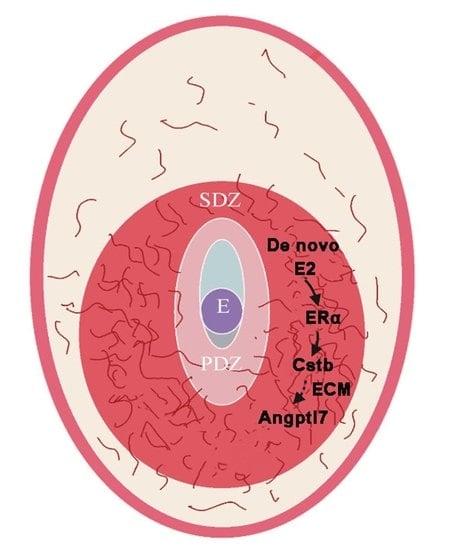
Expression and Regulation of a Novel Decidual Cells-Derived Estrogen Target during Decidualization
Abstract
1. Introduction
2. Results
2.1. Cstb Expression in Mouse Uterus during Early Pregnancy
2.2. Regulatory Role of Estrogen on Cstb Expression
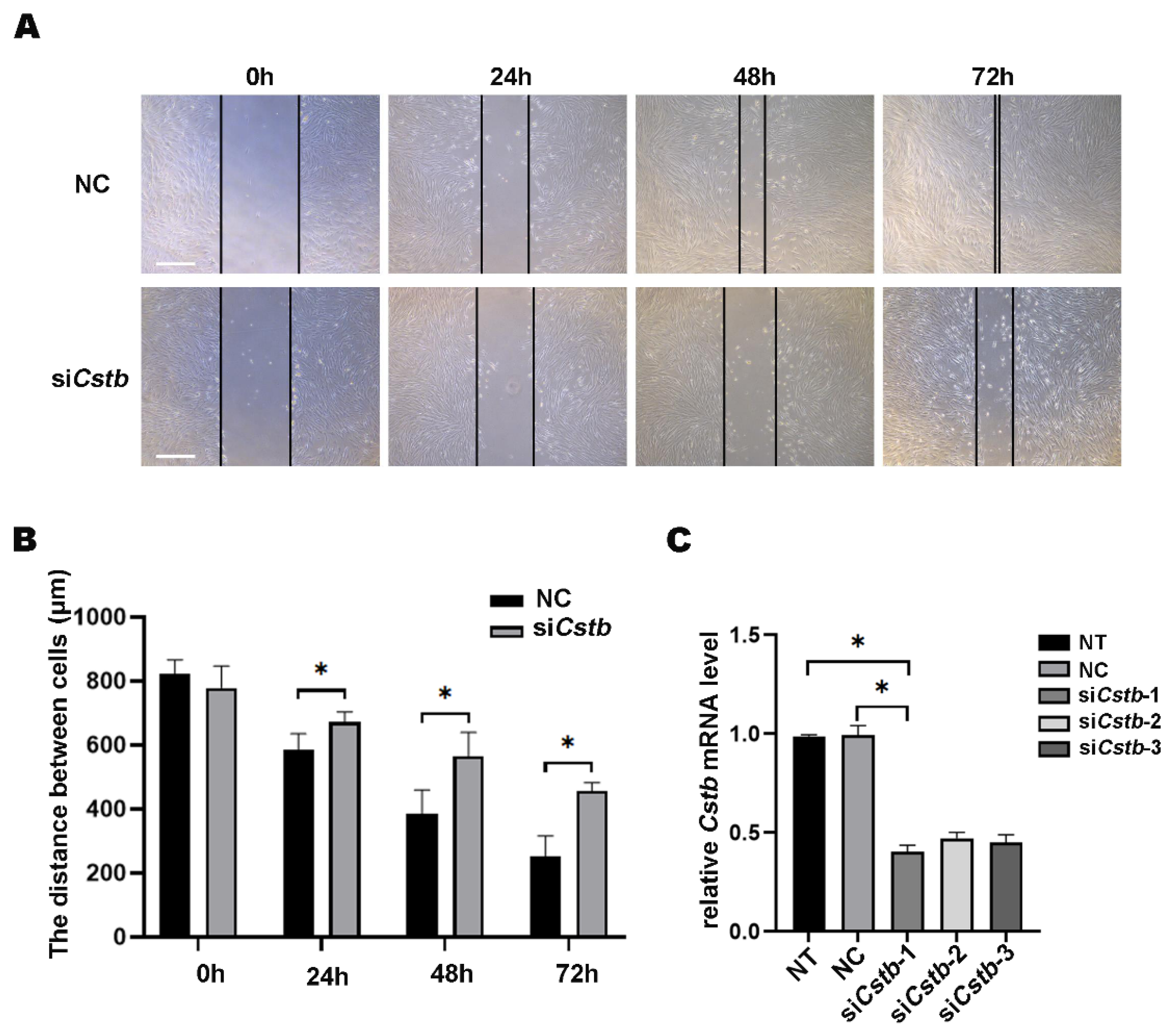
2.3. The Function of Cstb during Decidualization
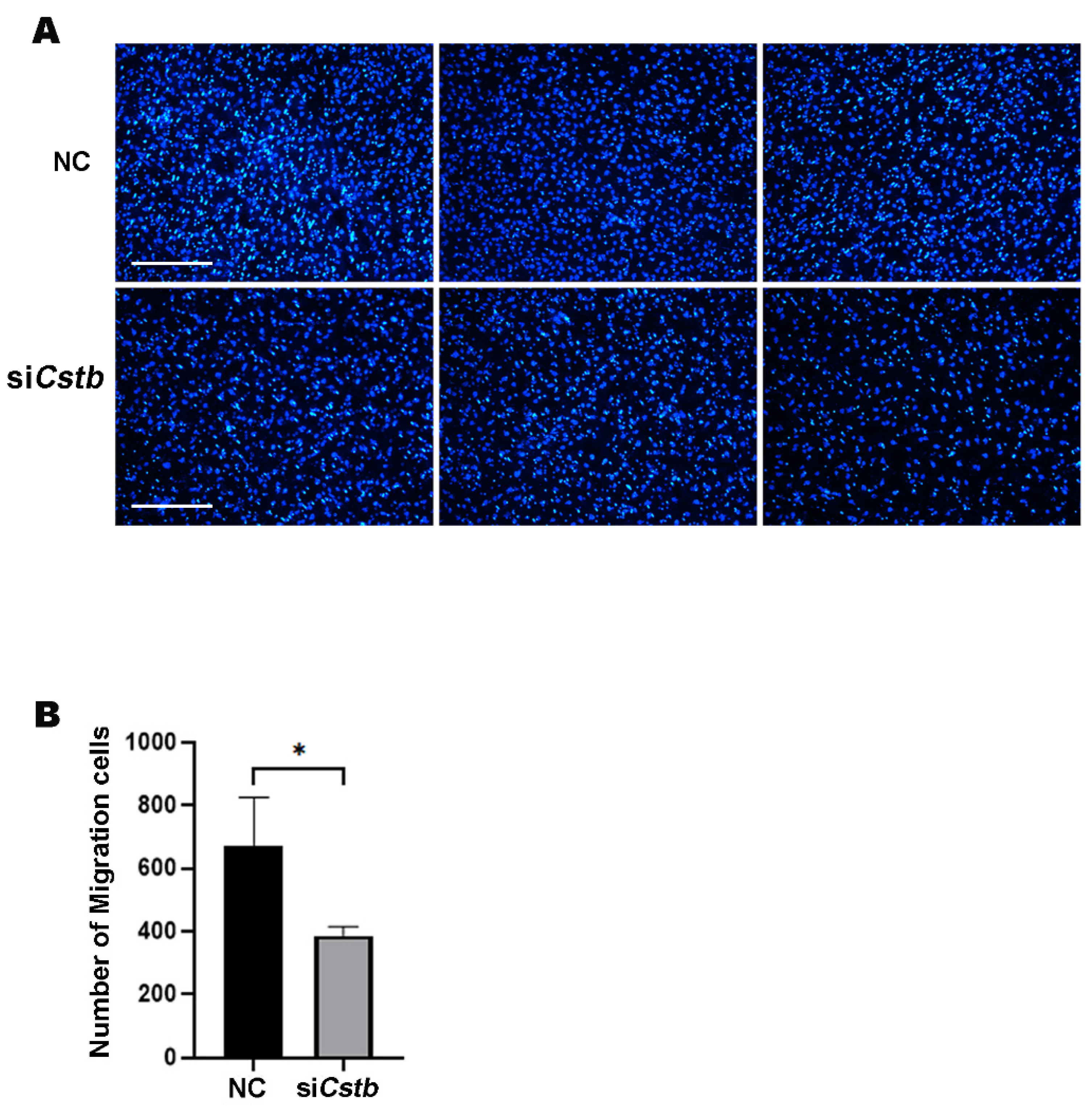
2.4. Cstb Promotes Migration of Stromal Cells in Mouse
2.5. Function of Cstb during Mouse In Vitro Decidualization
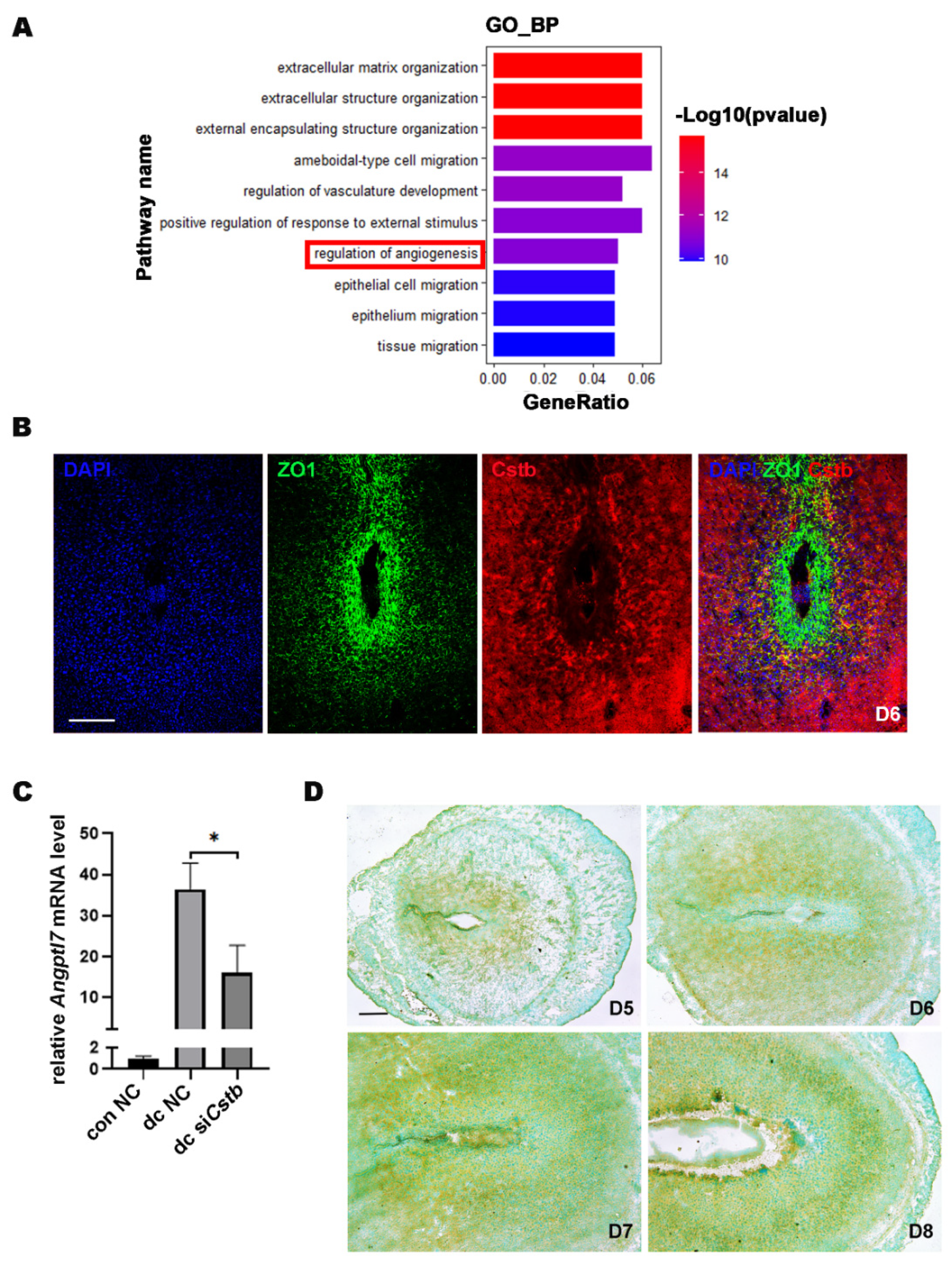
2.6. Cstb Expression Is Associated with Angiogenesis in the Decidual Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.3. In Situ Hybridization
4.4. Isolation and Treatment of Endometrial Stromal Cells
4.5. Immunohistochemistry
4.6. Immunofluorescence
4.7. Migration Analysis by Millicell Transwell Chamber
4.8. Wound Healing Assay
4.9. RNA-Seq and Data Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Angptl7 | Angiopoietin-like 7 |
| Cstb | Cystatin B |
| DAPI | 4′,6-diamidino-2-phenylindole dihydrochloride |
| E2 | 17β-Estradiol |
| ECM | Extracellular matrix |
| ERα | Estrogen receptor α |
| Esr1 | Estrogen receptor 1 |
| ICI | ICI182780 |
| P4 | Progesterone |
| PBS | Phosphate Buffered Saline |
| PDZ | Primary decidual zone |
| PPT | Propyl pyrazole triol |
| Prl8a2 | Prolactin family 8, subfamily a, member 2 |
| Rpl7 | Ribosomal protein L7 |
| SDZ | Secondary decidual zon |
| VEGF | Vascular endothelial growth factor |
| ZO1 | Zonula occludens-1 |
References
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [PubMed]
- Liang, X.H.; Deng, W.B.; Li, M.; Zhao, Z.A.; Wang, T.S.; Feng, X.H.; Cao, Y.J.; Duan, E.K.; Yang, Z.M. Egr1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4. J. Biol. Chem. 2014, 289, 23534–23545. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [PubMed]
