Urine-Derived Epithelial Cells as a New Model to Study Renal Metabolic Phenotypes of Patients with Glycogen Storage Disease 1a
Abstract
1. Introduction
2. Results
2.1. Characteristics of URECs
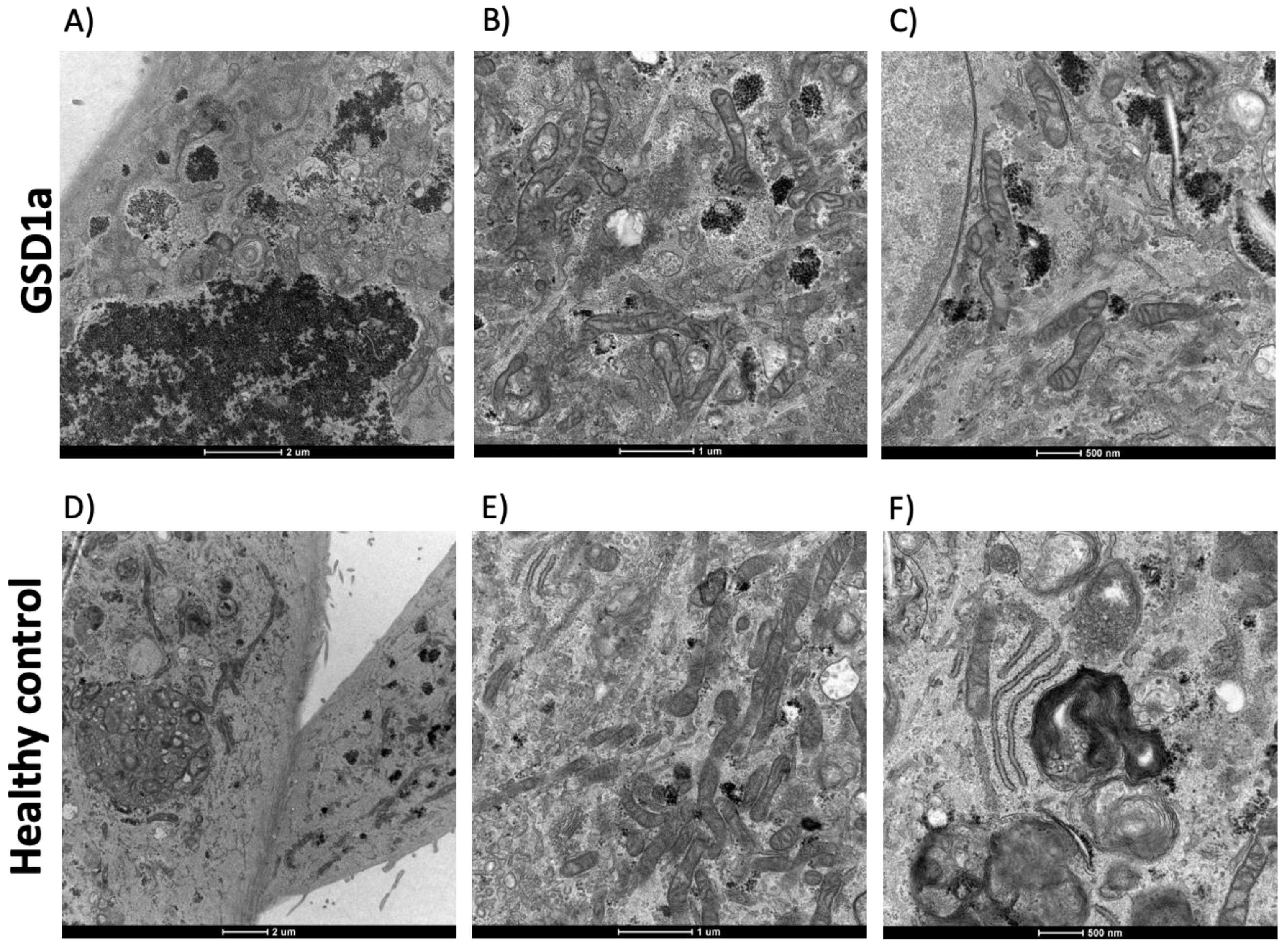
2.2. Analysis of ROS in GSD1a UREC
3. Discussion
4. Materials and Methods
4.1. Preparation and Culture of UREC
4.2. Morphological Analysis of Urine-Derived Renal Epithelial Cells
4.3. Immunofluorescence Protocol for URECs
4.4. Quantification of ROS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Froissart, R.; Piraud, M.; Boudjemline, A.M.; Vianey-saban, C.; Petit, F.; Hubert-buron, A.; Eberschweiler, P.T.; Gajdos, V.; Labrune, P. Glucose-6-Phosphatase Deficiency. Orphanet J. Rare Dis. 2011, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.Y.; Jun, H.S.; Mansfield, B.C. Glycogen Storage Disease Type I and G6Pase-β Deficiency: Etiology and Therapy. Nat. Rev. Endocrinol. 2010, 6, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Yiu, W.H.; Pan, C.J.; Ruef, R.A.; Peng, W.T.; Starost, M.F.; Mansfield, B.C.; Chou, J.Y. Angiotensin Mediates Renal Fibrosis in the Nephropathy of Glycogen Storage Disease Type Ia. Kidney Int. 2008, 73, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Visser, G.; Rake, J.; Labrune, P.; Leonard, J.; Moses, S.; Ullrich, K.; Wendel, U.; Smit, P. Consensus Guidelines for Management of Glycogen Storage Disease Type 1b—European Study on Glycogen Storage Disease Type 1. Eur. J. Pediatr. 2002, 161 (Suppl. S1), S120–S123. [Google Scholar] [CrossRef] [PubMed]
- Clar, J.; Gri, B.; Calderaro, J.; Birling, M.C.; Hérault, Y.; Smit, G.P.A.; Mithieux, G.; Rajas, F. Targeted Deletion of Kidney Glucose-6 Phosphatase Leads to Nephropathy. Kidney Int. 2014, 86, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Yiu, W.H.; Mead, P.A.; Jun, H.S.; Mansfield, B.C.; Chou, J.Y. Oxidative Stress Mediates Nephropathy in Type Ia Glycogen Storage Disease. Lab. Investig. 2010, 90, 620–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hijmans, B.S.; Boss, A.; van Dijk, T.H.; Soty, M.; Wolters, H.; Mutel, E.; Groen, A.K.; Derks, T.G.J.; Mithieux, G.; Heerschap, A.; et al. Hepatocytes Contribute to Residual Glucose Production in a Mouse Model for Glycogen Storage Disease Type Ia. Hepatology 2017, 66, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Chen, H.; Pan, C.; Ward, J.M.; Mosinger, B.; Lee, E.J.; Westphal, H.; Mansfield, B.C.; Chou, J.Y. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat. Genet. 1996, 13, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mutel, E.; Abdul-Wahed, A.; Ramamonjisoa, N.; Stefanutti, A.; Houberdon, I.; Cavassila, S.; Pilleul, F.; Beuf, O.; Gautier-Stein, A.; Penhoat, A.; et al. Targeted Deletion of Liver Glucose-6 Phosphatase Mimics Glycogen Storage Disease Type 1a Including Development of Multiple Adenomas. J. Hepatol. 2011, 54, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Resaz, R.; Vanni, C.; Segalerba, D.; Sementa, A.R.; Mastracci, L.; Grillo, F.; Murgia, D.; Bosco, M.C.; Chou, J.Y.; Barbieri, O.; et al. Development of Hepatocellular Adenomas and Carcinomas in Mice with Liver-Specific G6Pase-Aα Deficiency. DMM Dis. Model. Mech. 2014, 7, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.G.S.; Derks, T.G.J.; Huijkman, N.C.A.; Bos, T.; Kloosterhuis, N.J.; van de Kolk, K.C.W.A.; Wolters, J.C.; Koster, M.H.; Bongiovanni, L.; Thomas, R.E.; et al. Modeling Phenotypic Heterogeneity of Glycogen Storage Disease Type 1a Liver Disease in Mice by Somatic CRISPR/CRISPR-Associated Protein 9-Mediated Gene Editing. Hepatology 2021, 74, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Katagami, Y.; Kondo, T.; Suga, M.; Yada, Y.; Imamura, K.; Shibukawa, R.; Sagara, Y.; Okanishi, Y.; Tsukita, K.; Hirayama, K.; et al. Generation of a Human Induced Pluripotent Stem Cell Line, BRCi009-A, Derived from a Patient with Glycogen Storage Disease Type 1a. Stem Cell Res. 2020, 49, 102095. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes and CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Ziegler, W.H.; Lüdiger, S.; Hassan, F.; Georgiadis, M.E.; Swolana, K.; Khera, A.; Mertens, A.; Franke, D.; Wohlgemuth, K.; Dahmer-Heath, M.; et al. Primary URECs: A Source to Better Understand the Pathology of Renal Tubular Epithelia in Pediatric Hereditary Cystic Kidney Diseases. Orphanet J. Rare Dis. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Belche, V.; Schaller, K.; Grünert, S.C.; Kaech, A.; Baumgartner, M.R.; Kölker, S.; Hannibal, L.; Spiekerkoetter, U. Mitochondrial Damage in Renal Epithelial Cells Is Potentiated by Protein Exposure in Propionic Aciduria. J. Inherit. Metab. Dis. 2021, 44, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Schumann, A.; Berquez, M.; Chen, Z.; Nieri, D.; Failli, M.; Debaix, H.; Festa, B.P.; Tokonami, N.; Raimondi, A.; et al. Impaired Mitophagy Links Mitochondrial Disease to Epithelial Stress in Methylmalonyl-CoA Mutase Deficiency. Nat. Commun. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of Human Induced Pluripotent Stem Cells from Urine Samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef] [PubMed]


| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Age (years) | 40 | 44 | 29 |
| Diagnosis | Enzimatic | Enzimatic | Biochemical |
| Gender | Male | Male | Male |
| G6Pase Mutations | p.R83C p.R170Q | p.R83C p.Q20R | NA |
| eGFR mL/min/1.73 m2 | 119 | 110 | 140 |
| Proteinuria (g/24 h) * | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzini, L.; Iori, E.; Scannapieco, F.; Carraro, G.; Avogaro, A.; Vitturi, N. Urine-Derived Epithelial Cells as a New Model to Study Renal Metabolic Phenotypes of Patients with Glycogen Storage Disease 1a. Int. J. Mol. Sci. 2023, 24, 232. https://doi.org/10.3390/ijms24010232
Lenzini L, Iori E, Scannapieco F, Carraro G, Avogaro A, Vitturi N. Urine-Derived Epithelial Cells as a New Model to Study Renal Metabolic Phenotypes of Patients with Glycogen Storage Disease 1a. International Journal of Molecular Sciences. 2023; 24(1):232. https://doi.org/10.3390/ijms24010232
Chicago/Turabian StyleLenzini, Livia, Elisabetta Iori, Federico Scannapieco, Gianni Carraro, Angelo Avogaro, and Nicola Vitturi. 2023. "Urine-Derived Epithelial Cells as a New Model to Study Renal Metabolic Phenotypes of Patients with Glycogen Storage Disease 1a" International Journal of Molecular Sciences 24, no. 1: 232. https://doi.org/10.3390/ijms24010232
APA StyleLenzini, L., Iori, E., Scannapieco, F., Carraro, G., Avogaro, A., & Vitturi, N. (2023). Urine-Derived Epithelial Cells as a New Model to Study Renal Metabolic Phenotypes of Patients with Glycogen Storage Disease 1a. International Journal of Molecular Sciences, 24(1), 232. https://doi.org/10.3390/ijms24010232







