Natural Killer Cell Receptors and Endometriosis: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction and Quality Assessment
- Identification of the study—title, authors, year of publication, journal title, country of origin, study design, number of participants, and recruitment procedure and duration.
- Participant characteristics—sample size, age, BMI, parity, rAFS classification of endometriosis [23], previous treatments, and preoperative painful symptoms scores—of subjects in endometriosis and control groups.
- Methodological features—sample characteristics (endometriotic tissue, peritoneal fluid, peripheral venous blood, and eutopic endometrium), methodology used for NK cell marker characterization (51Cr cytotoxicity assay, ELISA, flow cytometry, immunohistochemistry, in situ hybridization, RNA extraction, and quantitative real-time Western blotting) and NK cell markers.
- Outcomes—comparison of NK cell markers between the different samples studied.
2.4. Data Synthesis
3. Results
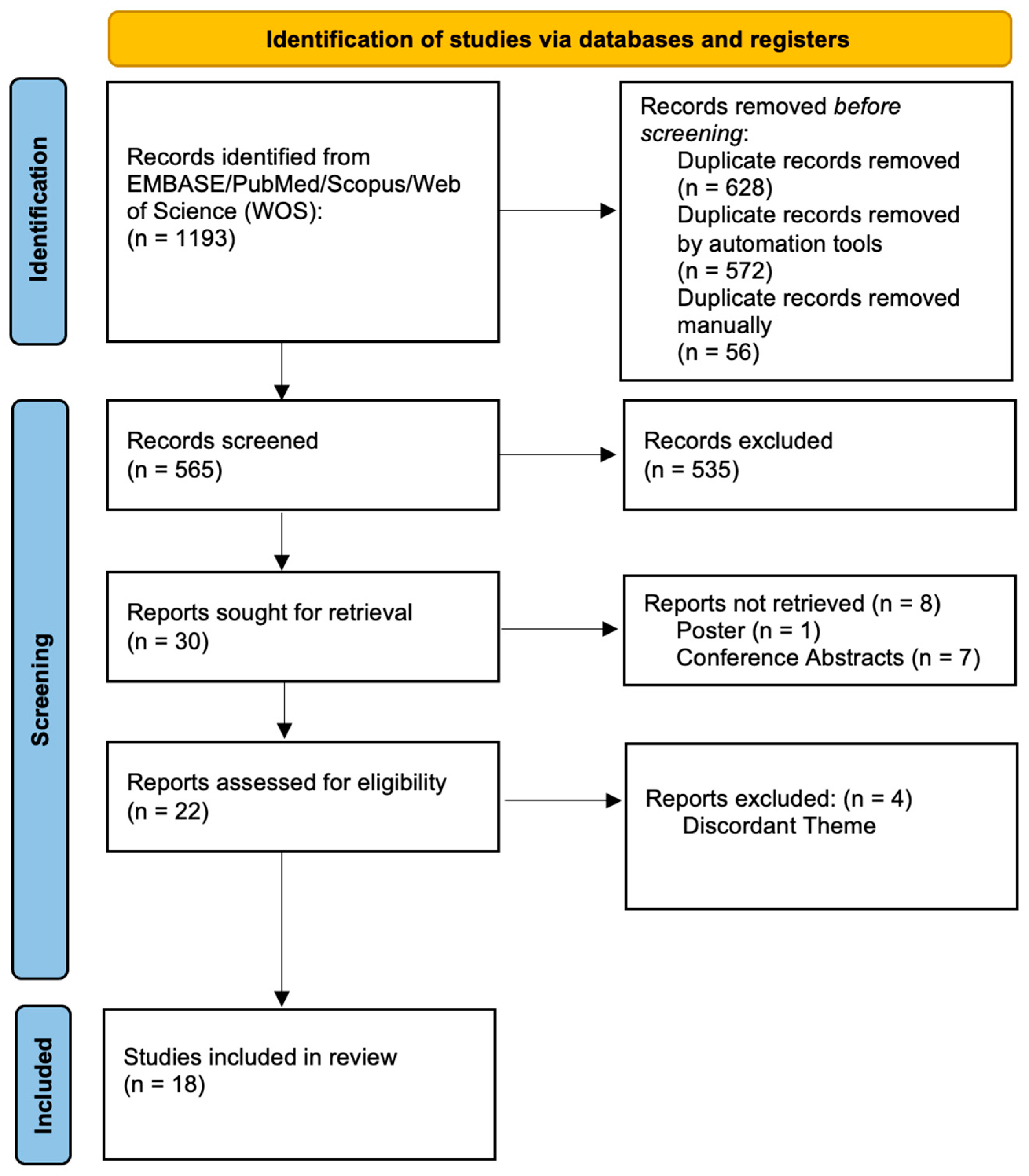
3.1. Study Selection
3.2. Study Quality Assessment and Risk of Bias
3.3. Heterogenicity of the Studies Assessed
| Samples | Design | n | Comparison | Methods | Markers | Results | Reference |
|---|---|---|---|---|---|---|---|
| PF | Case–control | 11 I/II EDT; 22 III/IV EDT; 11 controls | NK cytotoxicity and NK inhibition receptors in early and late EDT | FC cytotoxicity assay 51Cr | FITC anti-CD45/PE-anti-CD14 γ1 FITC/γ2a PE, FITC-anti-CD3/PE-anti-CD19, FITC-anti-CD3/PE-anti-CD56, FITC-anti-CD3/PE-anti-NKB1/PerCP-anti-CD56, FITC-anti-CD3/PE-anti-GL183/PerCP-anti-CD56, FITC-anti-CD3/PE-anti-EB6/PerCP-anti-CD56. | peritoneal cytotoxicity against K562 EDT (I/II/III/IV) vs. controls; KIR expression (NKB1, EB6) in III/IV EDT vs. controls; KIR expression (NKB1, EB6) in III/IV EDT vs. I/II EDT | Wu et al., 2000 [5] |
| PF | Case–control | 10 EDT; 10 controls | HLA-G inhibitory ligand expression in women with and without EDT | Western blotting | mAb aa 61-83 1 domain of HLA-G | No statistical significance between groups | Hornung et al., 2001 [6] |
| PB, PF | Case–control | 11 I/II EDT; 17 III/IV EDT; 6 controls | ICAM-1 and KIR expression in women with and without EDT | FC Western blotting | FITC-labeled anti-CD3 mAb, anti-CD4 mAb, PE-labeled anti-CD8 mAb, PE-labeled anti-CD19 mAb, FITC-labeled anti-CD16 mAb, FITC-labeled anti-CD14 mAb, PE-labeled anti-CD54 (ICAM-1) mAb, PE-labeled anti-CD158a, anti-CD158b, and CD94 | ICAM in PF macrophages EDT vs. controls; KIR2DL1+ NK among CD16+ NK in PB and PF of EDT vs. controls (more pronounced in III/IV EDT) | Maeda, Izumiya, Oguri et al., 2002 [7] |
| PB, PF | Case–control | 12 I/II EDT; 30 III/IV; 40 controls | KIR2DL1+ NK cell expression in women with and without EDT | FC Western blotting | FITC-anti-CD16 mAb PE-labeled anti-CD158a mAb, anti-CD158b mAb, PE anti-CD94 mAb | KIR2DL1+ NK cells in PB and PF of EDT vs. controls (more pronounced in III/IV EDT) | Maeda, Izumiya, Yamamoto et al., 2002 [8] |
