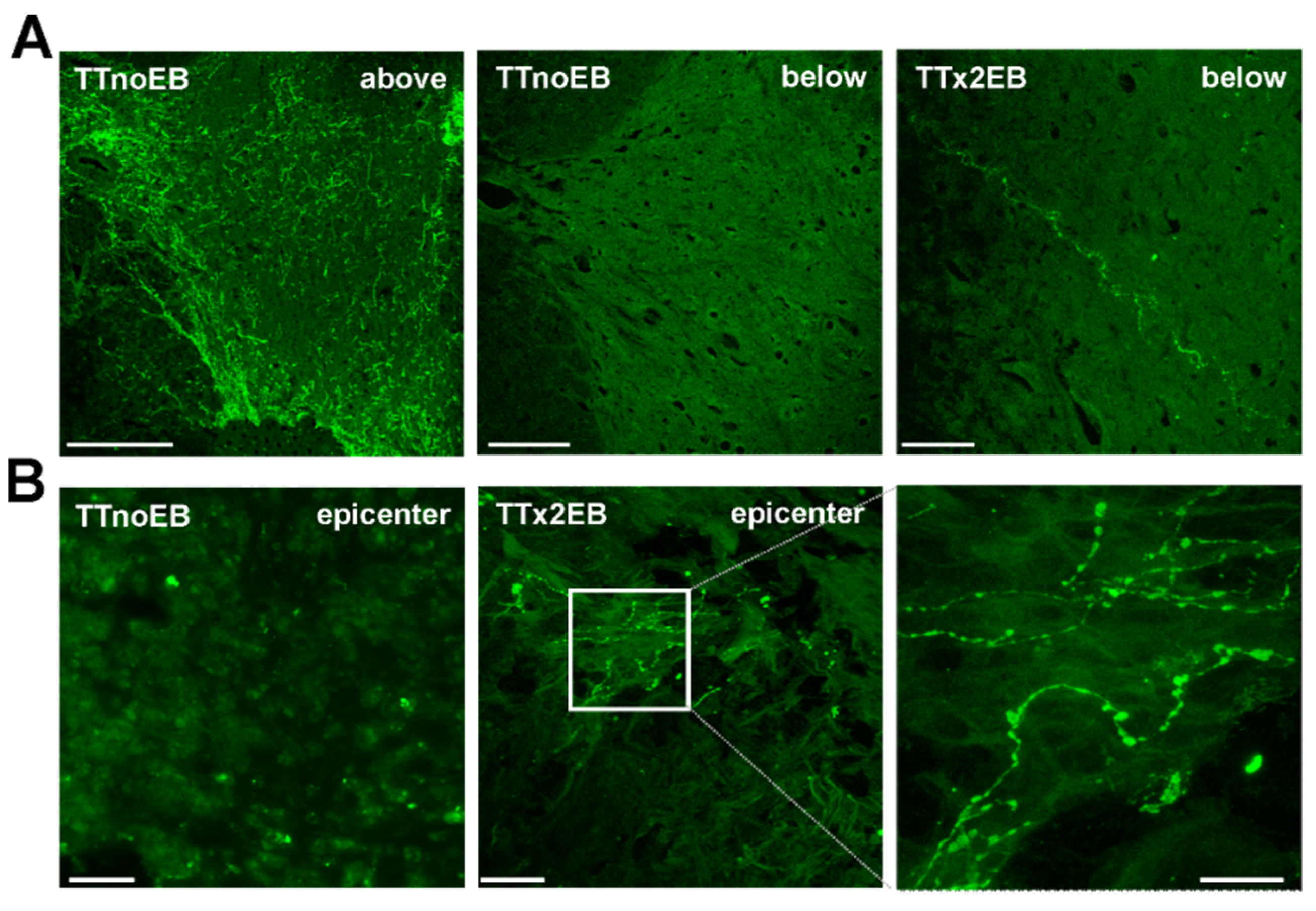
2.1. Chemically Induced White Matter Demyelination of the Transected Spinal Cord Was Followed by Subsequent Remyelination and Axonal Regrowth
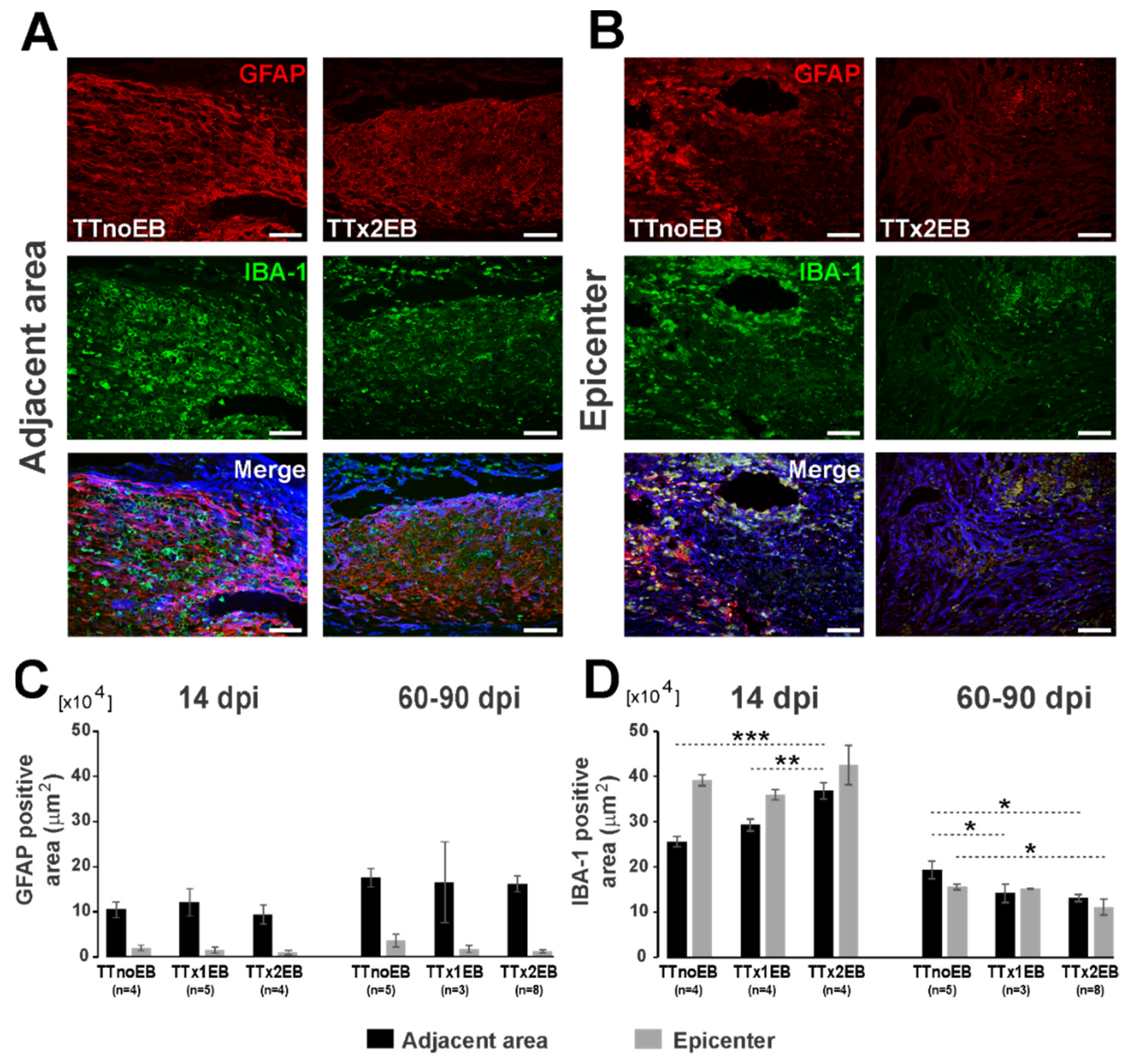
2.2. Forced Demyelination and Subsequent Remyelination Moderately Altered Astro- and Microglial Response to Spinal Cord Transection
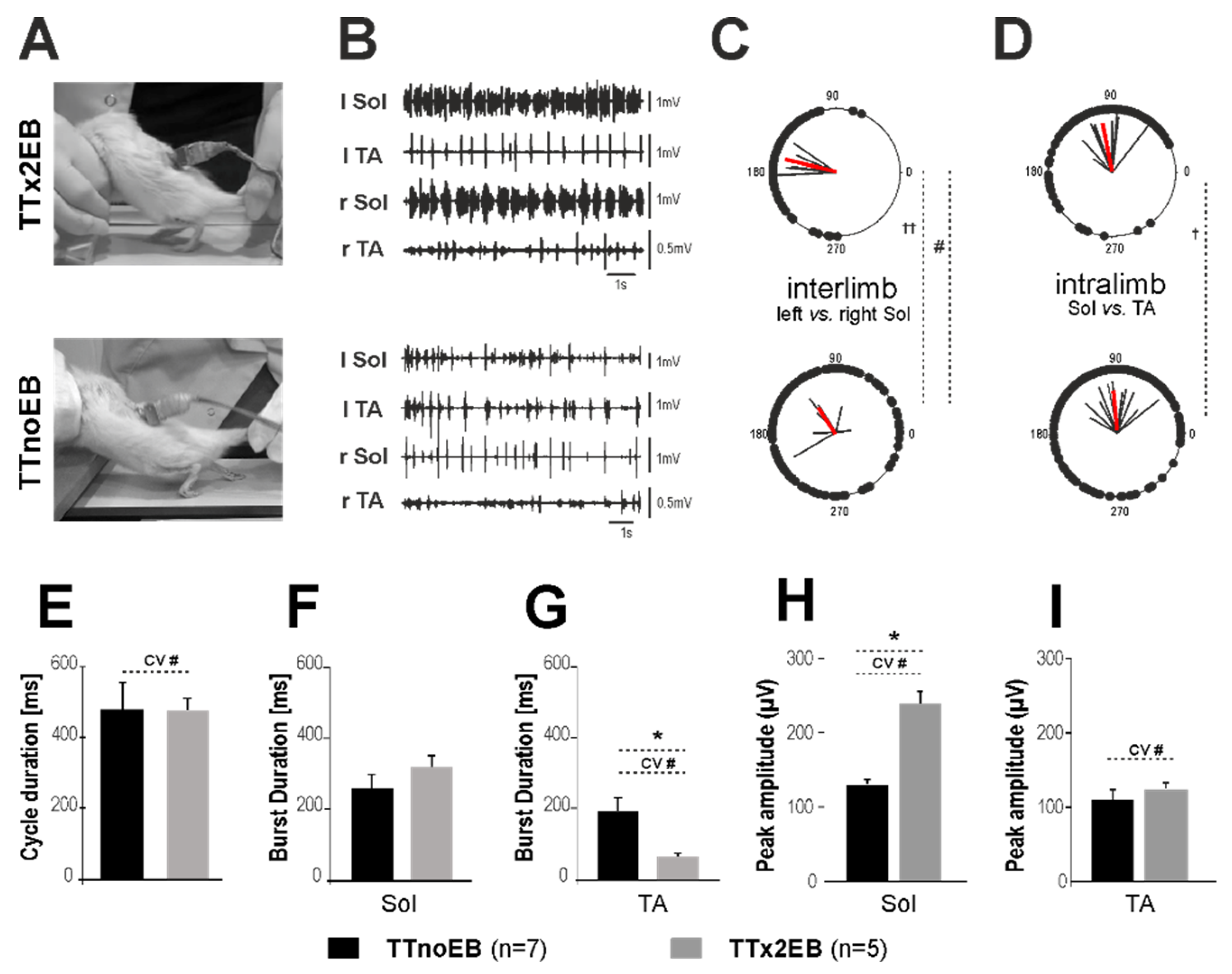
2.3. Locomotor Performance Was Improved in Rats with Forced Remyelination
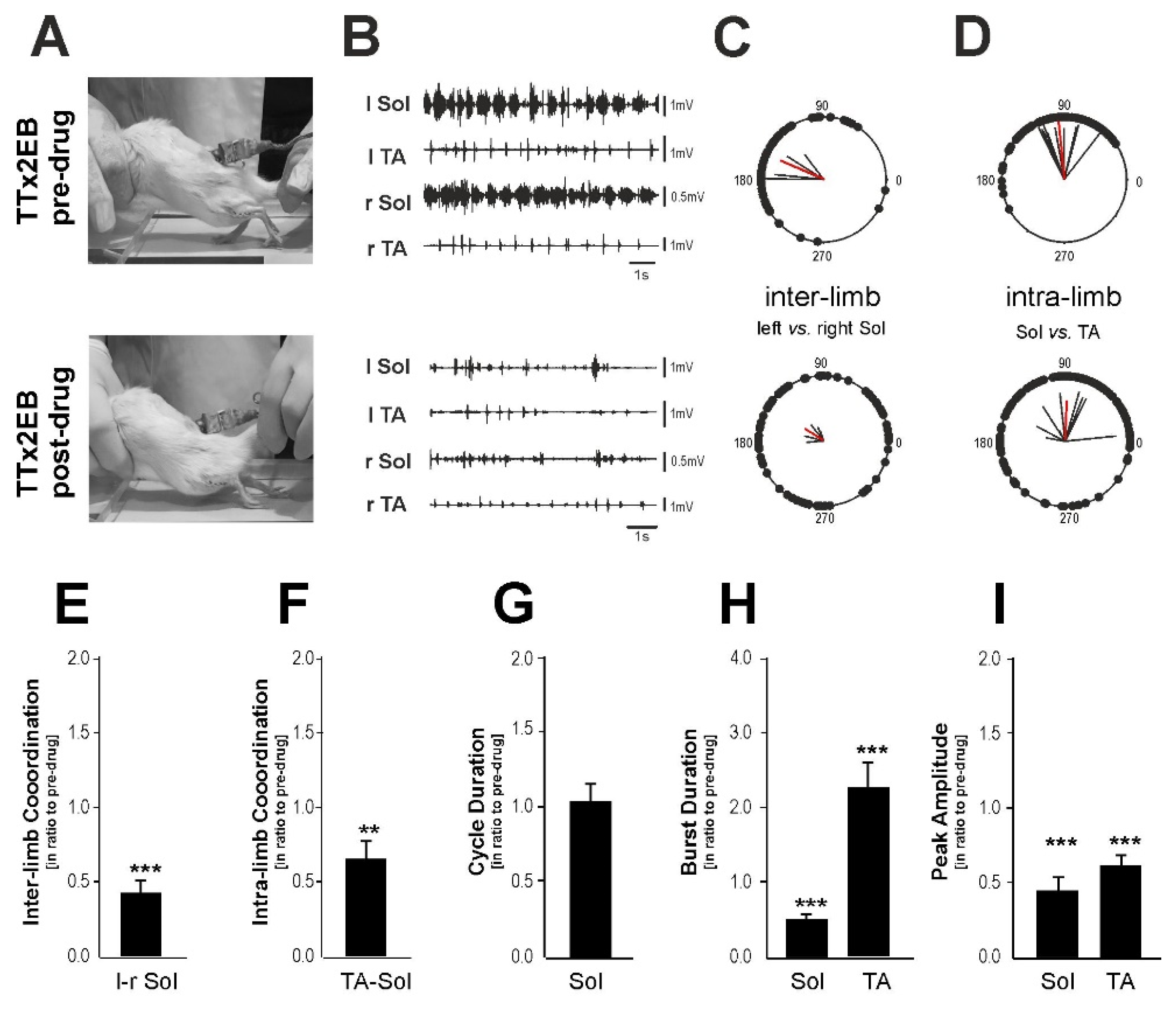
2.4. The Blockade of 5-HT2 Receptors Affected the Locomotor Performance of the Hind Limbs
3. Discussion
4. Materials and Methods
4.1. Animal Model and Ethics Statement
4.2. Complete Spinal Cord Transection
4.3. Induction of Multifocal Demyelination
4.4. Implantation of EMG Recording Electrodes
4.5. Video and Electromyographic Recordings
4.6. Evaluation of Hindlimb Locomotion Based on EMG Analysis
4.7. Blockade of 5-HT2 Receptors by Cyproheptadine Administration
4.8. Statistical Analysis of the Locomotor Performance Based on the EMG Data
4.9. Tissue Processing
4.10. Semi-Thin Resin Sections, Rank Analysis, and Electron Microscopy Analysis
4.11. Statistical Analysis of Immunohistochemical Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franklin, R.J. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Gallo, V. The translational biology of remyelination: Past, present, and future. Glia 2014, 62, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.J.; Assinck, P.; Plemel, J.R.; Tetzlaff, W. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 2020, 68, 227–245. [Google Scholar] [CrossRef]
- Powers, B.E.; Sellers, D.L.; Lovelett, E.A.; Cheung, W.; Aalami, S.P.; Zapertov, N.; Maris, D.O.; Horner, P.J. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc. Natl. Acad. Sci. USA 2013, 110, 4075–4080. [Google Scholar] [CrossRef]
- Sim, F.J.; Zhao, C.; Penderis, J.; Franklin, R.J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 2002, 22, 2451–2459. [Google Scholar] [CrossRef]
- Kotter, M.R.; Li, W.W.; Zhao, C.; Franklin, R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef]
