Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal
Abstract
1. Introduction
2. Results and Discussion
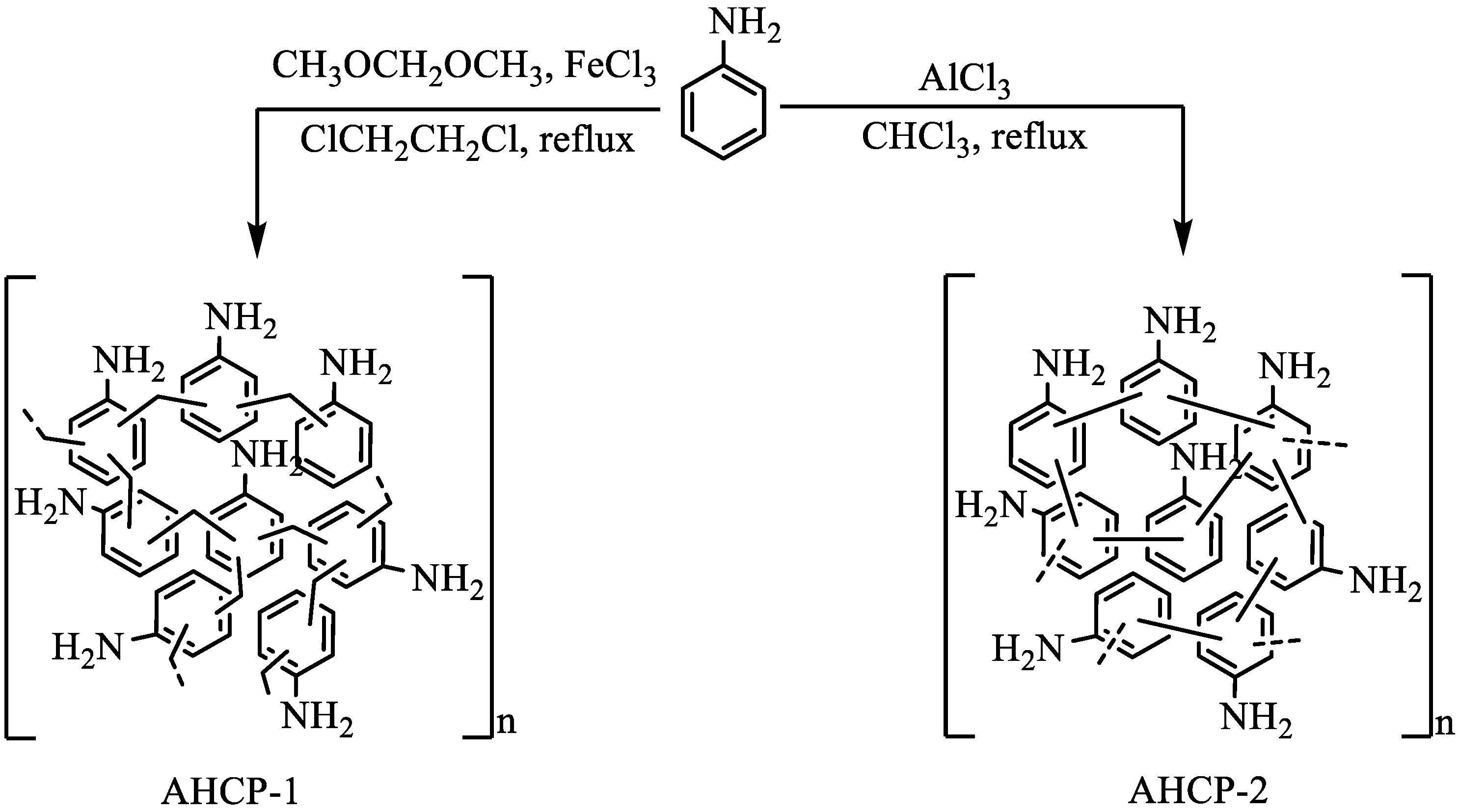
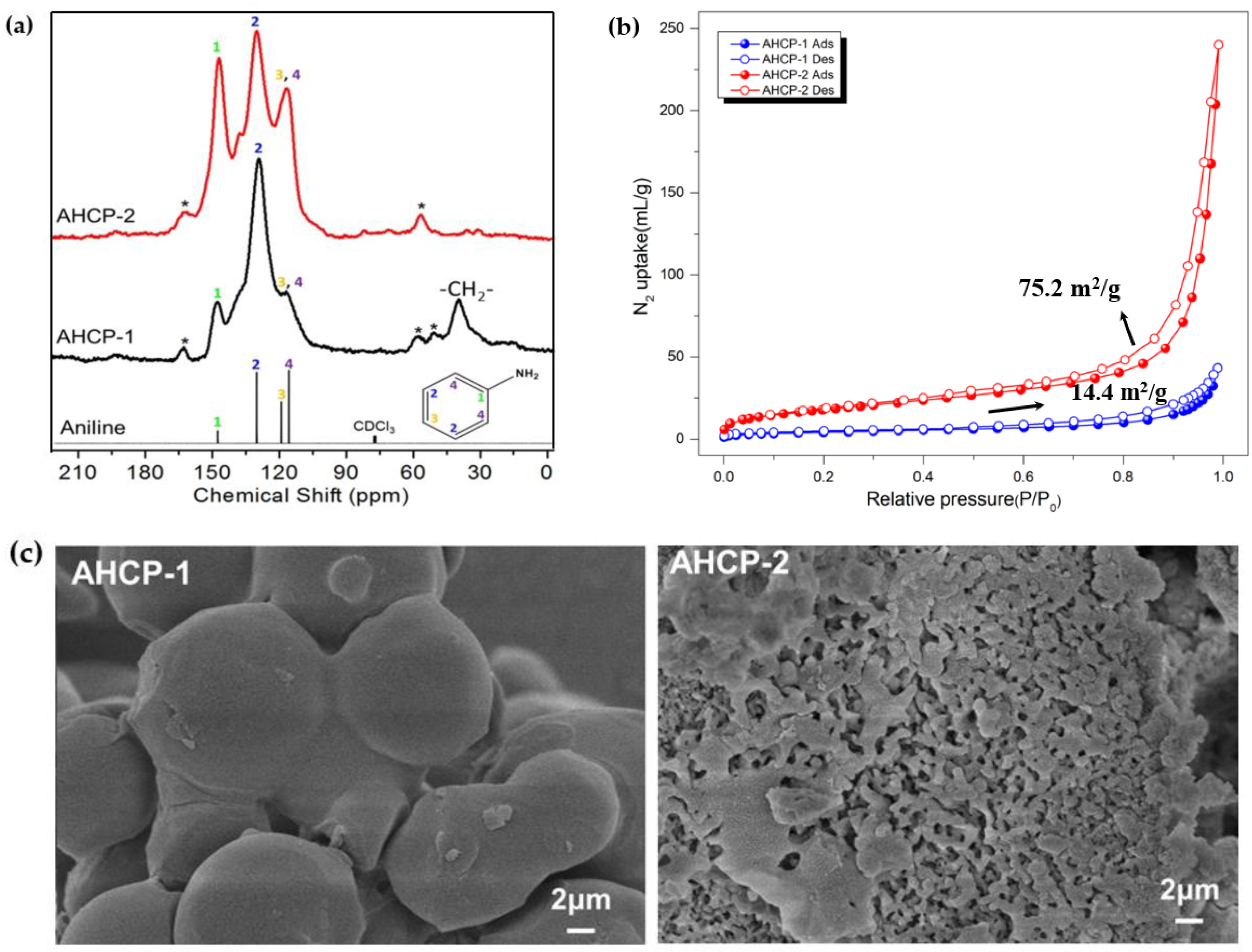
2.1. Synthesis and Structural Characterization
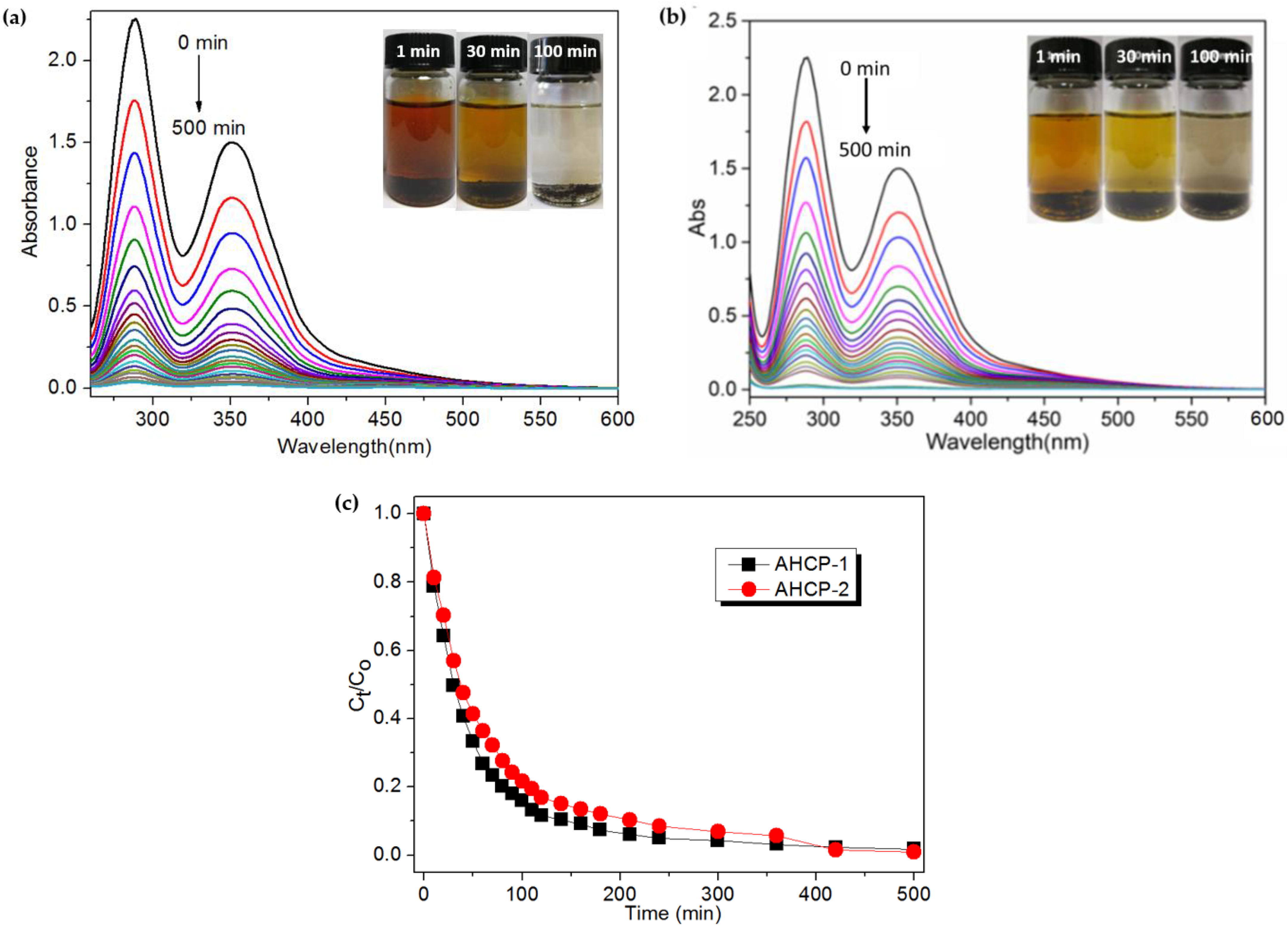
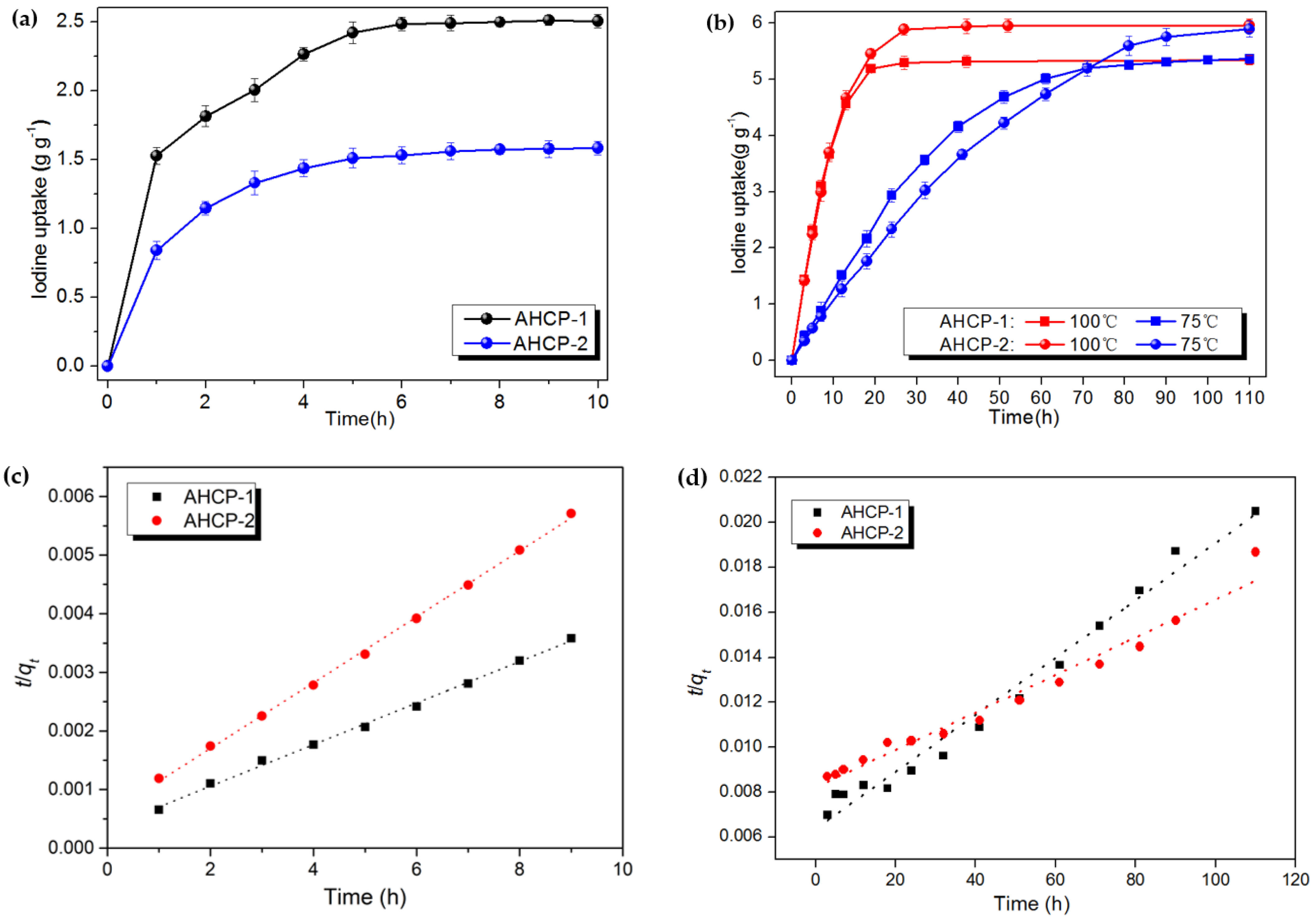
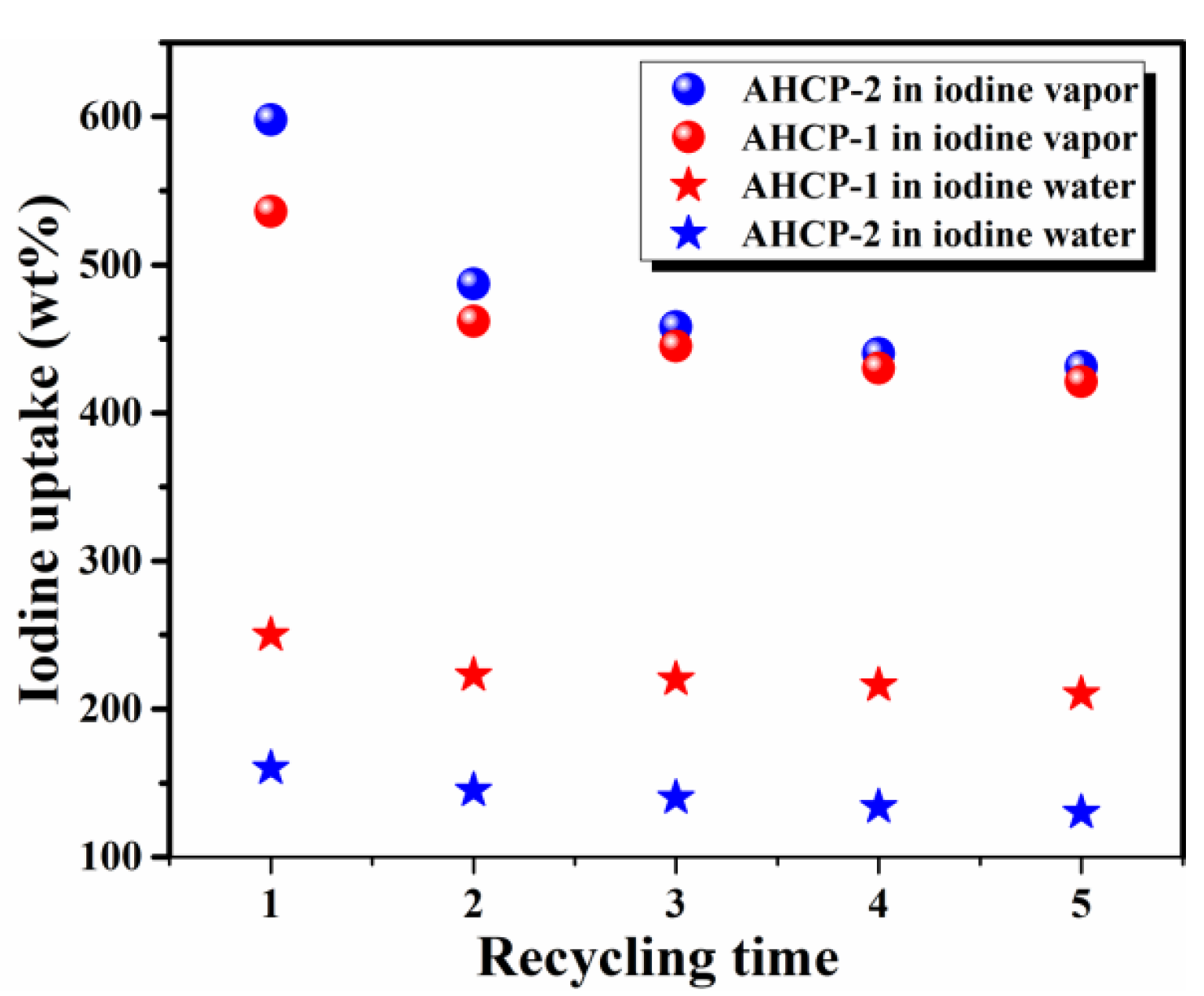
2.2. Iodine Adsorption
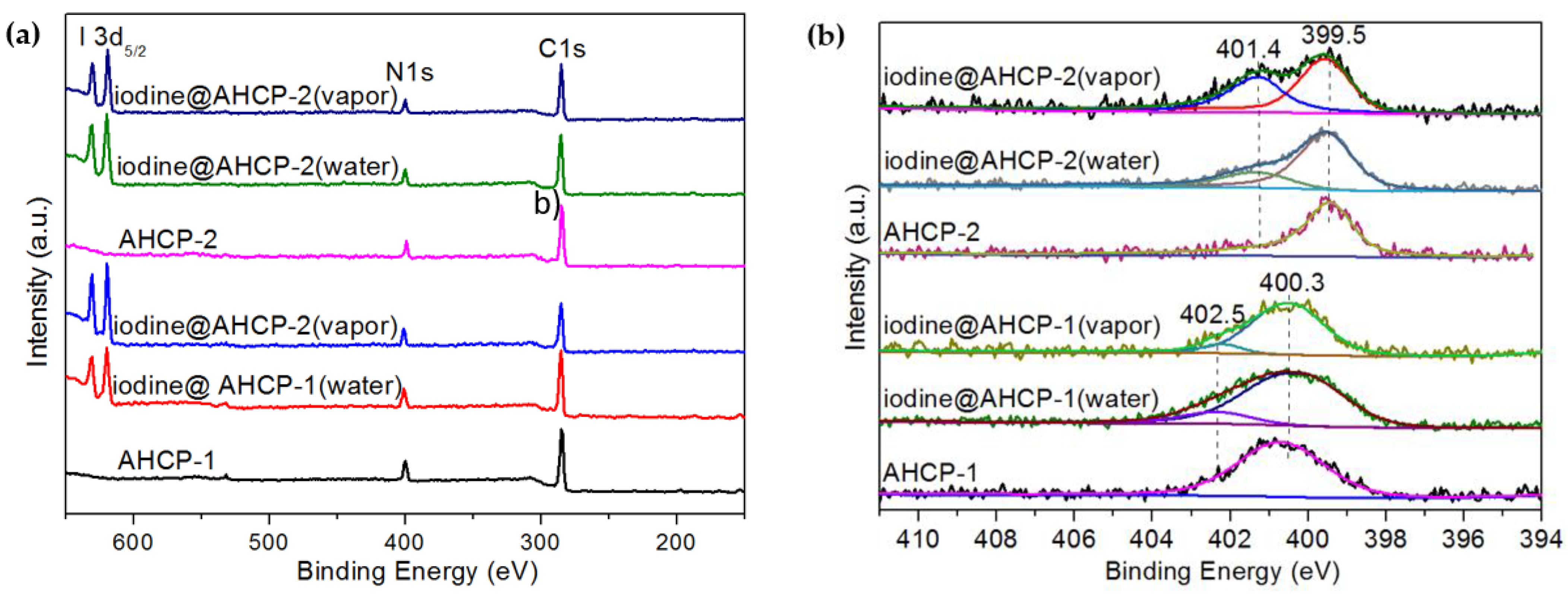
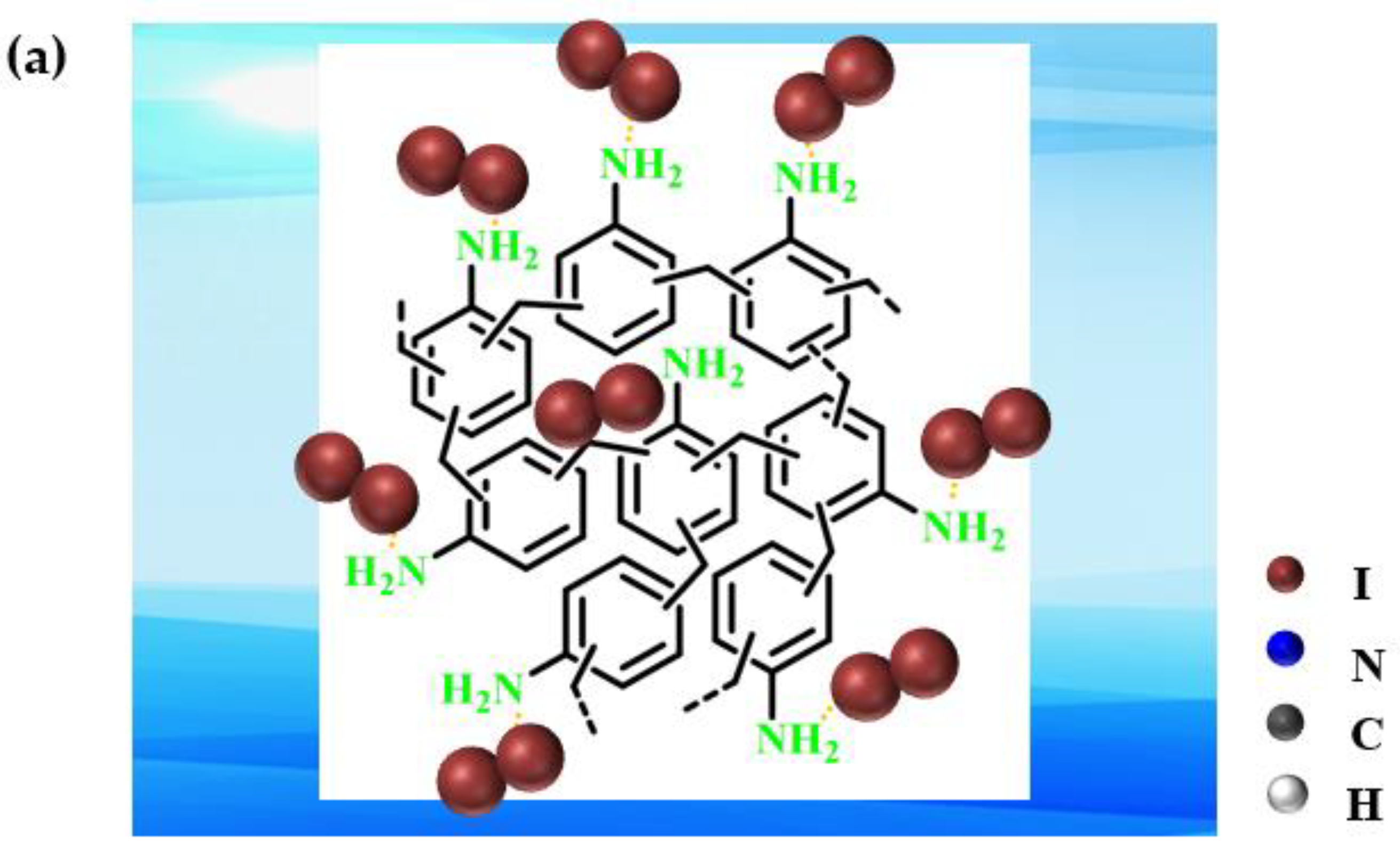
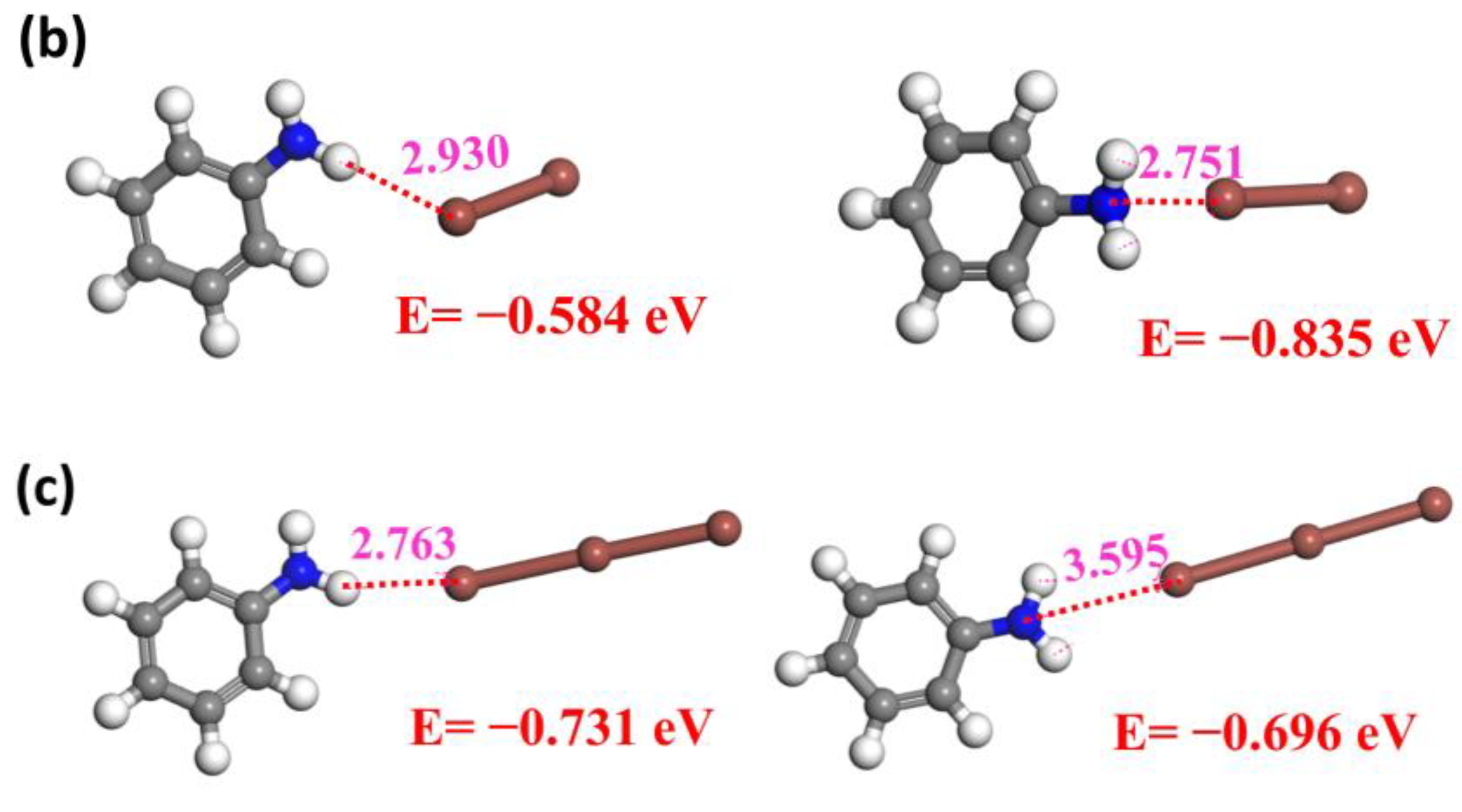
2.3. Mechanisms
3. Experimental Section
3.1. Preparation of AHCP-1
3.2. Preparation of AHCP-2
3.3. Structural Characterization
3.4. Iodine Adsorption
3.5. Iodine Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Z.J.; Chee, T.S.; Zhang, X.W.; Lei, L.C.; Xiao, C.L. Novel bismuth-based electrospinning materials for highly efficient capture of radioiodine. Chem. Eng. J. 2021, 412, 128687. [Google Scholar] [CrossRef]
- Ewing, R.C.; Von Hippel, F.N. Nuclear waste management in the United States-starting over. Science 2009, 325, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kanda, J. Tracking the Fukushima radionuclides. Science 2012, 336, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Kim, D.; Schweiger, M.J.; Kruger, A.A.; Thallapally, P.K. Removal of TcO4- ions from solution: Materials and future outlook. Chem. Soc. Rev. 2016, 45, 2724–2739. [Google Scholar] [CrossRef]
