Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii
Abstract
1. Introduction
2. Results
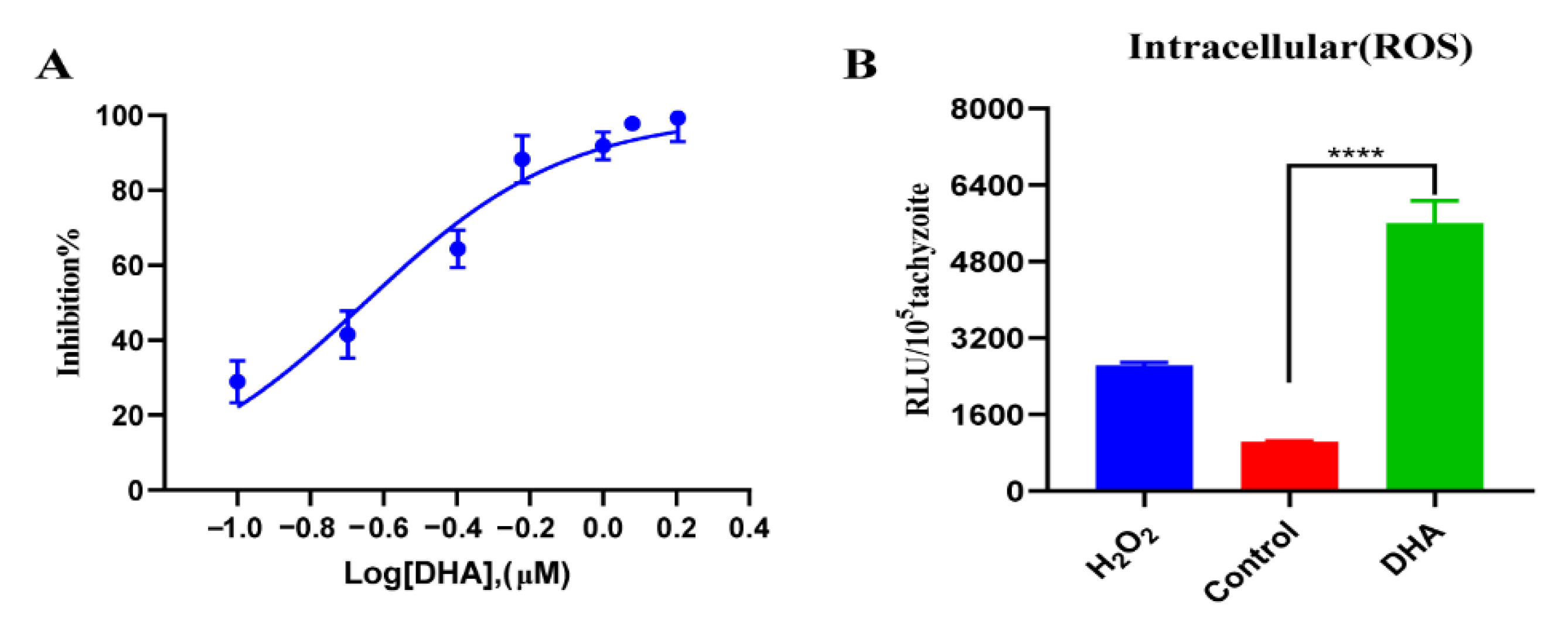
2.1. DHA Treatment Causes Growth Inhibition and Excessive ROS Accumulation in T. gondii Tachyzoites
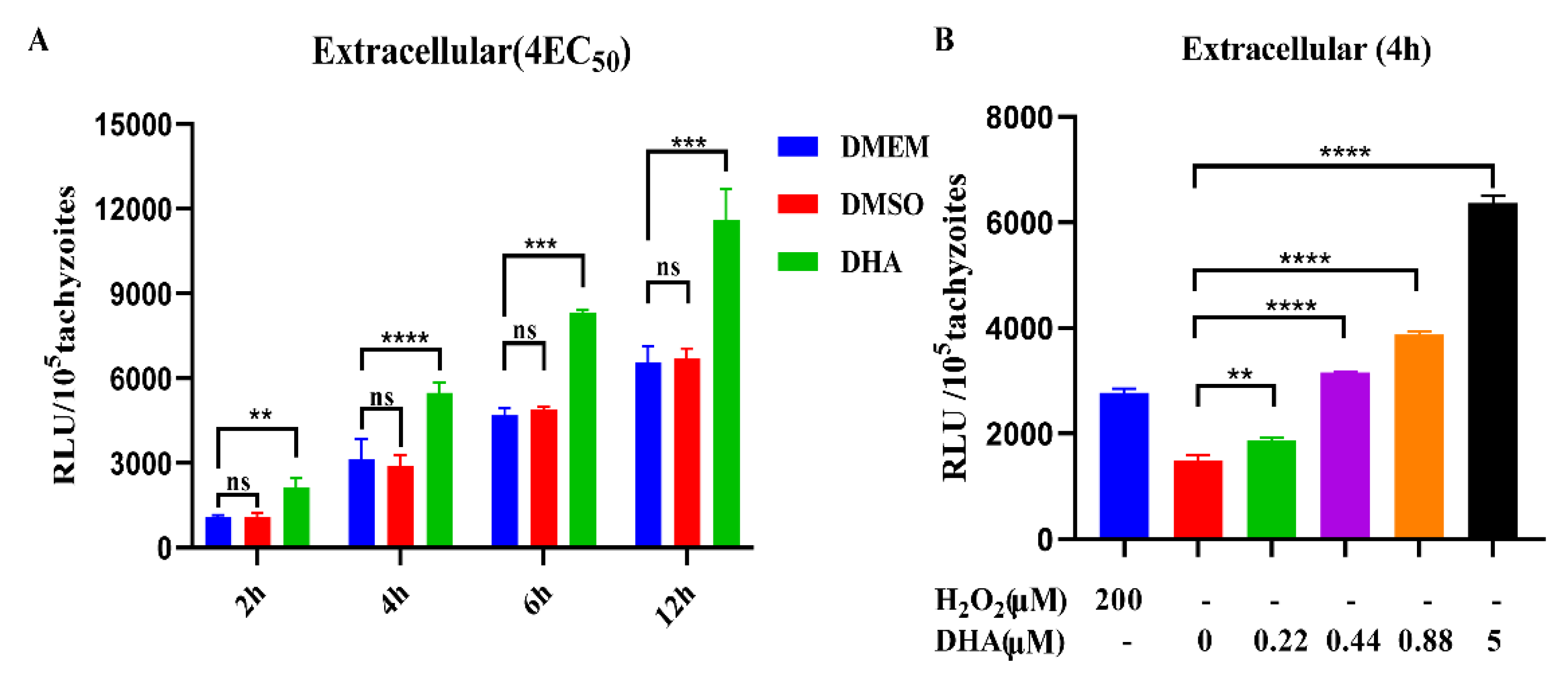
2.2. DHA Causes a Dose-Dependent Increase in ROS Levels in Tachyzoites
2.3. DHA Treatment Reduces Mitochondrial Membrane Potential
2.4. DHA Treatment Decreases the Transcription Levels of Important Antioxidant Genes in T. gondii
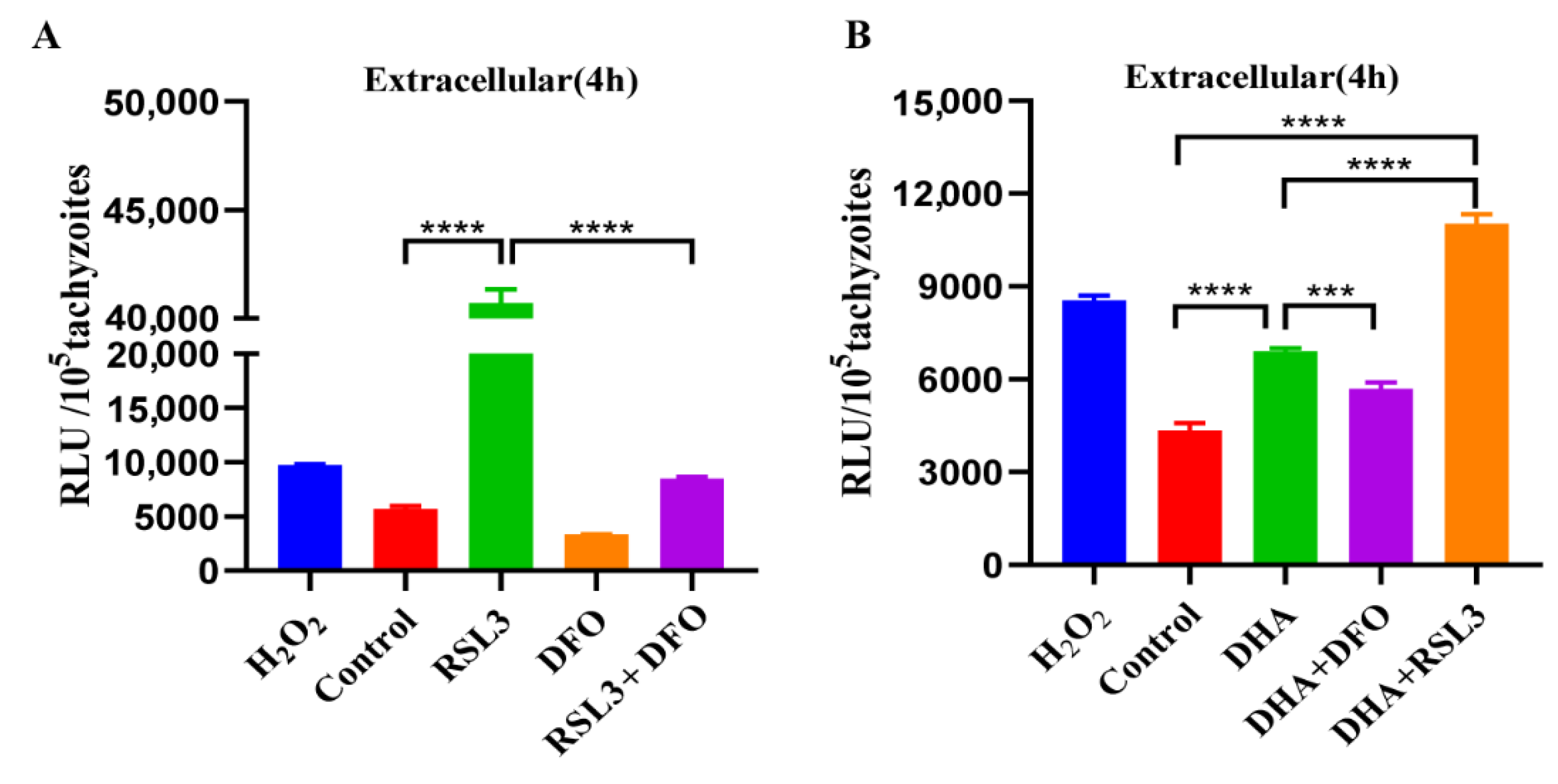
2.5. DHA and Ferroptosis Inducers Simultaneously Increase ROS in T. gondii
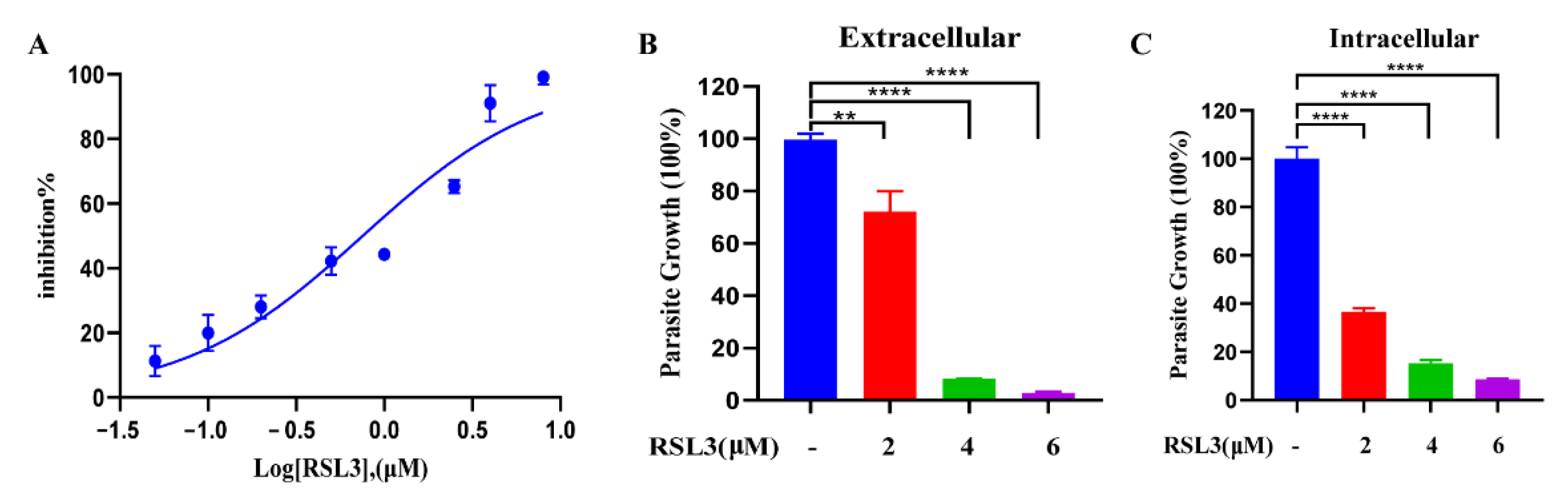
2.6. The Ferroptosis Inducer RSL3 Has a Potent Inhibitory Effect on Intracellular and Extracellular Tachyzoites In Vitro
2.7. Antiparasitic Effects of DHA with Ferroptosis Inducers and Inhibitors
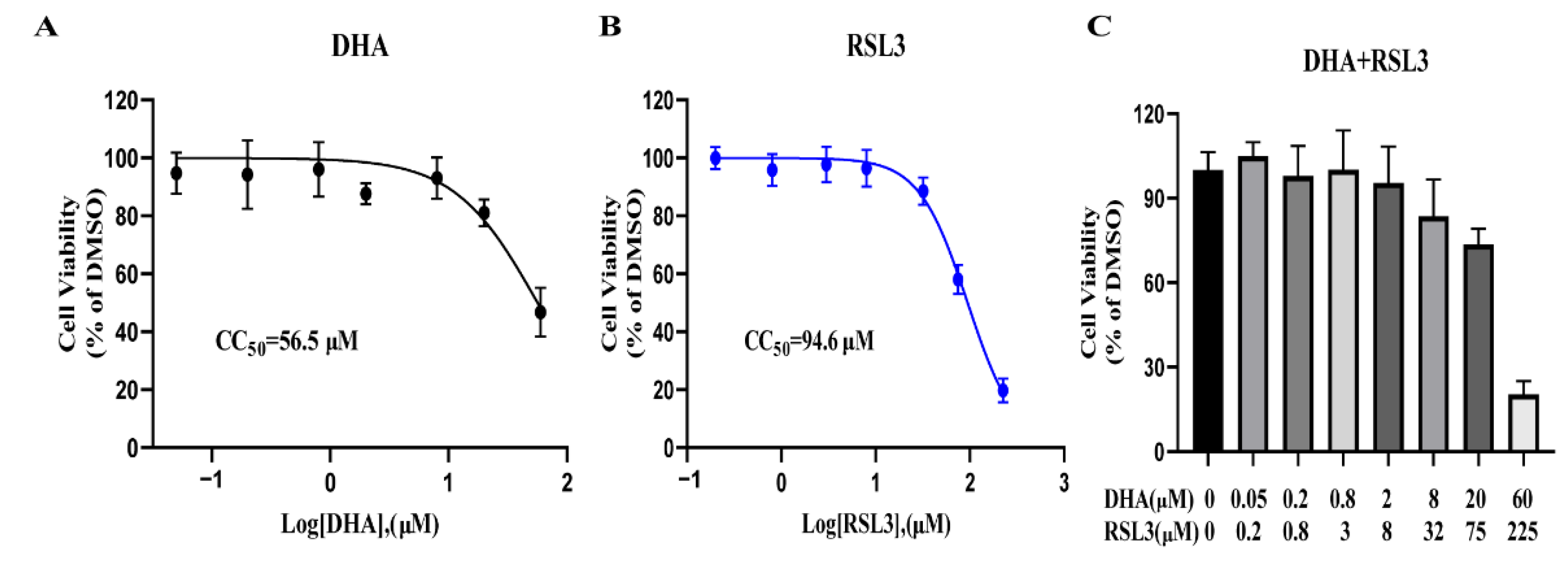
2.8. RSL3 and DHA Is Not Toxic to Host Cells at Antiparasitic Concentration
3. Discussion
4. Materials and Methods
4.1. Parasites and Cell Culture
4.2. Compounds
4.3. Growth Inhibition Assay
4.4. ROS Assay
4.5. Mitochondrial Membrane Potential (Δψ) Assay
4.6. Quantitative Real-Time (qPCR) Analysis of Antioxidant Genes
4.7. Cytotoxicity Test
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Amouei, A.; Hosseininejad, Z.; Daryani, A. The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wang, S.C.; Liu, H.H.; Ma, H.Y.; Li, Z.Y.; Wei, F.; Zhu, X.Q.; Liu, Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188. [Google Scholar] [CrossRef] [PubMed]
- Alday, H.P.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Devel. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef]