- Plemel, J.R.; Keough, M.B.; Duncan, G.J.; Sparling, J.S.; Yong, V.W.; Stys, P.K.; Tetzlaff, W. Remyelination after spinal cord injury: Is it a target for repair? Prog. Neurobiol. 2014, 117, 54–72. [Google Scholar] [CrossRef]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.J.; Assinck, P.; Liu, J.; Moulson, A.; Plemel, J.R.; Tetzlaff, W. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 2018, 9, 3066. [Google Scholar] [CrossRef]
- Lasiene, J.; Shupe, L.; Perlmutter, S.; Horner, P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J. Neurosci. 2008, 28, 3887–3896. [Google Scholar] [CrossRef]
- Powers, B.E.; Lasiene, J.; Plemel, J.R.; Shupe, L.; Perlmutter, S.I.; Tetzlaff, W.; Horner, P.J. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J. Neurosci. 2012, 32, 5120–5125. [Google Scholar] [CrossRef]
- Lee, S.; Leach, M.K.; Redmond, S.A.; Chong, S.Y.; Mellon, S.H.; Tuck, S.J.; Feng, Z.Q.; Corey, J.M.; Chan, J.R. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 2012, 9, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.F. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol. Appl. Neurobiol. 1982, 8, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Graca, D.L.; Blakemore, W.F. Delayed remyelination in rat spinal cord following ethidium bromide injection. Neuropathol. Appl. Neurobiol. 1986, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Penderis, J.; Shields, S.A.; Franklin, R.J. Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat central nervous system. Brain 2003, 126, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, B.; Bachelin, C.; Coulpier, F.; Gerschenfeld, G.; Deboux, C.; Zujovic, V.; Charnay, P.; Topilko, P.; Baron-Van Evercooren, A. Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling. Acta Neuropathol. 2019, 138, 457–476. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Franklin, R.J. Remyelination in experimental models of toxin-induced demyelination. Curr. Top. Microbiol. Immunol. 2008, 318, 193–212. [Google Scholar]
- Ulanska-Poutanen, J.; Mieczkowski, J.; Zhao, C.; Konarzewska, K.; Kaza, B.; Pohl, H.B.; Bugajski, L.; Kaminska, B.; Franklin, R.J.; Zawadzka, M. Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. Elife 2018, 7, e30325. [Google Scholar] [CrossRef]