- Das, A.; Mantena, S.R.; Kannan, A.; Evans, D.B.; Bagchi, M.K.; Bagchi, I.C. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12542–12547. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Aikawa, S.; Deng, W.; Bartos, A.; Walz, G.; Grahammer, F.; Huber, T.B.; Sun, X.; Dey, S.K. Primary decidual zone formation requires Scribble for pregnancy success in mice. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Gant, N.F.; Whalley, P.J.; Everett, R.B.; Worley, R.J.; MacDonald, P.C. Control of vascular reactivity in pregnancy. Am. J. Kidney Dis. 1987, 9, 303–307. [Google Scholar] [CrossRef]
- Lang, U.; Baker, R.S.; Braems, G.; Zygmunt, M.; Künzel, W.; Clark, K.E. Uterine blood flow--a determinant of fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110 (Suppl. 1), S55–S61. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.J.; Seol, J.W.; Jang, J.Y.; Cho, Y.S.; Kim, K.R.; Choi, Y.; Lydon, J.P.; Demayo, F.J.; Shibuya, M.; et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med. 2013, 5, 1415–1430. [Google Scholar] [CrossRef]
- Girling, J.E.; Rogers, P.A. Regulation of endometrial vascular remodelling: Role of the vascular endothelial growth factor family and the angiopoietin-TIE signalling system. Reproduction 2009, 138, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, L.H.; Li, H.Y.; Chang, S.P.; Liao, C.Y.; Tsui, K.H.; Sung, Y.J.; Chao, K.C. Roles of estrogen and progesterone in endometrial hemodynamics and vascular endothelial growth factor production. J. Chin. Med. Assoc. 2009, 72, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Butinar, M.; Prebanda, M.T.; Rajković, J.; Jerič, B.; Stoka, V.; Peters, C.; Reinheckel, T.; Krüger, A.; Turk, V.; Turk, B.; et al. Stefin B deficiency reduces tumor growth via sensitization of tumor cells to oxidative stress in a breast cancer model. Oncogene 2014, 33, 3392–3400. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Lim, S.Y.; Kutikhin, A.G.; Gordon-Weeks, A.N. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 207–228. [Google Scholar] [CrossRef]
