Role of Adenylyl Cyclase Type 7 in Functions of BV-2 Microglia
Abstract
1. Introduction
2. Results
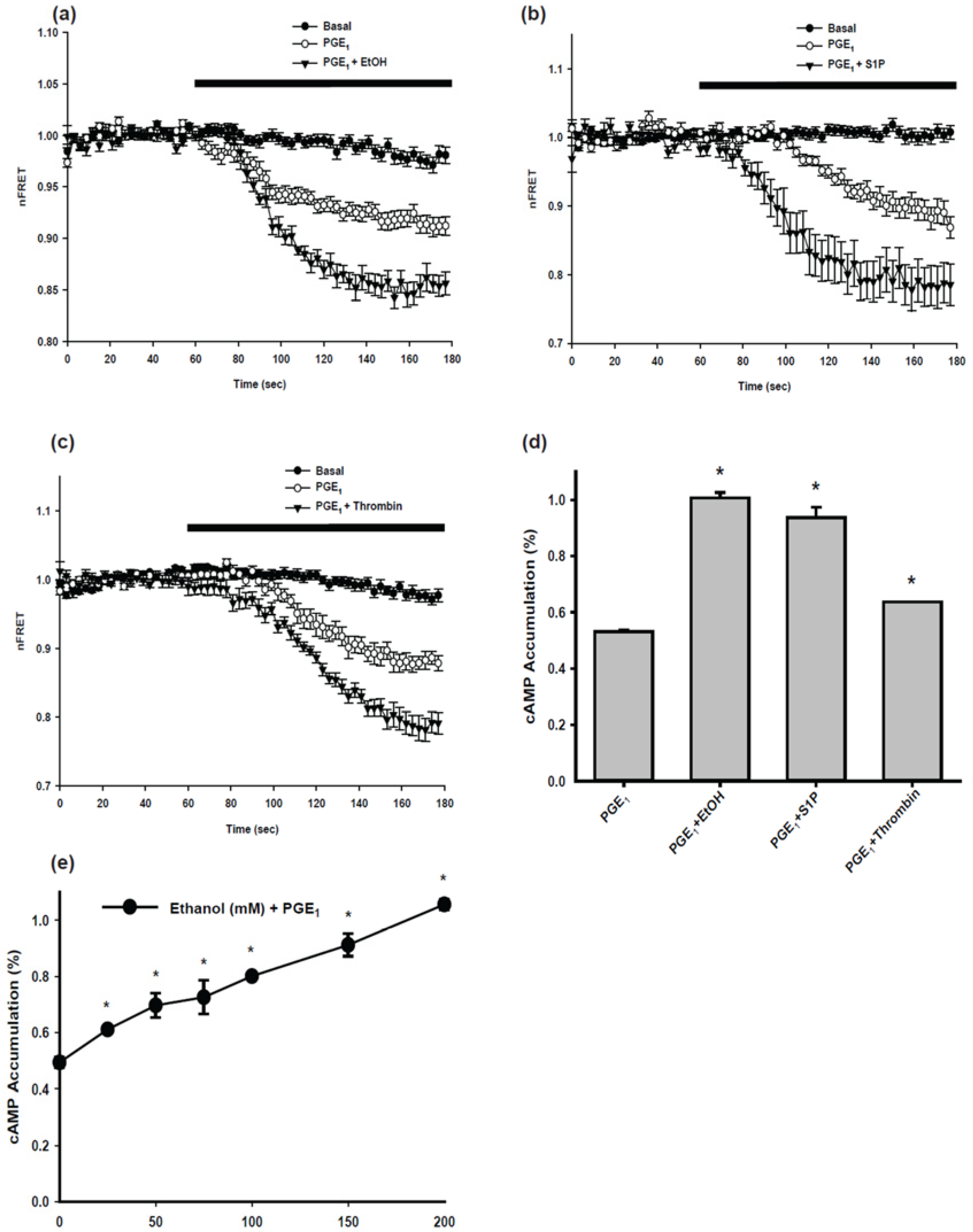
2.1. Cyclic AMP (cAMP) Signaling of Parental BV-2 Cells
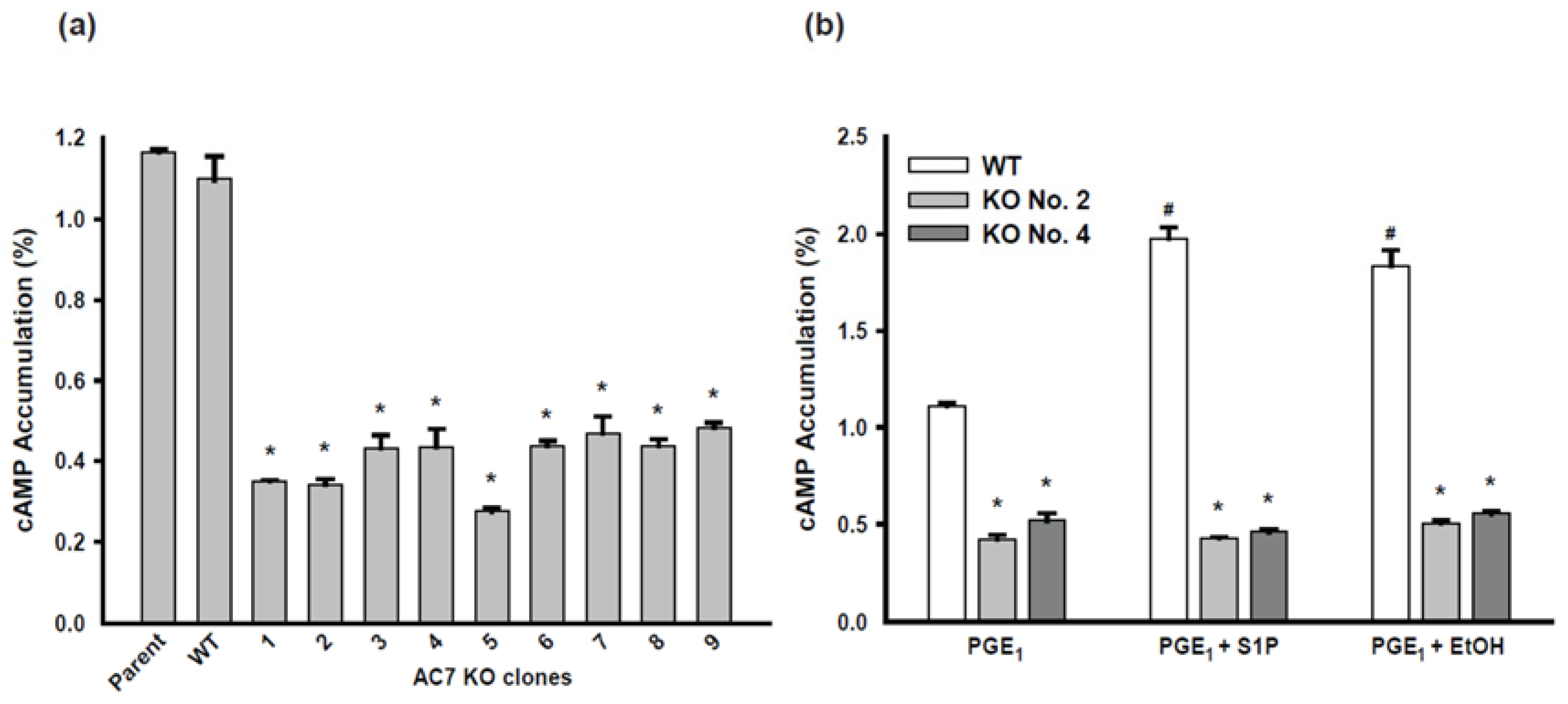
2.2. Isolation and Identification of AC7 KO Clones of BV-2 Cells
2.3. AC Activity of AC7 KO Clones of BV-2 Cells
2.4. Innate Immune Responses of AC7 KO Clones of BV-2 Cells
2.4.1. Phagocytic Activity of BV-2 Cells
2.4.2. Bacteria-Killing Activity of BV-2 Cells
2.4.3. Oxidative Burst Activity of BV-2 Cells
2.4.4. Classical Activation (M1) of BV-2 Cells
2.4.5. Alternative Activation (M2a) of BV-2 Cells
2.4.6. Regulatory Activation (M2b) of BV-2 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Transfection
4.3. Real-Time Assessment of Intracellular cAMP
4.4. cAMP Accumulation Assay
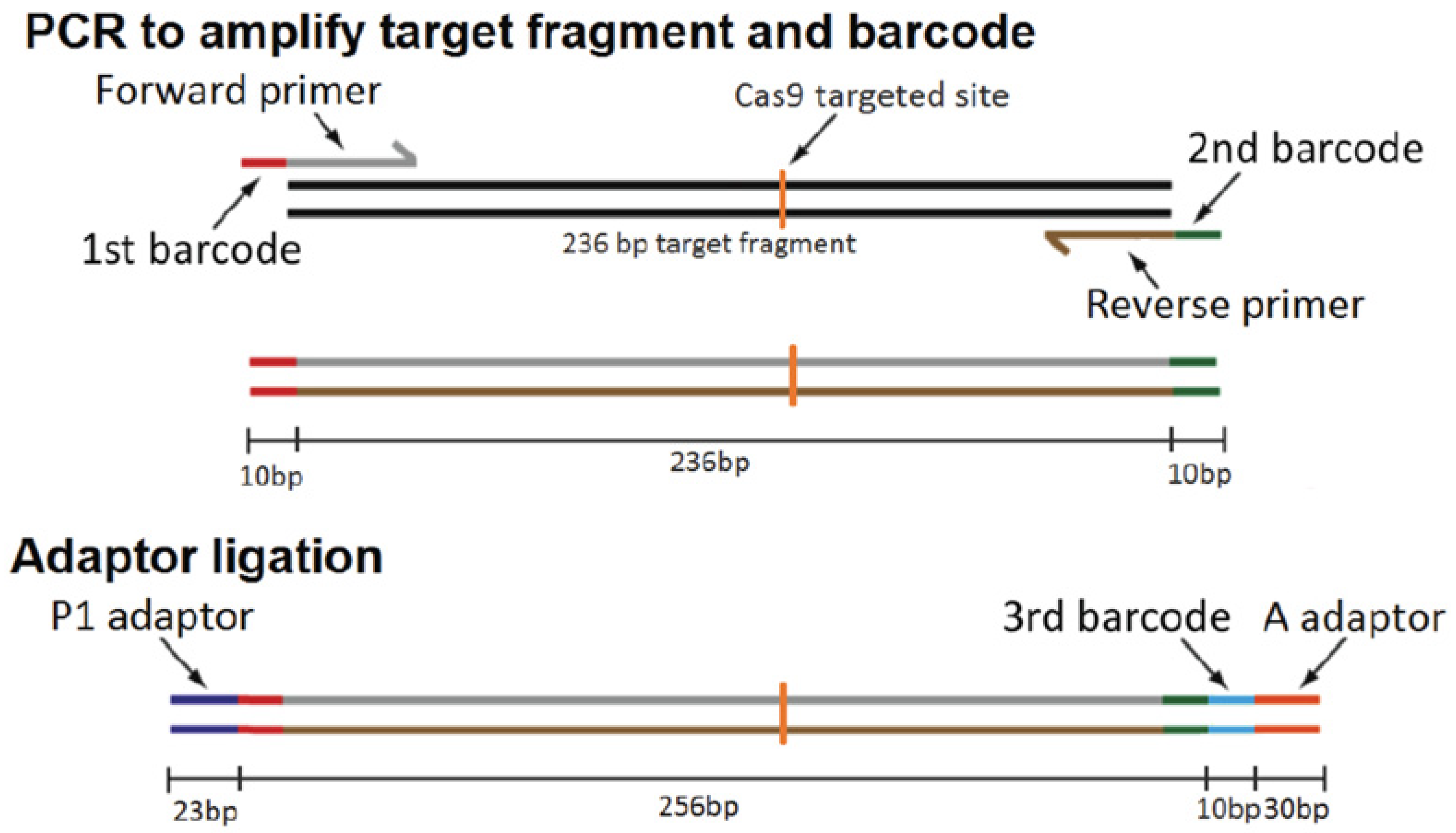
4.5. Isolation of Biallelic AC7 Knockout (KO) Clones of BV-2 Cells
Construction of CRISPR-Cas9 Screening Plasmids
4.6. Isolation of BV-2 Cells That Exhibit CRISPR-Cas9 Mediated Mutation
4.7. Next-Generation Sequencing (NGS) Analysis of BV-2 Cell Clones
4.8. Identification of BV-2 Cell Clones That Carry Biallelic AC7 KO Mutation
4.9. Authentication of BV-2 Cell Clones
4.10. Phagocytosis Assay
4.11. Bacterial Killing Assay
4.12. Oxidative Burst Assay
4.13. Microglia Activation
4.14. RNA Isolation, Reverse Transcription, and Quantitative PCR
4.15. Nitric Oxide (NO) Production
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, D.; Ero, C.; Markram, H. Cell Densities in the Mouse Brain: A Systematic Review. Front. Neuroanat. 2018, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Crews, F.T. Innate Immune Signaling and Alcohol Use Disorders. Handb. Exp. Pharmacol. 2018, 248, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. 2015, 37, 331–341, 344–351. [Google Scholar]
- Molina, P.E.; Katz, P.S.; Souza-Smith, F.; Ford, S.M.; Teng, S.X.; Dodd, T.Y.; Maxi, J.K.; Mayeux, J.P. Alcohol’s Burden on Immunity Following Burn, Hemorrhagic Shock, or Traumatic Brain Injury. Alcohol Res. 2015, 37, 263–278. [Google Scholar]