- Du, W.J.; Qin, Y.C.; Ni, C.L.; Dai, W.L.; Zou, J.P. Efficient capture of volatile iodine by thiophene-containing porous organic polymers. ACS Appl. Polym. Mater. 2020, 2, 5121–5128. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.R.; Xu, Y.H.; Jiang, D.L. Iodine capture in porous organic polymers and metal-organic frameworks materials. Mater. Horizons. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, L.; Zhao, Q.; Chen, G.Y.; Wang, Z.R.; Zhang, J.J.; Zhang, L.; Lei, J.H.; Duan, T. Novel synthesis of NaY-NH4F-Bi2S3 composite for enhancing iodine capture. Chem. Eng. J. 2022, 443, 69. [Google Scholar] [CrossRef]
- Wang, S.Y.; Luo, L.Y.; Li, Z.H.; Jin, C.X.; Wang, N.; Wang, D.X.; Wu, A.P.; Yan, H.J.; Wang, L.; Tian, C.G. Two-dimensional assembly made up of ZIF-8 particles for the high-efficient capture of the iodine and dyes. J. Hazard. Mater. 2022, 430, 128501. [Google Scholar] [CrossRef]
- Xiao, K.X.; Liu, H.; Li, Y.; Yang, G.Y.; Wang, Y.J.; Yao, H. Excellent performance of porous carbon from urea-assisted hydrochar of orange peel for toluene and iodine adsorption. Chem. Eng. J. 2020, 382, 122997. [Google Scholar] [CrossRef]
- Sun, H.X.; La, P.Q.; Yang, R.X.; Zhu, Z.Q.; Liang, W.D.; Yang, B.P.; Li, A.; Deng, W.Q. Innovative nanoporous carbons with ultrahigh uptakes for capture and reversible storage of CO2 and volatile iodine. J. Hazard. Mater. 2017, 321, 210–217. [Google Scholar] [CrossRef]
- Zhou, J.; Lan, T.; Li, T.C.; Chen, Q.; Bai, P.; Liu, F.; Yuan, Z.W.; Zheng, W.F.; Luo, X.L.; Yan, W.F.; et al. Highly efficient capture of iodine in spent fuel reprocessing off-gas by novelly porous copper-doped silica zeolites. Sep. Purif. Technol. 2022, 290, 120895. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Choi, D.Y.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energ. Environ. Sci. 2016, 9, 1050–1062. [Google Scholar] [CrossRef]
