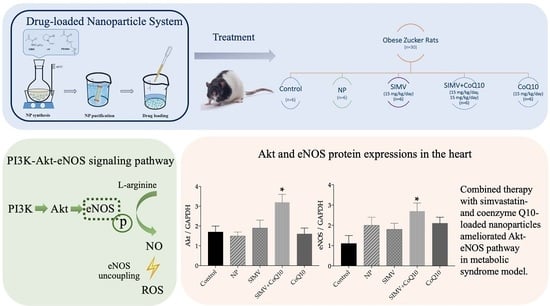
Combined Therapy with Simvastatin- and Coenzyme-Q10-Loaded Nanoparticles Upregulates the Akt-eNOS Pathway in Experimental Metabolic Syndrome
Abstract
1. Introduction
2. Results
2.1. Nanoparticle Characteristics
2.2. Weight Parameters
2.3. Lipid Profile and Lipid Peroxidation
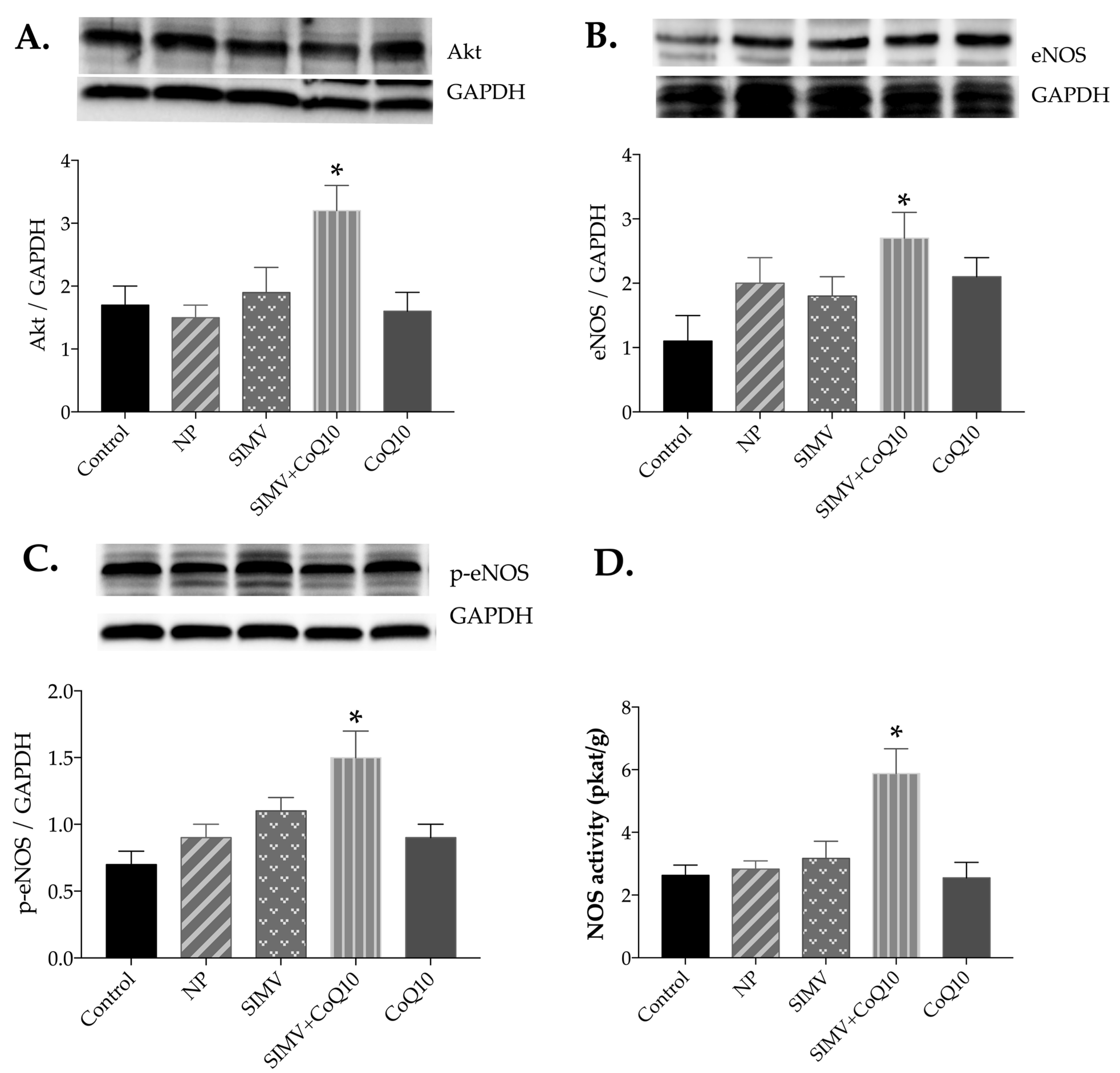
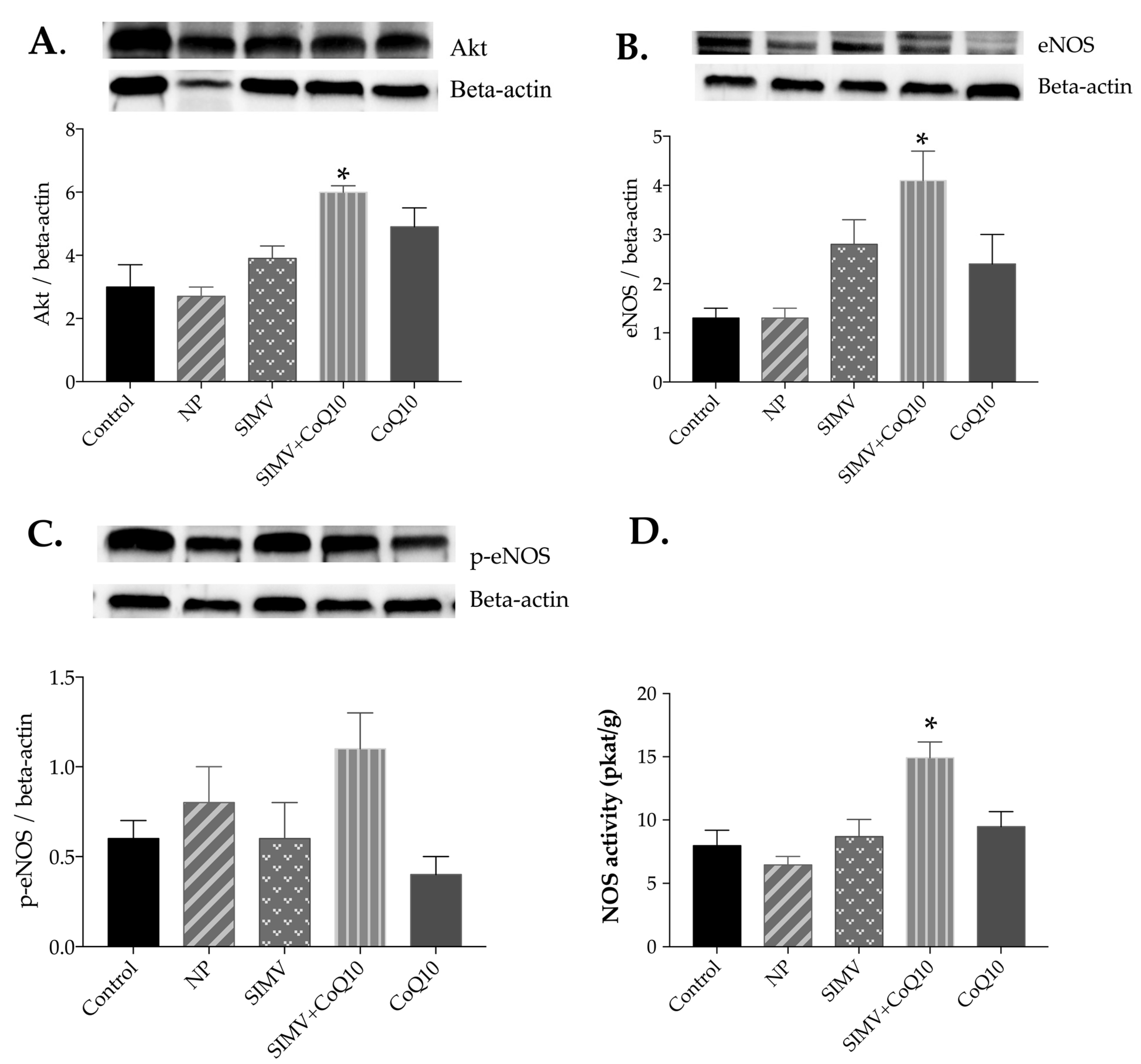
2.4. Protein Expressions of Akt, eNOS, and p-eNOS
2.5. Total NOS Activity
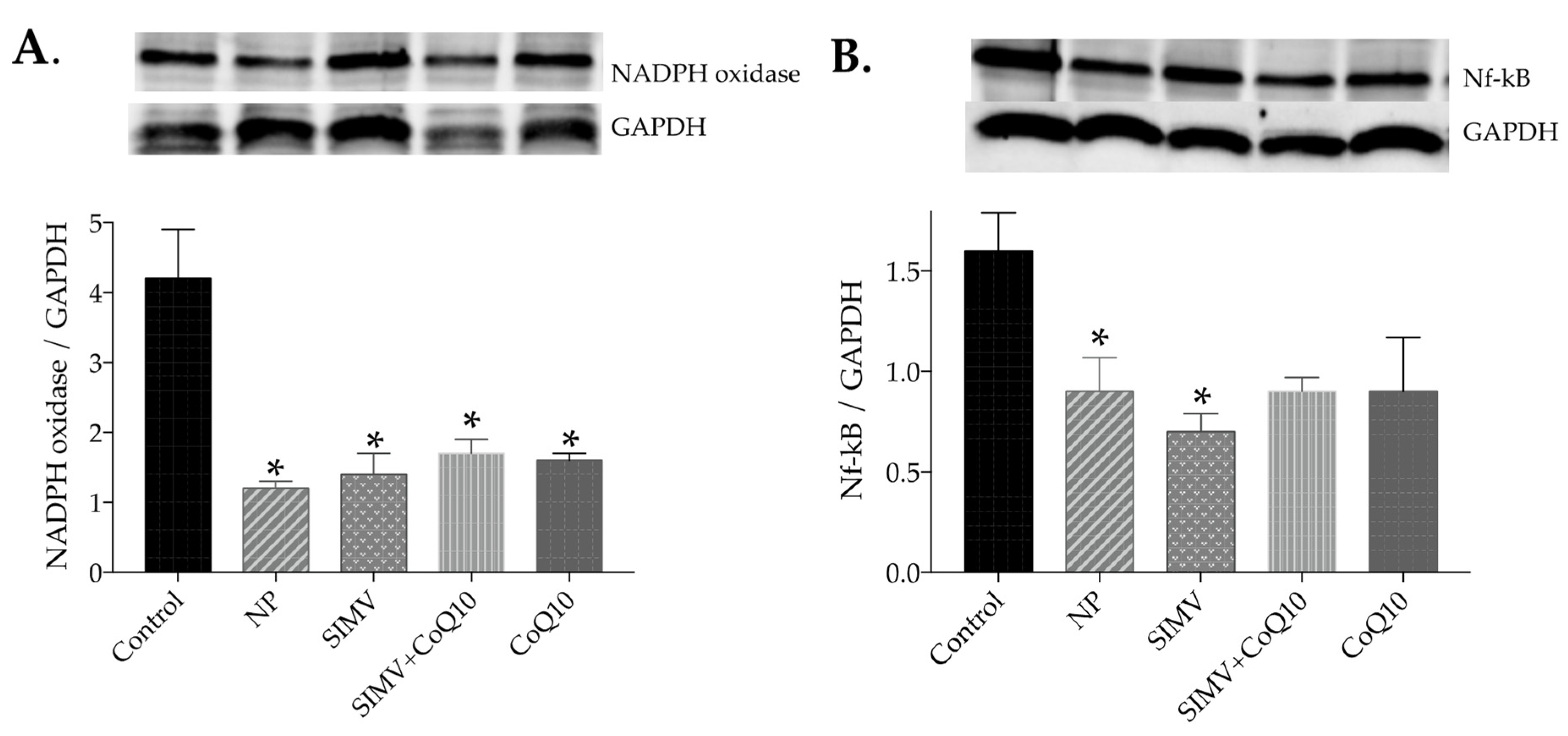
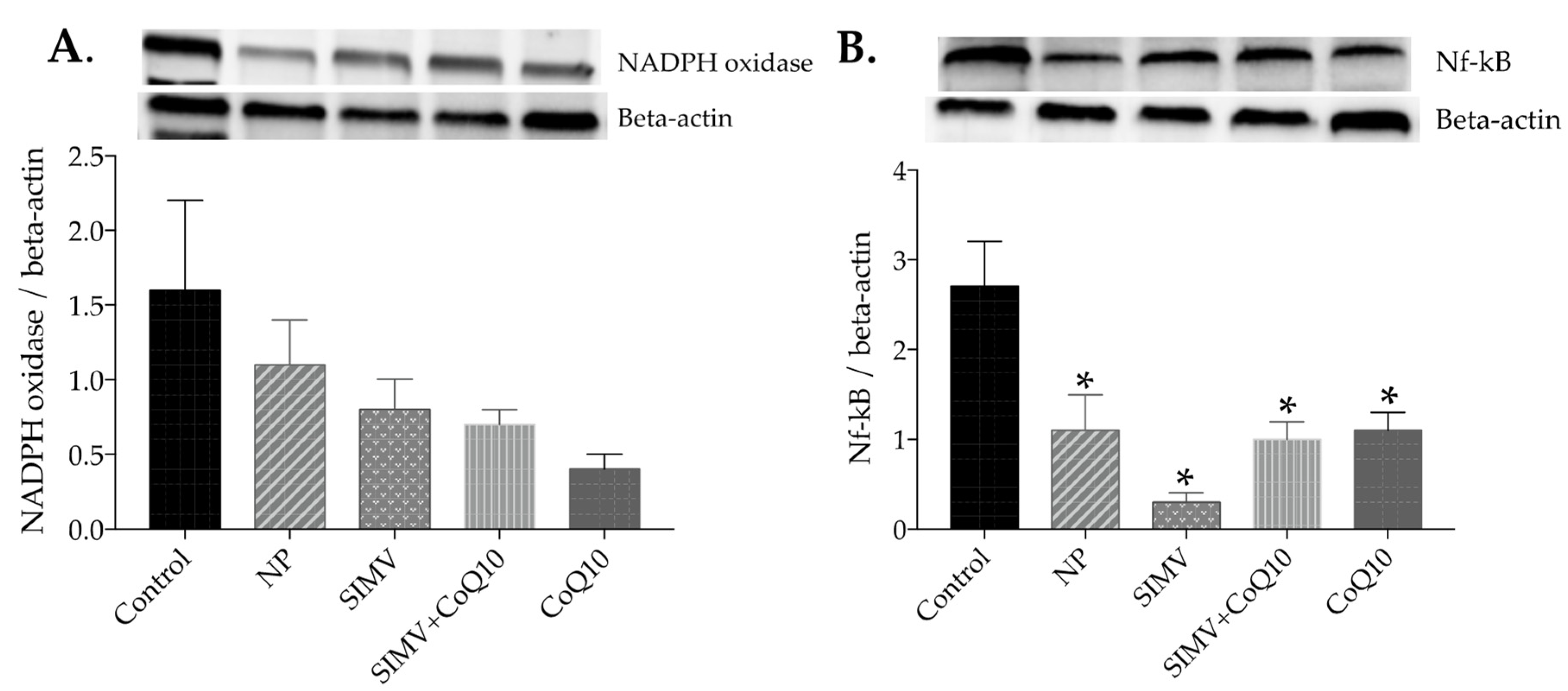
2.6. Protein Expressions of NADPH Oxidase and NF-kappaB
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Simvastatin Extraction
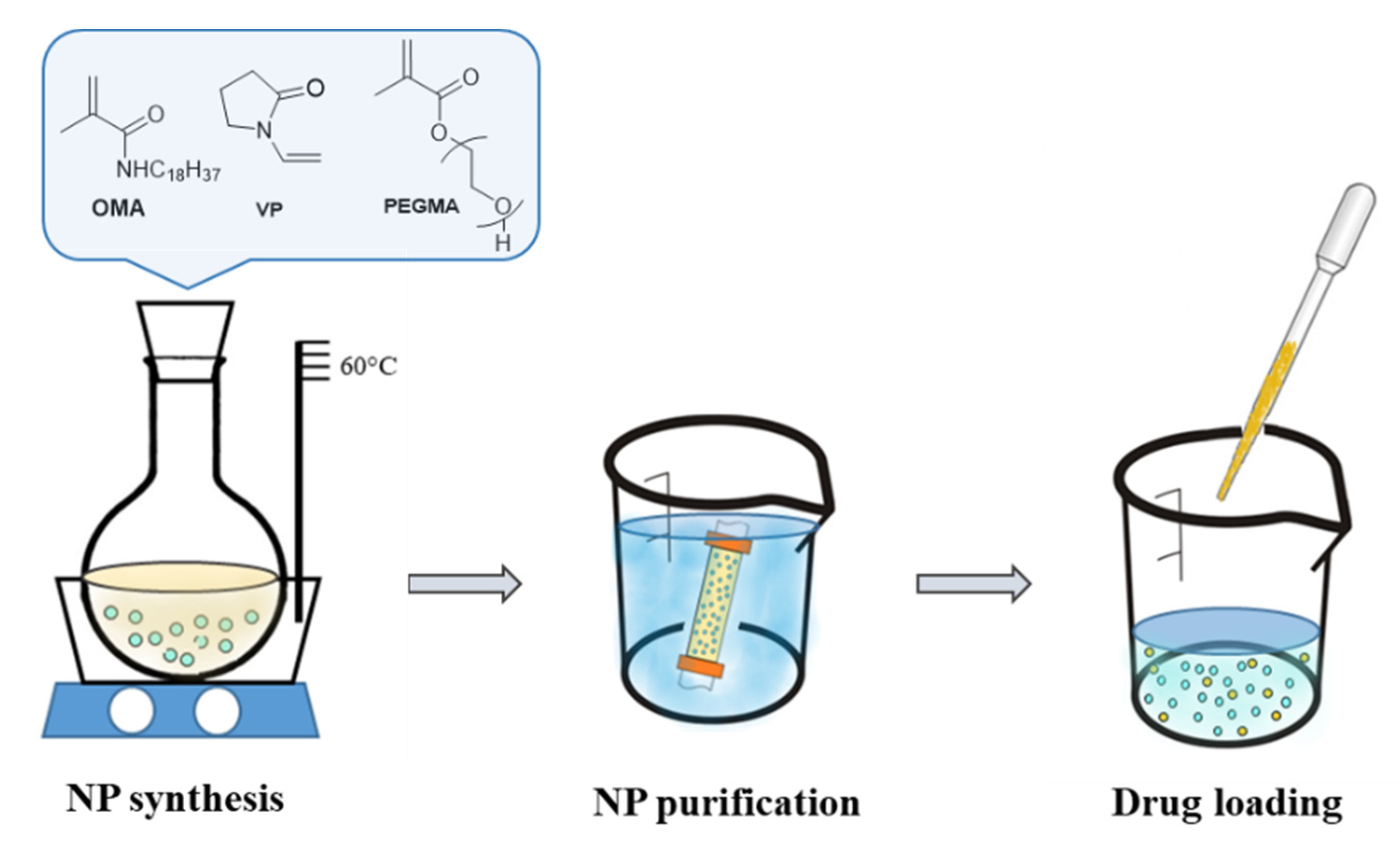
4.3. Synthesis and Loading of Polymeric Nanoparticles (NP)
4.4. Physicochemical Characterization of the Simvastatin- and Coenzyme-Q10-Loaded Polymeric Nanoparticles
4.5. Animals and Treatment
4.6. Weight Parameters
4.7. Conjugated Dienes
4.8. Lipid Profile
4.9. Western Blot Analysis
4.10. Total NOS Activity
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andreassi, M.G. Metabolic syndrome, diabetes and atherosclerosis: Influence of gene-environment interaction. Mutat. Res. 2009, 667, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, S.H.; Werner, C.M.; Laufs, U.; Böhm, M. Nitric oxide-donating statins: A new concept to boost the lipid-independent effects. Cardiovasc. Res. 2012, 94, 395–397. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Luo, M.; We, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxid. Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Deedwania, P.C. Mechanisms of endothelial dysfunction in the metabolic syndrome. Curr. Diab. Rep. 2003, 3, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Wang, T.; Cai, H. An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production. Circ. Res. 2009, 104, 50–59. [Google Scholar] [CrossRef]
- Cai, H.; Liu, D.; Garcia, J.G. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc. Res. 2008, 77, 30–34. [Google Scholar] [CrossRef]