- Silva, M.D.; Teixeira, C.; Gomes, P.; Borges, M. Promising Drug Targets and Compounds with Anti-Toxoplasma gondii Activity. Microorganisms 2021, 9, 1960. [Google Scholar] [CrossRef]
- Mack, D.G.; McLeod, R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: Comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob. Agents Chemother. 1984, 26, 26–30. [Google Scholar] [CrossRef]
- Araujo, F.G.; Suzuki, Y.; Remington, J.S. Use of rifabutin in combination with atovaquone, clindamycin, pyrimethamine, or sulfadiazine for treatment of toxoplasmic encephalitis in mice. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 394–397. [Google Scholar] [CrossRef]
- Fernandez-Martin, J.; Leport, C.; Morlat, P.; Meyohas, M.C.; Chauvin, J.P.; Vilde, J.L. Pyrimethamine-clarithromycin combination for therapy of acute Toxoplasma encephalitis in patients with AIDS. Antimicrob. Agents Chemother. 1991, 35, 2049–2052. [Google Scholar] [CrossRef]
- Felix, J.P.F.; Lira, R.P.C.; Zacchia, R.S.; Toribio, J.M.; Nascimento, M.A.; Arieta, C.E.L. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: Randomized controlled clinical trial. Am. J. Ophthalmol. 2014, 157, 762–766.e1. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Klayman, D.L.; Milhous, W.K. Antimalarial activity of new water-soluble dihydroartemisinin derivatives. J. Med. Chem. 1987, 30, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Straimer, J.; Gnädig, N.F.; Witkowski, B.; Amaratunga, C.; Duru, V.; Ramadani, A.P.; Dacheux, M.; Khim, N.; Zhang, L.; Lam, S.; et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347, 428–431. [Google Scholar] [CrossRef]