- Zhang, X.R.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.H.; Schroder, M. Adsorption of iodine in metal-organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Dong, X.L.; Wang, H.; Ma, D.X.; Tan, K.; Jensen, S.; Deibert, B.J.; Butler, J.; Cure, J.; Shi, Z.; et al. Capture of organic iodides from nuclear waste by metal-organic framework-based molecular traps. Nat. Commun. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, X.H.; Pang, M.B.; Dong, X.T.; Zhao, S.; Zhang, W. Iodine capture using Zr-based metal-organic frameworks (Zr-MOFs): Adsorption performance and mechanism. ACS Appl. Mater. Inter. 2020, 12, 20429–20439. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Pan, T.T.; Lei, Q.; Chen, C.L.; Dong, X.L.; Yuan, Y.Y.; Shen, J.; Cai, Y.C.; Zhou, C.H.; Pinnau, I.; et al. Ionic functionalization of multivariate covalent organic frameworks to achieve an exceptionally high iodine-capture capacity. Angew. Chem. Int. Edit. 2021, 60, 22432–22440. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.P.; Jiang, W.M.; Jiang, Q.H.; Jiang, D.L. Exceptional iodine capture in 2D covalent organic frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Pan, T.T.; Lei, Q.; Chen, C.L.; Dong, X.L.; Yuan, Y.Y.; Al Maksoud, W.; Zhao, L.; Cavallo, L.; Pinnau, I.; et al. Efficient and simultaneous capture of iodine and methyl iodide achieved by a covalent organic framework. Nat. Commun. 