- Nguyen, A.; Cai, H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 6530–6535. [Google Scholar] [CrossRef]
- Du, X.L.; Edelstein, D.; Dimmeler, S.; Ju, Q.; Sui, C.; Brownlee, M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Investig. 2001, 108, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Okon, E.B.; Chung, A.W.; Rauniyar, P.; Padilla, E.; Tejerina, T.; McManus, B.M.; Luo, H.; van Breemen, C. Compromised arterial function in human type 2 diabetic patients. Diabetes 2005, 54, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.D.; McMillin, S.L.; Riehle, C.; Tanner, J.; Palionyte, M.; Hillas, E.; Jones, D.; Cooksey, R.C.; Birnbaum, M.J.; McClain, D.A.; et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ. Res. 2009, 104, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Kagota, S.; McGuire, J.J.; Wakuda, H.; Yoshikawa, N.; Nakamura, K.; Shinozuka, K. Enhanced Nitric Oxide Synthase Activation via Protease-Activated Receptor 2 Is Involved in the Preserved Vasodilation in Aortas from Metabolic Syndrome Rats. J. Vasc. Res. 2015, 52, 232–243. [Google Scholar] [CrossRef]
- Huffman, G.B. Which Statin Is the Best Choice for Which Patient? Am. Fam. Physician 2002, 65, 1211–1215. [Google Scholar]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Laufs, U. Beyond lipid-lowering: Effects of statins on endothelial nitric oxide. Eur. J. Clin. Pharmacol. 2003, 58, 719–731. [Google Scholar] [CrossRef]
- Kano, H.; Hayashi, T.; Sumi, D.; Esaki, T.; Asai, Z.; Thakur, N.K.; Jayachandran, M.; Iguchi, A. A HMG-CoA reductase inhibitor improved regression of atherosclerosis in the rabbit aorta without affecting serum lipid levels: Possible relevance of up-regulation of endothelial NO synthase mRNA. Biochem. Biophys. Res. Commun. 1999, 259, 414–419. [Google Scholar] [CrossRef]
- Cebova, M.; Rehakova, R.; Kosutova, M.; Pechanova, O. Simvastatin Does Not Affect Nitric Oxide Generation Increased by Sesame Oil in Obese Zucker Rats. Oxid. Med. Cell. Longev. 2018, 2018, 5413423. [Google Scholar] [CrossRef]
- Laufs, U.; Gertz, K.; Huang, P.; Nickenig, G.; Böhm, M.; Dirnagl, U.; Endres, M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke 2000, 31, 2442–2449. [Google Scholar] [CrossRef]