| PB, PF | Case–control | 18 I/II EDT; 70 III/IV EDT; 104 controls | CD158a+ cells (KIR subtype) expression in women with and without EDT | FC Western blotting | FITC-labeled anti-CD16 mAB, PE-labeled anti-CD158a and anti-CD158b mAbs, PE-labeled anti-CD94 mAbs | CD158a+ cells in PF of EDT (I/II/III/IV and III/IV) vs. controls; CD158a+ cells in PB of EDT (I/II and III/IV) | Maeda et al., 2004 [9] |
| PB, PF | Case–control | 6 I/II EDT; 18 III/IV; 25 controls | ITIM and ITAM KIR expression in women with and without EDT | FC Western blotting | FITC-labeled anti-CD56 mAb PE-labeled anti-CD158a mAb, anti-CD158b mAb, Anti-CD158a mAb, anti-CD158b mAb | ITIM-KIR > ITAM-KIR in PB EDT; CD158a+CD56+ NK cells in PB and PF EDT vs. controls | Matsuoka et al., 2005 [10] |
| Endometrium Peritoneal EDT | Case–control | 15 EDT and 12 controls (IHC); 24 endometrium EDT and 14 peritoneal fluid EDT and 17 controls (RNA ISH) | HLA-G expression in eutopic and ectopic endometrium in women with and without EDT | IHC RNA ISH | mAb4H84 cDNA probe | HLA-G protein and gene transcripts found in >90% glandular peritoneal fluid EDT but not in stromal endometrial epithelium (controls and EDT) | Barrier et al., 2006 [12] |
| PF | Case–control | 26 I/II EDT; 20 III/IV EDT; 24 controls | CD56+ cell expression, Fas antigen CD95 and early activation molecule CD69 in PF of women with and without EDT | FC | Anti-CD45FITC/CD14PE, IgG1FITC/IgG2aPE, anti-CD69FITC, anti-95FITC and anti-CD56PE | CD56+ dim expression in I/II EDT vs. controls; CD95 in I/II EDT vs. controls; CD69+CD56+ in I/II EDT vs. controls; CD69+CD56+ in III/IV EDT vs. controls | Eidukaite et al., 2006 [11] |
| PF | Case–control | 17 I/II EDT; 14 III/IV EDT; 27 controls | HLA-G expression in PF of women with and without EDT | ELISA | Anti-sHLA-G | No statistical significance between groups | Eidukaite and Tamosiunas, 2008 [13] |
| PB, PF, Endometrium Peritoneal EDT | Case–control | 20 III/IV EDT; 13 controls | (HLA)-E receptor CD94/NKG2A expression in women with and without EDT | FC, RT-PCR | Anti-CD56 (clone C218), anti-NKG2A (clone Z199), PE-conjugated anti-NKG2A, PE-conjugated anti-NKG2C, fluorescein-conjugated anti-CD56, peridin chlorophyll protein-conjugated anti-CD3, anti-CD45, cytokeratin 20 | CD94/NKG2A cells in PF in EDT vs. controls; HLA-E mRNA in peritoneal EDT | Galandrini et al., 2008 [14] |
| Endometrium | Case–control | 15 EDT; 15 controls | HLA-I and HLA-II expression in women with and without EDT | IHC | IgG2a (HLA-I), IgG1 (HLA-II) | HLA-I and HLA-II expression in EDT endometrial stroma and glands vs. controls | Baka et al., 2011 [15] |