- Uyeda, A.; Muramatsu, R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int. J. Mol. Sci. 2020, 21, 8116. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S. Real-time imaging of axonally transported subresolution organelles in vertebrate myelinated axons. J. Neurosci. Methods 1989, 26, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Yezierski, R.P.; Devon, R.M.; Vicedomini, J.P.; Broton, J.G. Effects of dorsal column demyelination on evoked potentials in nucleus gracilis. J. Neurotrauma 1992, 9, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Felts, P.A.; Smith, K.J. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992, 574, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Chari, D.M.; Blakemore, W.F. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia 2002, 37, 307–313. [Google Scholar] [CrossRef]
- Woodruff, R.H.; Franklin, R.J. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: A comparative study. Glia 1999, 25, 216–228. [Google Scholar] [CrossRef]
- Sławińska, U.; Majczyński, H.; Dai, Y.; Jordan, L.M. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J. Physiol. 2012, 590, 1721–1736. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Miazga, K.; Majczyński, H.; Jordan, L.M.; Zawadzka, M.; Sławińska, U. Noradrenergic Components of Locomotor Recovery Induced by Intraspinal Grafting of the Embryonic Brainstem in Adult Paraplegic Rats. Int. J. Mol. Sci. 2020, 21, 5520. [Google Scholar] [CrossRef]
- Sławińska, U.; Miazga, K.; Cabaj, A.M.; Leszczyńska, A.N.; Majczyński, H.; Nagy, J.I.; Jordan, L.M. Grafting of fetal brainstem 5-HT neurons into the sublesional spinal cord of paraplegic rats restores coordinated hindlimb locomotion. Exp. Neurol. 2013, 247, 572–581. [Google Scholar] [CrossRef]
- Totoiu, M.O.; Keirstead, H.S. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 2005, 486, 373–383. [Google Scholar] [CrossRef]
- Lytle, J.M.; Wrathall, J.R. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 2007, 25, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.A.; Blakemore, W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain 2008, 131, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D., 3rd; Lebkowski, J.S. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Frame, J.; Siegenthaler, M.; Nistor, G.; Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010, 28, 152–163. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Antri, M.; Orsal, D.; Barthe, J.Y. Locomotor recovery in the chronic spinal rat: Effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002, 16, 467–476. [Google Scholar] [CrossRef]