- Harris, M.B.; Blackstone, M.A.; Sood, S.G.; Li, C.; Goolsby, J.M.; Venema, V.J.; Kemp, B.E.; Venema, R.C. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Brouet, A.; Sonveaux, P.; Dessy, C.; Moniotte, S.; Balligand, J.-L.; Feron, O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide; mediated effects of statins. Circ. Res. 2001, 89, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Kureishi, Y.; Luo, Z.; Shiojima, I.; Bialik, A.; Fulton, D.; Lefer, D.J.; Sessa, W.C.; Walsh, K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000, 6, 1004–1010. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, X.; Meng, X.; Lu, L.; Mai, D.; Qu, S. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in parkinson disease models. Front. Mol. Neurosci. 2018, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O. Remodelling of the heart and vessels in experimental hypertension: Advances in protection. J. Hypertens. 2010, 28, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Dayar, E.; Pechanova, O. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090. [Google Scholar] [CrossRef]
- Potgieter, M.; Pretorius, E.; Pepper, M.S. Primary and secondary coenzyme Q10 deficiency: The role of therapeutic supplementation. Nutr. Rev. 2013, 71, 180–188. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Anwar, M.; Warsi, M.H.; Mallick, N.; Akhter, S.; Gahoi, S.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Enhanced bioavailability of nano-sized chitosan-atorvastatin conjugate after oral administration to rats. Eur. J. Pharm. Sci. 2011, 44, 241–249. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madrakian, T.; Ghavami, S. Preparation and Characterization of Simvastatin Nanocapsules: Encapsulation of Hydrophobic Drugs in Calcium Alginate. Methods Mol. Biol. 2020, 2125, 47–56. [Google Scholar]