| PF | Case–control | 3 I/II EDT; 18 III/IV EDT; 28 controls | Expression of NK Cell surface antigens (CD16 and CD56+ cells), NCRs (NKp46/40/30) and cytokine production (TNF-, IFN- etc.) of PF NK cells in women with and without EDT | FC | Anti-CD45 PerCP-Cy5.5/anti-CD56 PE/anti-CD16 fluorescein isothiocyanate, anti-CD45 PerCP-Cy5.5/anti-CD56 FITC/anti-CD335 (NKp46) PE, anti-CD45 PerCP-Cy5.5/anti-CD56 FITC/anti-CD336 (NKp44) PE, and anti-CD45 PerCP-Cy5.5/anti-CD56 FITC/anti-CD337 (NKp30) PE | NKp46+ NK cells in III/IV EDT vs. controls; CD56dim/NKp46+ cells in III/IV EDT vs. controls; TNF- producing NK cells in III/IV EDT vs. controls; IFN- producing NK cells in III/IV EDT vs. controls | Funamizu et al., 2014 [16] |
| PF | Case–control | 121 EDT; 81 controls | Levels of soluble NKG2D ligands (MICA, MICB and ULBP-2) in women with and without EDT | ELISA | ELISA (R&D Systems, Inc., Minneapolis, MN, USA) | MICA in EDT vs. controls; MICB in EDT vs. controls; MICA, MICB, ULBP-2 in deep infiltrating EDT | González-Foruria et al., 2015 [17] |
| Serum, PF, Endometrium EDT | Cross-sectional observational/Case–control | 60 I/II EDT; 83 III/IV EDT; 77 controls (ELISA) 26 EDT; 22 controls (IHC) | Soluble HLA-G expression in endometrium, EDT, PF, and serum in women with and without EDT | ELISA IHC | mAb 4H84 MEM-G/9 mouse mAb | sHLA-G in serum but not PF of III/IV EDT vs. controls; HLA-G protein expression in EDT but not eutopic endometrium | Rached et al., 2019 [18] |
| Endometrium EDT PB | Case–control Quasi-experimental | 15 EDT; 15 controls | PD-1/PDL-1 expression in women with and without EDT, post and prior estrogen and cytokine treatment | IHC FC Western blotting | Rabbit anti-human PD-1, CD4, CD8, PD-L1. anti-GAPDH CD279 (PD-1)-PE, CD274 (B7-H1)-PE, CD8-FITC, and CD4-PerCP-Cyanine5.5 | PD-1/PDL-1 and PD-1 in eutopic and ectopic endometrium EDT vs. controls; PD-1/PDL-1 and PD-1 in PB EDT vs. controls; PD-1/PDL-1 and PD-1 in eutopic endometrium EDT post 17-estradiol treatment vs. controls | Wu et al., 2019 [24] |
| Endometrium EDT PF | Case–control | 20 EDT; 13 controls | NKG2D expression and its ligands in women with and without EDT | FC RT-PCR Western blotting | FITC-conjugated mouse, anti-human CD16 mAb, PE-Cy5-conjugated mouse, anti-human CD56 mAb PE-mouse, anti-human NKp30 mAb, PE-mouse, anti-human NKp44 mAb, PE-mouse, anti-human NKp46 mAb, PE-mouse, and anti-human NKG2D mAb | NKp30 CD56+ in PF in EDT; NKp46 CD16+ in PF in EDT; NKG2D CD56+ in PF in EDT; ULBP-2 in eutopic endometrium in EDT vs. controls; ULBP-3 in ectopic endometrium in EDT vs. controls and eutopic EDT | Xu, 2019 [19] |
| PB | Case–control | 147 EDT; 117 controls | HLA-C and KIR polymorphism combinations in women with and without EDT | PCR | Commercial kits (HLAssure SE kit, Accutype Software, SSO typing kit) | HLA-C*03:03:01 occurrence in EDT vs. controls; KIR centromeric A/A haplotypes in EDT vs. controls; KIR2DS2-positive individuals in EDT vs. controls | Chou et al., 2020 [20] |