2022, 13, 2878. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, G.; Ma, J.T.; Jia, Q. Pyrrolidinone-based hypercrosslinked polymers for reversible capture of radioactive iodine. Sep. Purif. Technol. 2019, 210, 995–1000. [Google Scholar] [CrossRef]
- Xu, X.H.; Li, Y.X.; Zhou, L.; Liu, N.; Wu, Z.Q. Precise fabrication of porous polymer frameworks using rigid polyisocyanides as building blocks: From structural regulation to efficient iodine capture. Chem. Sci. 2022, 13, 1111–1118. [Google Scholar] [CrossRef]
- Dai, D.H.; Yang, J.; Zou, Y.C.; Wu, J.R.; Tan, L.L.; Wang, Y.; Li, B.; Lu, T.; Wang, B.; Yang, Y.W. Macrocyclic arenes-based conjugated macrocycle polymers for highly selective CO2 capture and iodine adsorption. Angew. Chem. Int. Edit. 2021, 60, 8967–8975. [Google Scholar] [CrossRef]
- Yan, Z.J.; Yuan, Y.; Tian, Y.Y.; Zhang, D.M.; Zhu, G.S. Highly efficient enrichment of volatile iodine by charged porous aromatic frameworks with three sorption sites. Angew. Chem. Int. Edit. 2015, 54, 12733–12737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, J.; Xiong, S.H.; Lu, P.G.; Tang, J.T.; He, J.Q.; Javaid, M.U.; Pan, C.Y.; Yu, G.P. Ferrocene-based porous organic polymers for high-affinity iodine capture. Chem. Eng. J. 2020, 380, 122420. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Hypercrosslinked polymers: A review. Polym. Rev. 2018, 58, 1–41. [Google Scholar] [CrossRef]