- Gonzalez-Reimers, E.; Santolaria-Fernandez, F.; Martin-Gonzalez, M.C.; Fernandez-Rodriguez, C.M.; Quintero-Platt, G. Alcoholism: A systemic proinflammatory condition. World J. Gastroenterol. 2014, 20, 14660–14671. [Google Scholar] [CrossRef]
- Henriques, J.F.; Portugal, C.C.; Canedo, T.; Relvas, J.B.; Summavielle, T.; Socodato, R. Microglia and alcohol meet at the crossroads: Microglia as critical modulators of alcohol neurotoxicity. Toxicol. Lett. 2018, 283, 21–31. [Google Scholar] [CrossRef]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Peters-Golden, M. Putting on the brakes: Cyclic AMP as a multipronged controller of macrophage function. Sci. Signal. 2009, 2, pe37. [Google Scholar] [CrossRef]
- Ghosh, M.; Xu, Y.; Pearse, D.D. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J. Neuroinflammation 2016, 13, 9. [Google Scholar] [CrossRef]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef]
- Jiang, L.I.; Wang, J.E.; Sternweis, P.C. Regions on Adenylyl Cyclase VII Required for Selective Regulation by the G13 Pathway. Mol. Pharmacol. 2013, 83, 587–593. [Google Scholar] [CrossRef]
- Sadana, R.; Dessauer, C.W. Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef]
- Duan, B.; Davis, R.; Sadat, E.L.; Collins, J.; Sternweis, P.C.; Yuan, D.; Jiang, L.I. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J. Immunol. 2010, 185, 335–344. [Google Scholar] [CrossRef]
- Yoshimura, M.; Tabakoff, B. Selective effects of ethanol on the generation of cAMP by particular members of the adenylyl cyclase family. Alcohol. Clin. Exp. Res. 1995, 19, 1435–1440. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Jiang, L.I.; Collins, J.; Davis, R.; Fraser, I.D.; Sternweis, P.C. Regulation of cAMP responses by the G12/13 pathway converges on adenylyl cyclase VII. J. Biol. Chem. 2008, 283, 23429–23439. [Google Scholar] [CrossRef]