| PF | Case-control | 32 EDT; 30 controls | NKp46 co-expression patterns in women with and without EDT | FC | anti-TNF-α-BV421, anti-IFN-γ-PE- Cy7, anti-IL-4-PerCP-Cy5, anti-IL-10- APC, anti-TGF-β-PE | CD56+/NKp46+ in PF in EDT; NKG2C+/NKp46+ in PF in EDT; CD16+/NKp46+ in PF in EDT; NKG2D+/NKp46+ in PF in EDT No significant co-expression of inhibitory receptors (CD158a and NKG2A) and NKp46 IFN-γ-producing NK cells in PF in EDT | Saeki et al., 2022 [25] |
3.4. NK Cell Receptors in Peritoneal Fluid
3.5. NK Cell Receptors in Endometrium and Endometriotic Lesions
3.6. NK Cell Receptors in Peripheral Blood and Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafrir, A.; Farland, L.; Shah, D.; Harris, H.; Kvaskoff, M.; Zondervan, K.; Missmer, S. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110. [Google Scholar] [PubMed]
- Riccio, L.D.G.C.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yang, J.-H.; Chao, K.-H.; Hwang, J.-L.; Yang, Y.-S.; Ho, H.-N. Increase in the expression of killer cell inhibitory receptors on peritoneal natural killer cells in women with endometriosis. Fertil. Steril. 2000, 74, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Hornung, D.; Fujii, E.; Lim, K.-H.; Vigne, J.-L.; McMaster, M.T.; Taylor, R.N. Histocompatibility leukocyte antigen-G is not expressed by endometriosis or endometrial tissue. Fertil. Steril. 2001, 75, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Izumiya, C.; Oguri, H.; Kusume, T.; Yamamoto, Y.; Fukaya, T. Aberrant expression of intercellular adhesion molecule-1 and killer inhibitory receptors induces immune tolerance in women with pelvic endometriosis. Fertil. Steril. 2002, 77, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Izumiya, C.; Yamamoto, Y.; Oguri, H.; Kusume, T.; Fukaya, T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil. Steril. 2002, 77, 297–302. [Google Scholar] [CrossRef]
- Maeda, N.; Izumiya, C.; Kusum, T.; Masumoto, T.; Yamashita, C.; Yamamoto, Y.; Oguri, H.; Fukaya, T. Killer Inhibitory Receptor CD158a Overexpression Among Natural Killer Cells in Women With Endometriosis is Undiminished by Laparoscopic Surgery and Gonadotropin Releasing Hormone Agonist Treatment*. Am. J. Reprod. Immunol. 2004, 51, 364–372. [Google Scholar] [CrossRef]
- Matsuoka, S.; Maeda, N.; Izumiya, C.; Yamashita, C.; Nishimori, Y.; Fukaya, T. Expression of Inhibitory-Motif Killer Immunoglobulin-Like Receptor, KIR2DL1, is Increased in Natural Killer Cells from Women with Pelvic Endometriosis. Am. J. Reprod. Immunol. 2005, 53, 249–254. [Google Scholar] [CrossRef]
- Eidukaite, A.; Siaurys, A.; Tamosiunas, V. Aberrant Expression of CD95 and CD69 Molecules among CD56+ Cells in Women with Endometriosis. Am. J. Reprod. Immunol. 2006, 55, 276–281. [Google Scholar] [CrossRef]