- Deng, H.; Huang, X.; Jin, C.; Jin, C.M.; Quan, Z.S. Synthesis, in vitro and in vivo biological evaluation of dihydroartemisinin derivatives with potential anti-Toxoplasma gondii agents. Bioorg. Chem. 2020, 94, 103467. [Google Scholar] [CrossRef]
- Harding, C.R.; Sidik, S.M.; Petrova, B.; Gnädig, N.F.; Okombo, J.; Herneisen, A.L.; Ward, K.E.; Markus, B.M.; Boydston, E.A.; Fidock, D.A.; et al. Genetic screens reveal a central role for heme metabolism in artemisinin susceptibility. Nat. Commun. 2020, 11, 4813. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188.e1. [Google Scholar] [CrossRef]
- Chen, Y.; Mi, Y.; Zhang, X.; Ma, Q.; Song, Y.; Zhang, L.; Wang, D.; Xing, J.; Hou, B.; Li, H.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Li, Y.; Ren, X.; Zhou, Y.; Hu, W.; Zhou, C.; Jing, Q.; Yang, C.; Wang, L.; et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021, 12, 705. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Z.; Chen, L.; Zhou, Y.; Zou, P.; Feng, C.; Wang, L.; Liang, G. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016, 381, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Zhang, R.; Wang, F.; Wang, T.; Jiao, Y. The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. Onco. Targets Ther. 2020, 13, 5429–5441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Ghoochani, A.; Hsu, E.C.; Aslan, M.; Rice, M.A.; Nguyen, H.M.; Brooks, J.D.; Corey, E.; Paulmurugan, R.; Stoyanova, T. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res. 2021, 81, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, W.; Wang, H.; Tang, T.; Ma, J.; Cui, Z.; Shi, H.; Qin, T.; Zhou, H.; Li, L.; et al. Ferroptosis plays an essential role in the antimalarial mechanism of low-dose dihydroartemisinin. Biomed. Pharmacother. 2022, 148, 112742. [Google Scholar] [CrossRef]