- Jiang, L.I.; Collins, J.; Davis, R.; Lin, K.M.; DeCamp, D.; Roach, T.; Hsueh, R.; Rebres, R.A.; Ross, E.M.; Taussig, R.; et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J. Biol. Chem. 2007, 282, 10576–10584. [Google Scholar] [CrossRef]
- Mosser, D.M.; Gonçalves, R. Activation of murine macrophages. Curr. Protoc. Immunol. 2015, 111, 14.2.1–14.2.10. [Google Scholar] [CrossRef]
- Atwood, B.K.; Lopez, J.; Wager-Miller, J.; Mackie, K.; Straiker, A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genom. 2011, 12, 14. [Google Scholar] [CrossRef]
- Yoshimura, M.; Tabakoff, B. Ethanol’s actions on cAMP-mediated signaling in cells transfected with type VII adenylyl cyclase. Alcohol. Clin. Exp. Res. 1999, 23, 1457–1461. [Google Scholar] [CrossRef]
- Gupta, R.; Qualls-Creekmore, E.; Yoshimura, M. Real-time monitoring of intracellular cAMP during acute ethanol exposure. Alcohol. Clin. Exp. Res. 2013, 37, 1456–1465. [Google Scholar] [CrossRef]
- Hill, R.A.; Xu, W.; Yoshimura, M. Role of an adenylyl cyclase isoform in ethanol’s effect on cAMP regulated gene expression in NIH 3T3 cells. Biochem. Biophys. Rep. 2016, 8, 162–167. [Google Scholar] [CrossRef][Green Version]
- Steininger, T.S.; Stutz, H.; Kerschbaum, H.H. Beta-adrenergic stimulation suppresses phagocytosis via Epac activation in murine microglial cells. Brain Res. 2011, 1407, 1–12. [Google Scholar] [CrossRef]
- Ballinger, M.N.; Welliver, T.; Straight, S.; Peters-Golden, M.; Swanson, J.A. Transient increase in cyclic AMP localized to macrophage phagosomes. PLoS ONE 2010, 5, e13962. [Google Scholar] [CrossRef][Green Version]
- Colton, C.A.; Snell-Callanan, J.; Chernyshev, O.N. Ethanol induced changes in superoxide anion and nitric oxide in cultured microglia. Alcohol. Clin. Exp. Res. 1998, 22, 710–716. [Google Scholar] [CrossRef]
- Aroor, A.R.; Baker, R.C. Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol 1998, 15, 277–280. [Google Scholar] [CrossRef]
- Ramos, A.; Joshi, R.S.; Szabo, G. Innate immune activation: Parallels in alcohol use disorder and Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 910298. [Google Scholar] [CrossRef]