- Barrier, B.; Kendall, B.; Ryan, C.; Sharpe-Timms, K. HLA-G is expressed by the glandular epithelium of peritoneal endometriosis but not in eutopic endometrium. Hum. Reprod. 2005, 21, 864–869. [Google Scholar] [CrossRef]
- Eidukaite, A.; Tamosiunas, V. Soluble HLA-G in the peritoneal fluid of women with endometriosis. Fertil. Steril. 2008, 89, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Galandrini, R.; Porpora, M.G.; Stoppacciaro, A.; Micucci, F.; Capuano, C.; Tassi, I.; Di Felice, A.; Benedetti-Panici, P.; Santoni, A. Increased frequency of human leukocyte antigen-E inhibitory receptor CD94/NKG2A-expressing peritoneal natural killer cells in patients with endometriosis. Fertil. Steril. 2008, 89, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Baka, S.; Frangou-Plemenou, M.; Panagiotopoulou, E.; Makrakis, E.; Kaltsakas, G.; Hassiakos, D.; Kondi-Pafiti, A. The expression of human leukocyte antigens class I and II in women with endometriosis or adenomyosis. Gynecol. Endocrinol. 2010, 27, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, A.; Fukui, A.; Kamoi, M.; Fuchinoue, K.; Yokota, M.; Fukuhara, R.; Mizunuma, H. Expression of Natural Cytotoxicity Receptors on Peritoneal Fluid Natural Killer Cell and Cytokine Production by Peritoneal Fluid Natural Killer Cell in Women with Endometriosis. Am. J. Reprod. Immunol. 2014, 71, 359–367. [Google Scholar] [CrossRef] [PubMed]
- González-Foruria, I.; Santulli, P.; Chouzenoux, S.; Carmona, F.; Batteux, F.; Chapron, C. Soluble Ligands for the NKG2D Receptor Are Released during Endometriosis and Correlate with Disease Severity. PLoS ONE 2015, 10, e0119961. [Google Scholar] [CrossRef]
- Rached, M.R.; Coelho, V.; Marin, M.L.C.; Pincerato, K.; Fujita, A.; Kalil, J.E.; Abrão, M.S. HLA-G is upregulated in advanced endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 235, 36–41. [Google Scholar] [CrossRef]
- Xu, H. Expressions of natural cytotoxicity receptor, NKG2D and NKG2D ligands in endometriosis. J. Reprod. Immunol. 2019, 136, 102615. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Chen, C.-H.; Chen, M.-J.; Chang, C.-W.; Chen, P.-H.; Yu, M.-H.; Chen, Y.-J.; Tsai, E.-M.; Yang, P.-S.; Lin, S.-Y.; et al. Killer cell immunoglobulin-like receptors (KIR) and human leukocyte antigen-C (HLA-C) allorecognition patterns in women with endometriosis. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Andrews, W.C.; Buttram, V.C., Jr.; Weed, J.C.; Hammond, C.B.; Thomas, H.H.; Behrman, S.J. Revised American Fertility Society classification of endometriosis. Fertil. Steril. 1985, 43, 351–352. [Google Scholar]
- Wu, L.; Lv, C.; Su, Y.; Li, C.; Zhang, H.; Zhao, X.; Li, M. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol. Endocrinol. 2018, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Saeki, S.; Fukui, A.; Mai, C.; Takeyama, R.; Yamaya, A.; Shibahara, H. Co-expression of activating and inhibitory receptors on peritoneal fluid NK cells in women with endometriosis. J. Reprod. Immunol. 2022, 155, 103765. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef]