- Jones, J.M.; Player, D.J.; Samanta, S.; Rangasami, V.K.; Hilborn, J.; Lewis, M.P.; Oommen, O.P.; Mudera, V. Hyaluronan derived nanoparticle for simvastatin delivery: Evaluation of simvastatin induced myotoxicity in tissue engineered skeletal muscle. Biomater. Sci. 2019, 8, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.K.; Ratnam, D.V.; Chandraiah, G.; Ankola, D.D.; Rao, P.R.; Kumar, M.N. Oral nanoparticulate atorvastatin calcium is more efficient and safe in comparison to Lipicure in treating hyperlipidemia. Lipids 2008, 43, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and Nanoparticles for Statin Delivery: Current Use and Future Perspectives in Cardiovascular Disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef]
- Pechanova, O.; Barta, A.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Klimentova, J.; Tӧrӧk, J.; Zemancikova, A.; Cebova, M. Protective Effects of Nanoparticle-Loaded Aliskiren on Cardiovascular System in Spontaneously Hypertensive Rats. Molecules 2019, 24, 2710. [Google Scholar] [CrossRef]
- Pechanova, O.; Dayar, E.; Cebova, M. Therapeutic Potential of Polyphenols-Loaded Polymeric Nanoparticles in Cardiovascular System. Molecules 2020, 25, 3322. [Google Scholar] [CrossRef]
- Dayar, E.; Pechanova, O. Neuroprotective effects of natural polyphenol-loaded nanoparticles. Act. Nerv. Super Rediviva 2021, 63, 133–140. [Google Scholar]
- Török, J.; L’upták, I.; Matúsková, J.; Pechánová, O.; Zicha, J.; Kunes, J.; Simko, F. Comparison of the effect of simvastatin, spironolactone and L-arginine on endothelial function of aorta in hereditary hypertriglyceridemic rats. Physiol. Res. 2007, 56, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Matuskova, J.; Luptak, I.; Krajcirovicova, K.; Kucharska, J.; Gvozdjakova, A.; Babal, P.; Pechanova, O. Effect of simvastatin on remodeling of the left ventricle and aorta in L-NAME-induced hypertension. Life Sci. 2004, 74, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O.; Pelouch, V.; Krajcirovicova, K.; Mullerova, M.; Bednarova, K.; Adamcova, M.; Paulis, L. Effect of melatonin, captopril, spironolactone and simvastatin on blood pressure and left ventricular remodelling in spontaneously hypertensive rats. J. Hypertens. 2009, 27, 5–10. [Google Scholar] [CrossRef]