- Breznik, B.; Mitrović, A.; Lah, T.T.; Kos, J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie 2019, 166, 233–250. [Google Scholar] [CrossRef]
- Tian, C.; Öhlund, D.; Rickelt, S.; Lidström, T.; Huang, Y.; Hao, L.; Zhao, R.T.; Franklin, O.; Bhatia, S.N.; Tuveson, D.A.; et al. Cancer cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. 2020, 80, 1461–1474. [Google Scholar] [CrossRef]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the endometrium: Identification of conceptus-derived proteins during early pregnancy in cattle. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ozaki, Y.; Blomgren, K.; Tateyama, H.; Sugiura-Ogasawara, M.; Suzumori, K. Role of cathepsins and cystatins in patients with recurrent miscarriage. Mol. Hum. Reprod. 2005, 11, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and nuclear estrogen receptor alpha actions: From tissue specificity to medical implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef]
- Large, M.J.; DeMayo, F.J. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol. Cell. Endocrinol. 2012, 358, 155–165. [Google Scholar] [CrossRef]
- Shi, J.W.; Lai, Z.Z.; Yang, H.L.; Yang, S.L.; Wang, C.J.; Ao, D.; Ruan, L.Y.; Shen, H.H.; Zhou, W.J.; Mei, J.; et al. Collagen at the maternal-fetal interface in human pregnancy. Int. J. Biol. Sci. 2020, 16, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yan, J.Q.; Song, Z.; Liu, Y.F.; Song, M.J.; Qin, J.W.; Yang, Z.M.; Liang, X.H. Molecular characterization of lysyl oxidase-mediated extracellular matrix remodeling during mouse decidualization. FEBS Lett. 2017, 591, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Schatz, F.; Guzeloglu-Kayisli, O.; Arlier, S.; Kayisli, U.A.; Lockwood, C.J. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum. Reprod. Update 2016, 22, 497–515. [Google Scholar] [CrossRef]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue stiffness at the human maternal-fetal interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef]
- Divya; Chhikara, P.; Mahajan, V.S.; Datta Gupta, S.; Chauhan, S.S. Differential activity of cathepsin L in human placenta at two different stages of gestation. Placenta 2002, 23, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Baston-Buest, D.M.; Schanz, A.; Buest, S.; Fischer, J.C.; Kruessel, J.S.; Hess, A.P. The embryo’s cystatin C and F expression functions as a protective mechanism against the maternal proteinase cathepsin S in mice. Reproduction 2010, 139, 741–748. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Yang, H.; Pan, L.; Zhao, G.; Zhang, R.; Zhang, T.; Xiao, Z.; Tong, Y.; Zhang, Y.; Hu, R.; et al. Hepatitis B virus x protein (HBx) suppresses transcription factor EB (TFEB) resulting in stabilization of integrin beta 1 (ITGB1) in hepatocellular carcinoma cells. Cancers 2021, 13, 1181. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Katunuma, N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J. Med. Investig. 2005, 52, 1–9. [Google Scholar] [CrossRef]
- Grewal, S.; Carver, J.G.; Ridley, A.J.; Mardon, H.J. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. USA 2008, 105, 16189–16194. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Lambalk, C.B.; Huirne, J.; Mijatovic, V.; Repping, S.; Hamer, G.; Mastenbroek, S. High-quality human preimplantation embryos actively influence endometrial stromal cell migration. J. Assist. Reprod. Genet. 2018, 35, 659–667. [Google Scholar] [CrossRef]