- Ducournau, C.; Moiré, N.; Carpentier, R.; Cantin, P.; Herkt, C.; Lantier, I.; Betbeder, D.; Dimier-Poisson, I. Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep. Front. Immunol. 2020, 11, 2183. [Google Scholar] [CrossRef]
- Montazeri, M.; Sharif, M.; Sarvi, S.; Mehrzadi, S.; Ahmadpour, E.; Daryani, A. A Systematic Review of in vitro and in vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006–2016). Front. Microbiol. 2017, 8, 25. [Google Scholar] [CrossRef]
- Sarciron, M.E.; Saccharin, C.; Petavy, A.F.; Peyron, F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. Am. J. Trop. Med. Hyg. 2000, 62, 73–76. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Van Oppen, L.M.; Schöckel, L.; Bossenbroek, H.M.; Van Emst-de Vries, S.E.; Hermeling, J.C.; Grefte, S.; Kopitz, C.; Heroult, M.; HGMWillems, P.; et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Kwok, L.Y.; Schlüter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Kaasch, A.J.; Joiner, K.A. Targeting and subcellular localization of Toxoplasma gondii catalase. Identification of peroxisomes in an apicomplexan parasite. J. Biol. Chem. 2000, 275, 1112–1118. [Google Scholar] [CrossRef]