- Antri, M.; Mouffle, C.; Orsal, D.; Barthe, J.Y. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 2003, 18, 1963–1972. [Google Scholar] [CrossRef]
- Antri, M.; Barthe, J.Y.; Mouffle, C.; Orsal, D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci. Lett. 2005, 384, 162–167. [Google Scholar] [CrossRef]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef]
- Lavrov, I.; Dy, C.J.; Fong, A.J.; Gerasimenko, Y.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J. Neurosci. 2008, 28, 6022–6029. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Musienko, P.; Bogacheva, I.; Moshonkina, T.; Savochin, A.; Lavrov, I.; Roy, R.R.; Edgerton, V.R. Propriospinal bypass of the serotonergic system that can facilitate stepping. J. Neurosci. 2009, 29, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, U.; Miazga, K.; Jordan, L.M. 5-HT(2) and 5-HT(7) receptor agonists facilitate plantar stepping in chronic spinal rats through actions on different populations of spinal neurons. Front. Neural Circuits 2014, 8, 95. [Google Scholar] [PubMed]
- Franklin, R.J.; Gilson, J.M.; Blakemore, W.F. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J. Neurosci. Res. 1997, 50, 337–344. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Levine, J.M.; Blakemore, W.F. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia 1998, 22, 161–170. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef]
- Baer, A.S.; Syed, Y.A.; Kang, S.U.; Mitteregger, D.; Vig, R.; Ffrench-Constant, C.; Franklin, R.J.; Altmann, F.; Lubec, G.; Kotter, M.R. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 2009, 132, 465–481. [Google Scholar] [CrossRef]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoon, D.H.; Park, Y.G.; Lee, B.H. Effects of glial transplantation on functional recovery following acute spinal cord injury. J. Neurotrauma 2005, 22, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Shumsky, J.S.; Lepore, A.C.; Murray, M.; Fischer, I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J. Neurosci. 2005, 25, 9624–9636. [Google Scholar] [CrossRef]