- Douglas, N.C.; Tang, H.; Gomez, R.; Pytowski, B.; Hicklin, D.J.; Sauer, C.M.; Kitajewski, J.; Sauer, M.V.; Zimmermann, R.C. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology 2009, 150, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.H.; Chang, W.A.; Tsai, E.M.; Tsai, M.J.; Kuo, P.L. Investigating novel genes potentially involved in endometrial adenocarcinoma using next-generation sequencing and bioinformatic approaches. Int. J. Med. Sci. 2019, 16, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Toyono, T.; Usui, T.; Yokoo, S.; Taketani, Y.; Nakagawa, S.; Kuroda, M.; Yamagami, S.; Amano, S. Angiopoietin-like 7 is an anti-angiogenic protein required to prevent vascularization of the cornea. PLoS ONE 2015, 10, e0116838. [Google Scholar] [CrossRef]
- Parri, M.; Pietrovito, L.; Grandi, A.; Campagnoli, S.; De Camilli, E.; Bianchini, F.; Marchiò, S.; Bussolino, F.; Jin, B.; Sarmientos, P.; et al. Angiopoietin-like 7, a novel pro-angiogenetic factor over-expressed in cancer. Angiogenesis 2014, 17, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, B.; Li, M.; Luo, J.; Hong, Y.; He, Y.; Chen, S.; Yang, Z.; Liang, C.; Yang, Z. Caveolin-1 Regulation and Function in Mouse Uterus during Early Pregnancy and under Human In Vitro Decidualization. Int. J. Mol. Sci. 2022, 23, 3699. [Google Scholar] [CrossRef]
- Montagutelli, X. Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 2000, 11 (Suppl. 16), S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- Ni, H.; Sun, T.; Ding, N.Z.; Ma, X.H.; Yang, Z.M. Differential expression of microsomal prostaglandin e synthase at implantation sites and in decidual cells of mouse uterus. Biol. Reprod. 2002, 67, 351–358. [Google Scholar] [CrossRef]
- Hu, S.J.; Ren, G.; Liu, J.L.; Zhao, Z.A.; Yu, Y.S.; Su, R.W.; Ma, X.H.; Ni, H.; Lei, W.; Yang, Z.M. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008, 283, 23473–23484. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef]