- Sumi, D.; Hayashi, T.; Thankur, N.K.; Jayachandran, M.; Asai, Y.; Kano, H.; Matsui, H.; Iguchi, A. A HMG-CoA reductase inhibitor possesses a potent anti-atherosclerotic effect other than serum lipid lowering effects the relevance of endothelial nitric oxide synthase and superoxide anion scavenging action. Atherosclerosis 2001, 155, 347–357. [Google Scholar] [CrossRef]
- Sata, M.; Nishimatsu, H.; Suzuki, E.; Sugiura, S.; Yoshizumi, M.; Ouchi, Y.; Hirata, Y.; Nagai, R. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. 2001, 15, 2530–2532. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Kitajima, I.; Wang, Z. Effects of different statins on endothelial nitric oxide synthase and AKT phosphorylation in endothelial cells. Int. J. Cardiol. 2008, 127, 33–39. [Google Scholar] [CrossRef]
- Huang, B.; Li, F.A.; Wu, C.H.; Wang, D.L. The role of nitric oxide on rosuvastatin-mediated S-nitrosylation and translational proteomes in human umbilical vein endothelial cells. Proteome Sci. 2012, 10, 43. [Google Scholar] [CrossRef]
- Nangle, M.R.; Cotter, M.A.; Cameron, N.E. Effects of rosuvastatin on nitric oxide-dependent function in aorta and corpus cavernosum of diabetic mice: Relationship to cholesterol biosynthesis pathway inhibition and lipid lowering. Diabetes 2003, 52, 2396–2402. [Google Scholar] [CrossRef][Green Version]
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants 2021, 10, 906. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Kawashima, S.; Takeshita, S.; Yamashita, T.; Azumi, H.; Yasuhara, M.; Nishi, H.; Inoue, N.; Yokoyama, M. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001, 154, 87–96. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Romana, B.; Batger, M.; Prestidge, C.A.; Colombo, G.; Sonvico, F. Expanding the therapeutic potential of statins by means of nanotechnology enabled drug delivery systems. Curr. Top. Med. Chem. 2014, 14, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Korani, S.; Korani, M.; Bahrami, S.; Johnston, T.P.; Butler, A.E.; Banach, M.; Sahebkar, A. Application of nanotechnology to improve the therapeutic benefits of statins. Drug Discov. Today 2019, 24, 567–574. [Google Scholar] [CrossRef]