- Balamurugan, M.; Santharaman, P.; Madasamy, T.; Rajesh, S.; Sethy, N.K.; Bhargava, K.; Kotamraju, S.; Karunakaran, C. Recent trends in electrochemical biosensors of superoxide dismutases. Biosens. Bioelectron. 2018, 116, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Clayton, C.; Soldati, D. Toxoplasma gondii catalase: Are there peroxisomes in toxoplasma? J. Cell Sci. 2000, 113 Pt 13, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Britigan, B.E. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 1997, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ödberg-Ferragut, C.; Renault, J.P.; Viscogliosi, E.; Toursel, C.; Briche, I.; Engels, A.; Lepage, G.; Morgenstern-Badarau, I.; Camus, D.; Tomavo, S.; et al. Molecular cloning, expression analysis and iron metal cofactor characterisation of a superoxide dismutase from Toxoplasma gondii. Mol. Biochem. Parasitol. 2000, 106, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Son, E.S.; Song, K.J.; Shin, J.C.; Nam, H.W. Molecular cloning and characterization of peroxiredoxin from Toxoplasma gondii. Korean J. Parasitol. 2001, 39, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vercesi, A.E.; Rodrigues, C.O.; Uyemura, S.A.; Zhong, L.; Moreno, S.N. Respiration and oxidative phosphorylation in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 1998, 273, 31040–31047. [Google Scholar] [CrossRef]
- Huffman, A.M.; Ayariga, J.A.; Napier, A.; Robertson, B.K.; Abugri, D.A. Inhibition of Toxoplasma gondii Growth by Dihydroquinine and Its Mechanisms of Action. Front. Cell Infect. Microbiol. 2022, 12, 852889. [Google Scholar] [CrossRef]
- Zhao, X.; Hong, Y.; Drlica, K. Moving forward with reactive oxygen species involvement in antimicrobial lethality. J. Antimicrob. Chemother. 2015, 70, 639–642. [Google Scholar] [CrossRef]