- Plemel, J.R.; Chojnacki, A.; Sparling, J.S.; Liu, J.; Plunet, W.; Duncan, G.J.; Park, S.E.; Weiss, S.; Tetzlaff, W. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia 2011, 59, 1891–1910. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.; Mothe, A.; Wang, J.; Wang, S.; Tator, C.; Fehlings, M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012, 21, 2222–2238. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Tran, A.; Howell, C.J.; Busch, S.A.; Evans, T.A.; Stallcup, W.B.; Kang, S.H.; Bergles, D.E.; Lee, S.I.; Levine, J.M.; et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 2014, 34, 16369–16384. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.R.; Yahn, S.L.; Lyapichev, K.; Dajnoki, A.; Lee, D.H.; Rodriguez, M.; Cammer, N.; Pak, J.; Mehta, S.T.; Bodamer, O.; et al. Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol. 2018, 308, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Melik Parsadaniantz, S.; Franklin, R.J.; et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef]
- Pluchino, S.; Zanotti, L.; Rossi, B.; Brambilla, E.; Ottoboni, L.; Salani, G.; Martinello, M.; Cattalini, A.; Bergami, A.; Furlan, R.; et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005, 436, 266–271. [Google Scholar] [CrossRef]
- Einstein, O.; Friedman-Levi, Y.; Grigoriadis, N.; Ben-Hur, T. Transplanted neural precursors enhance host brain-derived myelin regeneration. J. Neurosci. 2009, 29, 15694–15702. [Google Scholar] [CrossRef]
- Zujovic, V.; Bachelin, C.; Baron-Van Evercooren, A. Remyelination of the central nervous system: A valuable contribution from the periphery. Neuroscientist 2007, 13, 383–391. [Google Scholar] [CrossRef]
- Ma, D.; Wang, B.; Zawadzka, M.; Gonzalez, G.; Wu, Z.; Yu, B.; Rawlins, E.L.; Franklin, R.J.M.; Zhao, C. A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System. J. Neurosci. 2018, 38, 9228–9239. [Google Scholar] [CrossRef]
- Talbott, J.F.; Cao, Q.; Enzmann, G.U.; Benton, R.L.; Achim, V.; Cheng, X.X.; Mills, M.D.; Rao, M.S.; Whittemore, S.R. Schwann cell-like differentiation by adult oligodendrocyte precursor cells following engraftment into the demyelinated spinal cord is BMP-dependent. Glia 2006, 54, 147–159. [Google Scholar] [CrossRef]