- Karavitis, J.; Kovacs, E.J. Macrophage phagocytosis: Effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J. Leukoc. Biol. 2011, 90, 1065–1078. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, J.; Son, E.; Mosa, A.; Cho, G.J.; Choi, W.S.; Ha, J.H.; Kim, I.K.; Lee, M.G.; Kim, C.Y.; et al. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J. Neuroimmunol. 2004, 156, 88–95. [Google Scholar] [CrossRef]
- Deng, X.S.; Deitrich, R.A. Ethanol metabolism and effects: Nitric oxide and its interaction. Curr. Clin. Pharmacol. 2007, 2, 145–153. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Nikolaev, V.O.; Bünemann, M.; Hein, L.; Hannawacker, A.; Lohse, M.J. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 2004, 279, 37215–37218. [Google Scholar] [CrossRef]
- Sorkin, A.; McClure, M.; Huang, F.; Carter, R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 2000, 10, 1395–1398. [Google Scholar] [CrossRef]
- Kou, J.; Yoshimura, M. Isoform-specific enhancement of adenylyl cyclase activity by n-alkanols. Alcohol. Clin. Exp. Res. 2007, 31, 1467–1472. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Kim, H.; Um, E.; Cho, S.R.; Jung, C.; Kim, H.; Kim, J.S. Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nat. Methods 2011, 8, 941–943. [Google Scholar] [CrossRef]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J.S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef]
- Heigwer, F.; Kerr, G.; Boutros, M. E-CRISP: Fast CRISPR target site identification. Nat. Methods 2014, 11, 122–123. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J.S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Pliatsika, V.; Rigoutsos, I. “Off-Spotter”: Very fast and exhaustive enumeration of genomic lookalikes for designing CRISPR/Cas guide RNAs. Biol. Direct 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Magor, G.W.; Gillinder, K.R.; Perkins, A.C. A high-throughput screening strategy for detecting CRISPR-Cas9 induced mutations using next-generation sequencing. BMC Genom. 2014, 15, 1002. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]