- Lavine, M.D.; Arrizabalaga, G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS ONE 2012, 7, e42107. [Google Scholar] [CrossRef]
- Rizk, Y.S.; Santos-Pereira, S.; Gervazoni, L.; Hardoim, D.D.J.; Cardoso, F.D.O.; de Souza, C.D.S.F.; Pelajo-Machado, M.; Carollo, C.A.; de Arruda, C.C.P.; Almeida-Amaral, E.E.; et al. Amentoflavone as an Ally in the Treatment of Cutaneous Leishmaniasis: Analysis of Its Antioxidant/Prooxidant Mechanisms. Front. Cell Infect. Microbiol. 2021, 11, 615814. [Google Scholar] [CrossRef]
- Akerman, S.E.; Müller, S. Peroxiredoxin-linked detoxification of hydroperoxides in Toxoplasma gondii. J. Biol. Chem. 2005, 280, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int. J. Mol. Sci. 2021, 22, 5705. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Cao, X.; Jiang, Y.; Shi, Y.; Ma, Y.; Hu, D.; Song, X. Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii. Int. J. Mol. Sci. 2023, 24, 229. https://doi.org/10.3390/ijms24010229
Huang M, Cao X, Jiang Y, Shi Y, Ma Y, Hu D, Song X. Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii. International Journal of Molecular Sciences. 2023; 24(1):229. https://doi.org/10.3390/ijms24010229
Chicago/Turabian StyleHuang, Mao, Xinru Cao, Yucong Jiang, Yuehong Shi, Yazhen Ma, Dandan Hu, and Xingju Song. 2023. "Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii" International Journal of Molecular Sciences 24, no. 1: 229. https://doi.org/10.3390/ijms24010229
APA StyleHuang, M., Cao, X., Jiang, Y., Shi, Y., Ma, Y., Hu, D., & Song, X. (2023). Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii. International Journal of Molecular Sciences, 24(1), 229. https://doi.org/10.3390/ijms24010229