- Fukui, A.; Mai, C.; Saeki, S.; Yamamoto, M.; Takeyama, R.; Kato, T.; Ukita, Y.; Wakimoto, Y.; Yamaya, A.; Shibahara, H. Pelvic endometriosis and natural killer cell immunity. Am. J. Reprod. Immunol. 2021, 85, e13342. [Google Scholar] [CrossRef]
- Brooks, A.G.; Boyington, J.C.; Sun, P.D. Natural killer cell recognition of HLA class I molecules. Rev. Immunogenet. 2000, 2, 433–448. [Google Scholar]
- Ibrahim, E.C.; Morange, M.; Dausset, J.; Carosella, E.D.; Paul, P. Heat shock and arsenite induce expression of the nonclassical class I histocompatibility HLA-G gene in tumor cell lines. Cell Stress Chaperon. 2000, 5, 207–218. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Rusak, M.; Eljaszewicz, A.; Bołkun, Ł.; Łuksza, E.; Łapuć, I.; Piszcz, J.; Singh, P.; Dąbrowska, M.; Bodzenta-Łukaszyk, A.; Kłoczko, J.; et al. Prognostic significance of PD-1 expression on peripheral blood CD4+ T cells in patients with newly diagnosed chronic lymphocytic leukemia. Pol. Arch. Intern. Med. 2015, 125, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, H.Y.; Zhao, X.X.; Chen, J.N.; Zhang, Y.W.; Huang, Y.; Xue, L.; Li, H.G.; Du, H.; Wu, X.Y.; et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Hum. Pathol. 2016, 53, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Coudert, J.D.; Scarpellino, L.; Gros, F.; Vivier, E.; Held, W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 2008, 111, 3571–3578. [Google Scholar] [CrossRef]
- Evert, J.H.-V.; Paap, R.; Nap, A.; van der Molen, R. The Promises of Natural Killer Cell Therapy in Endometriosis. Int. J. Mol. Sci. 2022, 23, 5539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, J.L.; Rosa, N.N.; Ângelo-Dias, M.; Martins, C.; Borrego, L.M.; Lima, J. Natural Killer Cell Receptors and Endometriosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 331. https://doi.org/10.3390/ijms24010331
Reis JL, Rosa NN, Ângelo-Dias M, Martins C, Borrego LM, Lima J. Natural Killer Cell Receptors and Endometriosis: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(1):331. https://doi.org/10.3390/ijms24010331
Chicago/Turabian StyleReis, José Lourenço, Natacha Nurdine Rosa, Miguel Ângelo-Dias, Catarina Martins, Luís Miguel Borrego, and Jorge Lima. 2023. "Natural Killer Cell Receptors and Endometriosis: A Systematic Review" International Journal of Molecular Sciences 24, no. 1: 331. https://doi.org/10.3390/ijms24010331
APA StyleReis, J. L., Rosa, N. N., Ângelo-Dias, M., Martins, C., Borrego, L. M., & Lima, J. (2023). Natural Killer Cell Receptors and Endometriosis: A Systematic Review. International Journal of Molecular Sciences, 24(1), 331. https://doi.org/10.3390/ijms24010331