| Clone | gRNA Target Sequence | |
|---|---|---|
| Deletion/Insertion | TCTCCACACGAAGCTTCCGACGG | |
| 1 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| 2 | −16 base | T----------------CGACGG |
| −1 base | TCTCCACACGAAGCTT-CGACGG | |
| 3 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| 4 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| +1 base | TCTCCACACGAAGCTTCGCGACGG | |
| 5 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| 6 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| −2 base | TCTCCACACGAAGCT--CGACGG | |
| 7 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| 8 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| 9 | −1 base | TCTCCACACGAAGCTT-CGACGG |
| Forward (3′-5′) | Reverse (3′-5′) | |
|---|---|---|
| IL-10 | ATAACTGCACCCACTTCCCA | GGGCATCACTTCTACCAGGT |
| iNOS | CAGAGGACCCAGAGACAAGC | CAGCCTGGCCAGATGTTCCT |
| Arginase I | GGAATCTGCATGGGCAACCT | GGTCTACGTCTCGCAAGCCA |
| β-actin | CCTTCTACAATGAGCTGCGTGT | CTGGATGGCTACGTACATGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Hill, R.A.; Yoshimura, M. Role of Adenylyl Cyclase Type 7 in Functions of BV-2 Microglia. Int. J. Mol. Sci. 2023, 24, 347. https://doi.org/10.3390/ijms24010347
Hu Y, Hill RA, Yoshimura M. Role of Adenylyl Cyclase Type 7 in Functions of BV-2 Microglia. International Journal of Molecular Sciences. 2023; 24(1):347. https://doi.org/10.3390/ijms24010347
Chicago/Turabian StyleHu, Yawen, Rebecca A. Hill, and Masami Yoshimura. 2023. "Role of Adenylyl Cyclase Type 7 in Functions of BV-2 Microglia" International Journal of Molecular Sciences 24, no. 1: 347. https://doi.org/10.3390/ijms24010347
APA StyleHu, Y., Hill, R. A., & Yoshimura, M. (2023). Role of Adenylyl Cyclase Type 7 in Functions of BV-2 Microglia. International Journal of Molecular Sciences, 24(1), 347. https://doi.org/10.3390/ijms24010347