- Monteiro de Castro, G.; Deja, N.A.; Ma, D.; Zhao, C.; Franklin, R.J. Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination. Am. J. Pathol. 2015, 185, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Hoffman, R.L. Schwann cell invasion of the central nervous system of the myelin mutants. J. Anat. 1997, 190 Pt 1, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Oudega, M.; Bates, M.L.; Wood, P.M.; Kleitman, N.; Bunge, M.B. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 2002, 22, 6670–6681. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.R.; Henao, M.; Pearse, D.D.; Bunge, M.B. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 2015, 24, 115–131. [Google Scholar] [CrossRef]
- Assinck, P.; Sparling, J.S.; Dworski, S.; Duncan, G.J.; Wu, D.L.; Liu, J.; Kwon, B.K.; Biernaskie, J.; Miller, F.D.; Tetzlaff, W. Transplantation of Skin Precursor-Derived Schwann Cells Yields Better Locomotor Outcomes and Reduces Bladder Pathology in Rats with Chronic Spinal Cord Injury. Stem Cell Rep. 2020, 15, 140–155. [Google Scholar] [CrossRef]
- Majczyński, H.; Cabaj, A.M.; Jordan, L.M.; Sławińska, U. Contribution of 5-HT2 Receptors to the Control of the Spinal Locomotor System in Intact Rats. Front. Neural Circuits 2020, 14, 14. [Google Scholar] [CrossRef]
- Leszczyńska, A.N.; Majczyński, H.; Wilczyński, G.M.; Sławińska, U.; Cabaj, A.M. Thoracic Hemisection in Rats Results in Initial Recovery Followed by a Late Decrement in Locomotor Movements, with Changes in Coordination Correlated with Serotonergic Innervation of the Ventral Horn. PLoS ONE 2015, 10, e0143602. [Google Scholar] [CrossRef]
- Saruhashi, Y.; Young, W.; Perkins, R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp. Neurol. 1996, 139, 203–213. [Google Scholar] [CrossRef]
- Saruhashi, Y.; Matsusue, Y.; Fujimiya, M. The recovery of 5-HT transporter and 5-HT immunoreactivity in injured rat spinal cord. Arch. Orthop. Trauma Surg. 2009, 129, 1279–1285. [Google Scholar] [CrossRef]