- Helkin, A.; Bruch, D.; Wilson, D.R.; Gruessner, A.C.; Bader, R.R.; Maier, K.G.; Gahtan, V. Intraluminal Delivery of Simvastatin Attenuates Intimal Hyperplasia After Arterial Injury. Vasc. Endovasc. Surg. 2019, 53, 379–386. [Google Scholar] [CrossRef]
- Jorat, M.V.; Tabrizi, R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Mottaghi, R.; Asemi, Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018, 17, 230. [Google Scholar] [CrossRef]
- Ferrero-Andrés, A.; Rosello, A.P.; Serafín, A.; Roselló-Catafau, J.; Folch-Puy, E.; Roselló, P. Polyethylene Glycol 35 (PEG35) Protects against Inflammation in Experimental Acute Necrotizing Pancreatitis and Associated Lung Injury. Int. J. Mol. Sci. 2020, 21, 917. [Google Scholar] [CrossRef]
- Dutheil, D.; Rioja-Pastor, I.; Tallineau, C.; Hauet, T.; Mauco, G.; Petit-Paris, I.; Goujon, J.-M. Protective Effect of PEG 35 000 Da on Renal Cells: Paradoxical Activation of JNK Signaling Pathway During Cold Storage. Am. J. Transplant. 2006, 6, 1529–1540. [Google Scholar] [CrossRef]
- Dutheil, D.; Underhaug Gjerde, A.; Petit-Paris, I.; Mauco, G.; Holmsen, H. Polyethylene glycols interact with membrane glycerophospholipids: Is this part of their mechanism for hypothermic graft protection? J. Chem. Biol. 2009, 2, 39–49. [Google Scholar] [CrossRef]
- Ferrero-Andrés, A.; Closa, D.; Roselló-Catafau, J.; Folch-Puy, E. Polyethylene Glycol 35 (PEG35) Modulates Exosomal Uptake and Function. Polymers 2020, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Ii, M.; Masuda, M.; Tabata, Y.; Hoshiga, M.; Ishizaka, N.; Asahi, M. Cardiac Regeneration by Statin-Polymer Nanoparticle-Loaded Adipose-Derived Stem Cell Therapy in Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, N.; Honda, Y.; Ukita, N.; Matsui, M.; Miura, Y.; Hoshina, K. Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine. Int. J. Mol. Sci. 2020, 21, 8702. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bao, P.; Li, L.; Wang, Y.; Xu, C.; Deng, M.; Zhang, J.; Zhao, X. Pitavastatin nanoparticle-engineered endothelial progenitor cells repair injured vessels. Sci. Rep. 2017, 7, 18067. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Li, P.; Zhou, Y.; Song, Y.; Shi, Y.; Feng, W.; Mo, X.; Gao, H.; An, Q.; et al. Rosuvastatin- and Heparin-Loaded Poly(l-lactide- co-caprolactone) Nanofiber Aneurysm Stent Promotes Endothelialization via Vascular Endothelial Growth Factor Type A Modulation. ACS Appl. Mater. Interfaces 2018, 10, 41012–41018. [Google Scholar] [CrossRef]
- Rikkou-Kalourkoti, M.; Panteli, P.A.; Patrickios, C.S. Synthesis and characterization of amphiphilic diblock copolymers of 2-(1-imidazolyl)ethyl methacrylate and styrene. Polym. Chem. 2014, 5, 4339–4347. [Google Scholar] [CrossRef]
- Dayar, E.; Cebova, M.; Lietava, J.; Panghyova, E.; Pechanova, O. Antioxidant Effect of Lonicera caerulea L. in the Cardiovascular System of Obese Zucker Rats. Antioxidants 2021, 10, 1199. [Google Scholar] [CrossRef]
- Paulis, L.; Pechanova, O.; Zicha, J.; Krajcirovicova, K.; Barta, A.; Pelouch, V.; Adamcova, M.; Simko, F. Melatonin prevents fibrosis but not hypertrophy development in the left ventricle of NG-nitro-L-arginine-methyl ester hypertensive rats. J Hypertens. Suppl. 2009, 27, 11–16. [Google Scholar] [CrossRef]
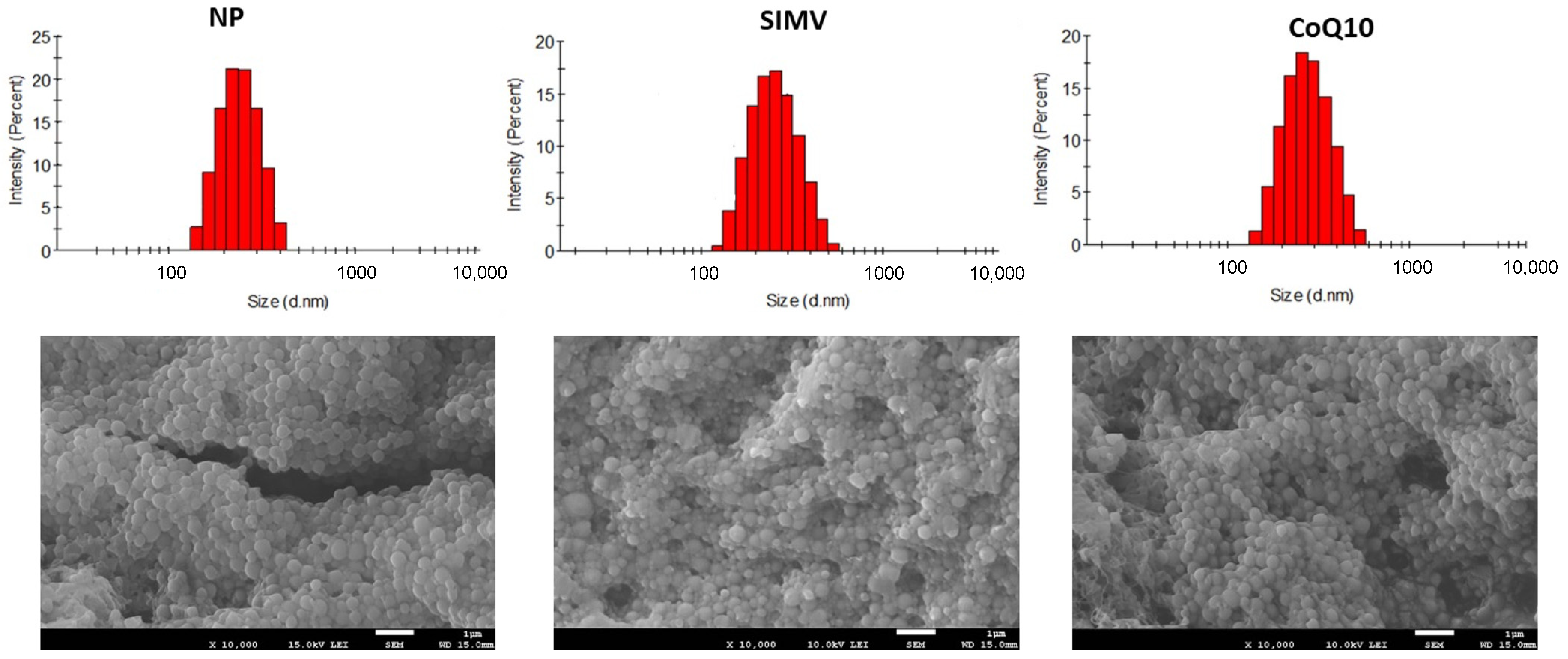
|
Diameter (nm) | PDI |
Zeta Potentials (g) | |
|---|---|---|---|
| NP | 233 ± 14 | 0.03 | −15.5 |
| SIMV | 347 ± 52 | 0.23 | −29.2 |
| CoQ10 | 270 ± 24 | 0.12 | −15.8 |
| BW (g) | HW (g) | LKW (g) | Relative HW (HW/TL) | Relative KW (g/100 g BW) | |
|---|---|---|---|---|---|
| Control | 687.5 ± 18.5 | 1.3 ± 0.1×10−2 | 1.66 ± 10−6 | 0.032 ± 0.1 × 10−2 | 24 × 10−6 ± 10−6 |
| NP | 637.3 ± 17.2 | 1.2 ± 0.3 × 10−3 | 1.68 ± 2 × 10−6 | 0.031 ± 0.3 × 10−3 | 27 × 10−6 ± 2 × 10−6 |
| SIMV | 636.1 ± 26.9 | 1.2 ± 0.1 × 10−2 | 1.8 ± 2 × 10−6 | 0.031 ± 0.1 × 10−2 | 29 × 10−6 ± 2 × 10−6 |
| SIMV+CoQ10 | 656.8 ± 8.3 | 1.3 ± 0.4 × 10−2 | 1.75 ± 3 × 10−6 | 0.033 ± 0.4 × 10−2 | 27 × 10−6 ± 3 × 10−6 |
| CoQ10 | 639.8 ± 17.2 | 1.3 ± 0.1 × 10−2 | 1.67 ± 10−6 | 0.031 ± 0.1 × 10−2 | 26 × 10−6 ± 10−6 |