- Lau, C.H.; Lu, T.D.; Sun, S.P.; Chen, X.F.; Carta, M.; Dawson, D.M. Continuous flow knitting of a triptycene hypercrosslinked polymer. Chem. Commun. 2019, 55, 8571–8574. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Zhai, T.L.; Wang, Z.; Cheng, G.; Ma, H.; Zhang, Q.P.; Zhao, Y.H.; Tan, B.; Zhang, C. Hyperporous carbon from triptycene-based hypercrosslinked polymer for iodine capture. Adv. Mater. Interf. 2019, 6, 1900249. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, G.; Jia, Q. One-pot synthesis of viologen-based hypercrosslinked polymers for efficient volatile iodine capture. Micropor. Mesopor. Mat. 2019, 279, 186–192. [Google Scholar] [CrossRef]
- Xiong, S.H.; Tang, X.; Pan, C.Y.; Li, L.; Tang, J.T.; Yu, G.P. Carbazole-bearing porous organic polymers with a mulberry-like morphology for efficient iodine capture. ACS Appl. Mater. Inter. 2019, 11, 27335–27342. [Google Scholar] [CrossRef]
- Falaise, C.; Volkringer, C.; Facqueur, J.; Bousquet, T.; Gasnot, L.; Loiseau, T. Capture of iodine in highly stable metal-organic frameworks: A systematic study. Chem. Commun. 2013, 49, 10320–10322. [Google Scholar] [CrossRef]
- Reda, A.T.; Zhang, D.X.; Xu, X.Y. LiAlO2-Melamine for efficient and rapid iodine capture. J. Environ. Chem. Eng. 2022, 10, 107842. [Google Scholar] [CrossRef]
- Kobinata, S.; Nagakura, S. Dipole moments of charge-transfer complexes between iodine and some aliphatic amines. J. Am. Chem. Soc. 1966, 88, 3905–3909. [Google Scholar] [CrossRef]