- Majczyński, H.; Maleszak, K.; Cabaj, A.; Sławińska, U. Serotonin-related enhancement of recovery of hind limb motor functions in spinal rats after grafting of embryonic raphe nuclei. J. Neurotrauma 2005, 22, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.J.; Jordan, L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000, 53, 689–710. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.B.; Sillar, K.T. Neuromodulation of vertebrate locomotor control networks. Physiology 2011, 26, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Pearse, D.D. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front. Neural Circuits 2014, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rios, M.; Guertin, P.A.; Rivera-Oliver, M. Neuromodulation of Spinal Locomotor Networks in Rodents. Curr. Pharm. Des. 2017, 23, 1741–1752. [Google Scholar] [CrossRef]
- Sławińska, U.; Jordan, L.M. Serotonergic influences on locomotor circuits. Curr. Opin. Physiol. 2019, 8, 63–69. [Google Scholar] [CrossRef]
- Hawthorne, A.L.; Hu, H.M.; Kundu, B.; Steinmetz, M.P.; Wylie, C.J.; Deneris, E.S.; Silver, J. The Unusual Response of Serotonergic Neurons after CNS Injury: Lack of Axonal Dieback and Enhanced Sprouting within the Inhibitory Environment of the Glial Scar. J. Neurosci. 2011, 31, 5605–5616. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.M.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef]
- Jin, Y.J.; Dougherty, S.E.; Wood, K.; Sun, L.; Cudmore, R.H.; Abdalla, A.; Kannan, G.; Pletnikov, M.; Hashemi, P.; Linden, D.J. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 2016, 91, 748–762. [Google Scholar] [CrossRef]
- Milner, R.; Campbell, I.L. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol. Cell. Neurosci. 2002, 20, 616–626. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Warren, P.M.; Alilain, W.J. Plasticity Induced Recovery of Breathing Occurs at Chronic Stages after Cervical Contusion. J. Neurotrauma 2019, 36, 1985–1999. [Google Scholar] [CrossRef] [PubMed]
- Feraboli-Lohnherr, D.; Orsal, D.; Yakovleff, A.; Gimenez y Ribotta, M.; Privat, A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: Specific role of serotonergic neurons. Exp. Brain Res. 1997, 113, 443–454. [Google Scholar] [CrossRef]
- Ribotta, M.G.; Provencher, J.; Feraboli-Lohnherr, D.; Rossignol, S.; Privat, A.; Orsal, D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J. Neurosci. 2000, 20, 5144–5152. [Google Scholar] [CrossRef]