| TG (mmol/L) | Total chol (mmol/L) | HDL (mg/dL) | LDL (mg/dL) | Hepatic CD (nmol/g Tissue) | |
|---|---|---|---|---|---|
| Control | 2.9 ± 0.2 | 7.7 ± 0.2 | 147.3 ± 10.1 | 70.9 ± 2.7 | 1424.1 ± 57.6 |
| NP | 5.5 ± 0.7 ** | 5.5 ± 0.4 * | 96.1 ± 11.2 ** | 53.9 ± 6.6 | 1202.3 ± 32.1 |
| SIMV | 3.0 ± 0.3 | 4.9 ± 0.5 * | 81.4 ± 7.6 *** | 42.7 ± 4.6 * | 1278.2 ± 31.7 |
| SIMV+CoQ10 | 3.3 ± 0.5 | 6.0 ± 0.4 | 135.5 ± 8.7 | 45.0 ± 3.3 * | 1132.3 ± 12.7 *** |
| CoQ10 | 2.9 ± 0.5 | 6.2 ± 0.5 | 143.7 ± 6.3 | 49.6 ± 4.1 * | 1039.0 ± 46.0 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şaman, E.; Cebova, M.; Barta, A.; Koneracka, M.; Zavisova, V.; Eckstein-Andicsova, A.; Danko, M.; Mosnacek, J.; Pechanova, O. Combined Therapy with Simvastatin- and Coenzyme-Q10-Loaded Nanoparticles Upregulates the Akt-eNOS Pathway in Experimental Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 276. https://doi.org/10.3390/ijms24010276
Şaman E, Cebova M, Barta A, Koneracka M, Zavisova V, Eckstein-Andicsova A, Danko M, Mosnacek J, Pechanova O. Combined Therapy with Simvastatin- and Coenzyme-Q10-Loaded Nanoparticles Upregulates the Akt-eNOS Pathway in Experimental Metabolic Syndrome. International Journal of Molecular Sciences. 2023; 24(1):276. https://doi.org/10.3390/ijms24010276
Chicago/Turabian StyleŞaman, Ezgi, Martina Cebova, Andrej Barta, Martina Koneracka, Vlasta Zavisova, Anita Eckstein-Andicsova, Martin Danko, Jaroslav Mosnacek, and Olga Pechanova. 2023. "Combined Therapy with Simvastatin- and Coenzyme-Q10-Loaded Nanoparticles Upregulates the Akt-eNOS Pathway in Experimental Metabolic Syndrome" International Journal of Molecular Sciences 24, no. 1: 276. https://doi.org/10.3390/ijms24010276
APA StyleŞaman, E., Cebova, M., Barta, A., Koneracka, M., Zavisova, V., Eckstein-Andicsova, A., Danko, M., Mosnacek, J., & Pechanova, O. (2023). Combined Therapy with Simvastatin- and Coenzyme-Q10-Loaded Nanoparticles Upregulates the Akt-eNOS Pathway in Experimental Metabolic Syndrome. International Journal of Molecular Sciences, 24(1), 276. https://doi.org/10.3390/ijms24010276