- Dawson, R.; Stevens, L.A.; Drage, T.C.; Snape, C.E.; Smith, M.W.; Adams, D.J.; Cooper, A.I. Impact of water coadsorption for carbon dioxide capture in microporous polymer sorbents. J. Am. Chem. Soc. 2012, 134, 10741–10744. [Google Scholar] [CrossRef]
- Lee, J.S.M.; Briggs, M.E.; Hu, C.C.; Cooper, A.I. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 2018, 46, 277–289. [Google Scholar] [CrossRef]
- Babu, R.S.; Vinodh, R.; de Barros, A.L.F.; Samyn, L.M.; Prasanna, K.; Maier, M.A.; Alves, C.H.F.; Kim, H.J. Asymmetric supercapacitor based on carbon nanofibers as the anode and two-dimensional copper cobalt oxide nanosheets as the cathode. Chem. Eng. J. 2019, 366, 390–403. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhu, Z.M.; Zhang, W.Y.; Wang, Y. A nitrogen-rich fluorescent conjugated microporous polymer with triazine and triphenylamine units for high iodine capture and nitro aromatic compound detection. J. Mater. Chem. 2017, A5, 7612–7617. [Google Scholar] [CrossRef]
- Li, X.M.; Peng, Y.; Jia, Q. Construction of hypercrosslinked polymers with dual nitrogen-enriched building blocks for efficient iodine capture. Sep. Purif. Technol. 2020, 236, 116260. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhang, C.; Liu, M.; Hu, C.; Chen, G.F. Preparation of biimidazole-based porous organic polymers for ultrahigh iodine capture and formation of liquid complexes with iodide/polyiodide ions. J. Mater. Chem. A 2020, 8, 2820–2826. [Google Scholar] [CrossRef]