- Sławińska, U.; Majczyński, H.; Djavadian, R. Recovery of hindlimb motor functions after spinal cord transection is enhanced by grafts of the embryonic raphe nuclei. Exp. Brain Res. 2000, 132, 27–38. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Errea, O.; van Wijngaarden, P.; Gonzalez, G.A.; Kerninon, C.; Jarjour, A.A.; Lewis, H.J.; Jones, C.A.; Nait-Oumesmar, B.; Zhao, C.; et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol. 2015, 211, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.K.; Jarjour, A.A.; Nait Oumesmar, B.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A.; et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Simons, M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron 2022, 110, 3549–3565. [Google Scholar] [CrossRef]
- Aigrot, M.S.; Barthelemy, C.; Moyon, S.; Dufayet-Chaffaud, G.; Izagirre-Urizar, L.; Gillet-Legrand, B.; Tada, S.; Bayon-Cordero, L.; Chara, J.C.; Matute, C.; et al. Genetically modified macrophages accelerate myelin repair. EMBO Mol. Med. 2022, 14, e14759. [Google Scholar] [CrossRef]
- Zar, J.H. Circular distribution. In Biostatistical Analysis, 5th ed.; McElroy, W.D., Swanson, C.P., Eds.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 605–668. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzka, M.; Yeghiazaryan, M.; Niedziółka, S.; Miazga, K.; Kwaśniewska, A.; Bekisz, M.; Sławińska, U. Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 495. https://doi.org/10.3390/ijms24010495
Zawadzka M, Yeghiazaryan M, Niedziółka S, Miazga K, Kwaśniewska A, Bekisz M, Sławińska U. Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(1):495. https://doi.org/10.3390/ijms24010495
Chicago/Turabian StyleZawadzka, Małgorzata, Marine Yeghiazaryan, Sylwia Niedziółka, Krzysztof Miazga, Anna Kwaśniewska, Marek Bekisz, and Urszula Sławińska. 2023. "Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 1: 495. https://doi.org/10.3390/ijms24010495
APA StyleZawadzka, M., Yeghiazaryan, M., Niedziółka, S., Miazga, K., Kwaśniewska, A., Bekisz, M., & Sławińska, U. (2023). Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury. International Journal of Molecular Sciences, 24(1), 495. https://doi.org/10.3390/ijms24010495