- Odell, I.D.; Cook, D. Immunofluorescence techniques. J. Investig. Derm. 2013, 133, e4. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Primer Sequences | Size (bp) | Application | Accession Number |
|---|---|---|---|---|
| Cstb | CCTAGTTGGATCTGTCTTCA AGCCACTATCTGTCTCTTG | 201 | ISH | NM_007793.3 |
| Cstb | AGGTGAAGTCCCAGCTTGAAT GTCTGATAGGAAGACAGGGTCA | 196 | RT | NM_007793.3 |
| Dtprp | AGCCAGAAATCACTGCCACT TGATCCATGCACCCATAAAA | 119 | RT | NM_001289919.1 |
| Angptl7 | TAAACGCAAGACACAGCTCAA TGCATGATGTCAATCTGGTTGT | 237 | ISH | NM_001039554.3 |
| Angptl7 | TGACTGTTCTTCCCTGTACCA CAAGGCCACTCTTACGTCTCT | 158 | RT | NM_001039554.3 |
| Rpl7 | GCAGATGTACCGCACTGAGATTCACCTTTGGGCTTACTCCATTGATA | 129 | RT | NM_011291.5 |
| Cstb-1 | CAGCTTGAATCGAAAGAAA | siRNA | ||
| Cstb-2 | GCACTTGAGGGTGTTTCAA | siRNA | ||
| Cstb-3 | CCTATCAGACCAACAAAGA | siRNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Chen, Y.; Yang, Z.; Liang, S.; Zhu, S.; Liang, X. Expression and Regulation of a Novel Decidual Cells-Derived Estrogen Target during Decidualization. Int. J. Mol. Sci. 2023, 24, 302. https://doi.org/10.3390/ijms24010302
Lu L, Chen Y, Yang Z, Liang S, Zhu S, Liang X. Expression and Regulation of a Novel Decidual Cells-Derived Estrogen Target during Decidualization. International Journal of Molecular Sciences. 2023; 24(1):302. https://doi.org/10.3390/ijms24010302
Chicago/Turabian StyleLu, Lin, Yingni Chen, Zhenshan Yang, Shijin Liang, Songqi Zhu, and Xiaohuan Liang. 2023. "Expression and Regulation of a Novel Decidual Cells-Derived Estrogen Target during Decidualization" International Journal of Molecular Sciences 24, no. 1: 302. https://doi.org/10.3390/ijms24010302
APA StyleLu, L., Chen, Y., Yang, Z., Liang, S., Zhu, S., & Liang, X. (2023). Expression and Regulation of a Novel Decidual Cells-Derived Estrogen Target during Decidualization. International Journal of Molecular Sciences, 24(1), 302. https://doi.org/10.3390/ijms24010302