- Sigen, A.; Zhang, Y.W.; Li, Z.P.; Xia, H.; Xue, M.; Liu, X.M.; Mu, Y. Highly efficient and reversible iodine capture using a metalloporphyrin-based conjugated microporous polymer. Chem. Commun. 2014, 50, 8495–8498. [Google Scholar]
- Li, R.P.; Zhao, Y.F.; Chen, Y.; Liu, Z.M.; Han, B.X.; Li, Z.Y.; Wang, J.J. Imidazolate ionic liquids for high-capacity capture and reliable storage of iodine. Commun. Chem. 2018, 1, 69. [Google Scholar] [CrossRef]
- Guo, Z.X.; Sun, P.L.; Zhang, X.; Lin, J.B.; Shi, T.; Liu, S.F.; Sun, A.B.; Li, Z.B. Amorphous porous organic polymers based on schiff-base chemistry for highly efficient iodine capture. Chem.-Asian J. 2018, 13, 2046–2053. [Google Scholar] [CrossRef]
- Guo, X.H.; Tian, Y.; Zhang, M.C.; Li, Y.; Wen, R.; Li, X.; Li, X.F.; Xue, Y.; Ma, L.J.; Xia, C.Q.; et al. Mechanistic insight into hydrogen-bond-controlled crystallinity and adsorption property of covalent organic frameworks from flexible building blocks. Chem. Mater. 2018, 30, 2299–2308. [Google Scholar] [CrossRef]
- Behera, B.; Das, P.K. Blue- and red-shifting hydrogen bonding: A gas phase FTIR and Ab initio study of RR’ CO center dot center dot center dot DCCI3 and RR’ S center dot center dot center dot DCCI3 Complexes. J. Phys. Chem. A 2018, 122, 4481–4489. [Google Scholar] [CrossRef]
- Hughes, J.T.; Sava, D.F.; Nenoff, T.M.; Navrotsky, A. Thermochemical evidence for strong iodine chemisorption by ZIF-8. J. Am. Chem. Soc. 2013, 135, 16256–16259. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wang, S.X.; Hu, P.; Huang, L.Y.; Xue, C.; Yang, Z.J.; Zhou, X.T.; Wang, Y.Q.; Ji, H.B. Efficient selective removal of Pb(II) by using 6-aminothiouracil-modified Zr-based organic frameworks: From experiments to mechanisms. ACS Appl. Mater. Inter. 2020, 12, 7162–7178. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Narayanan Nair, A.K.; Sun, S.Y. Sorption and diffusion of methane and carbon dioxide in amorphous poly (alkyl acrylates): A molecular simulation study. J. Phys. Chem. B 2020, 124, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Mao, C.; Zhou, Z.; Wang, Q.; Zhou, X.; Liao, Z.; Deng, R.; Liu, D.; Beiyuan, J.; Lv, D.; et al. Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal. Int. J. Mol. Sci. 2023, 24, 370. https://doi.org/10.3390/ijms24010370
Liu B, Mao C, Zhou Z, Wang Q, Zhou X, Liao Z, Deng R, Liu D, Beiyuan J, Lv D, et al. Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal. International Journal of Molecular Sciences. 2023; 24(1):370. https://doi.org/10.3390/ijms24010370
Chicago/Turabian StyleLiu, Biying, Chaochao Mao, Zian Zhou, Qiannan Wang, Xiong Zhou, Zhijie Liao, Ran Deng, Defei Liu, Jingzi Beiyuan, Daofei Lv, and et al. 2023. "Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal" International Journal of Molecular Sciences 24, no. 1: 370. https://doi.org/10.3390/ijms24010370
APA StyleLiu, B., Mao, C., Zhou, Z., Wang, Q., Zhou, X., Liao, Z., Deng, R., Liu, D., Beiyuan, J., Lv, D., Li, J., Huang, L., Chen, X., & Yuan, W. (2023). Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal. International Journal of Molecular Sciences, 24(1), 370. https://doi.org/10.3390/ijms24010370






