Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area
Abstract
1. Introduction
2. Results
2.1. Physical and Chemical Properties of SS/PVA
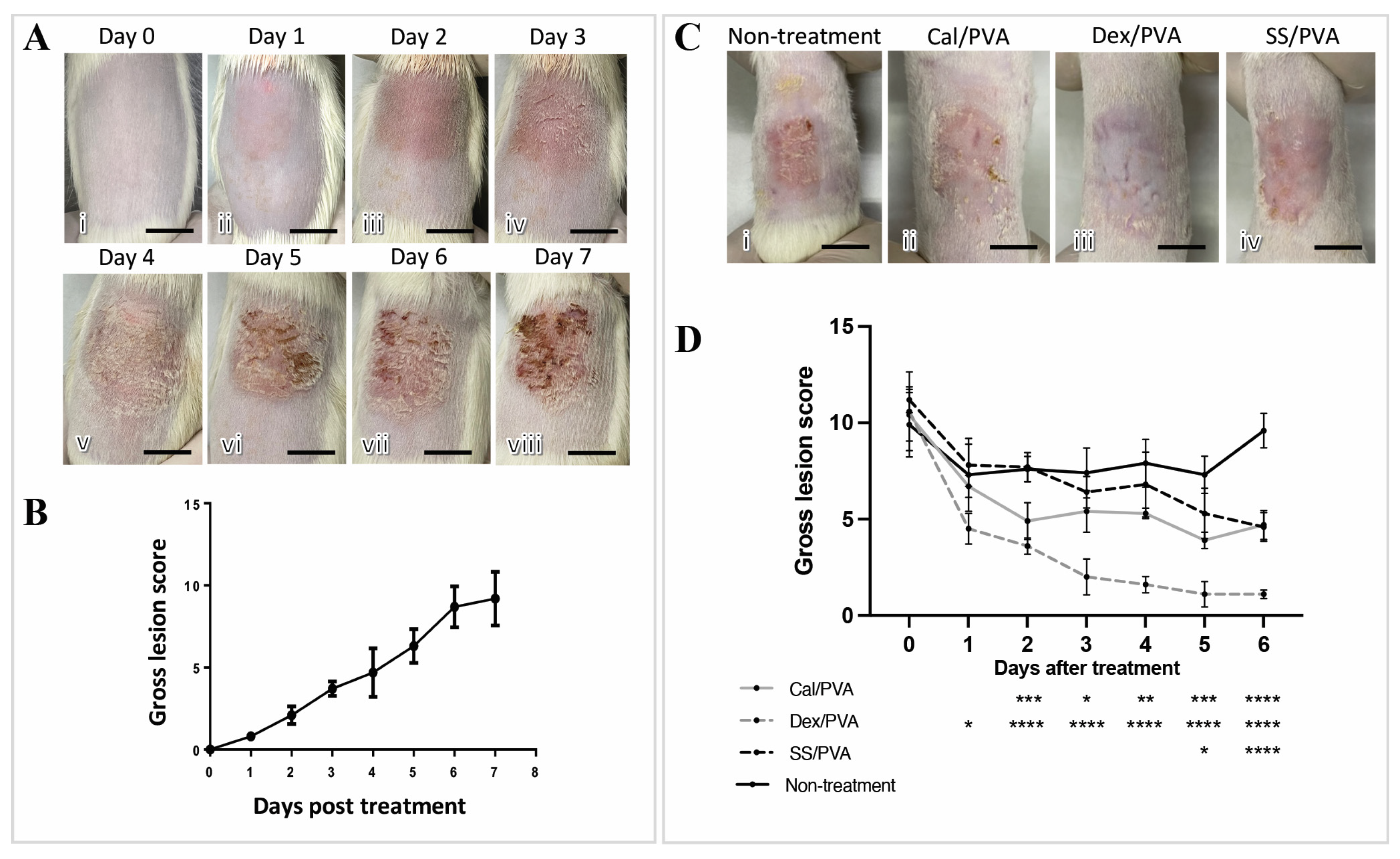
2.2. SS/PVA Reduced the Severity of Psoriatic Gross Skin Lesions in Imiquimod-Induced Psoriasis
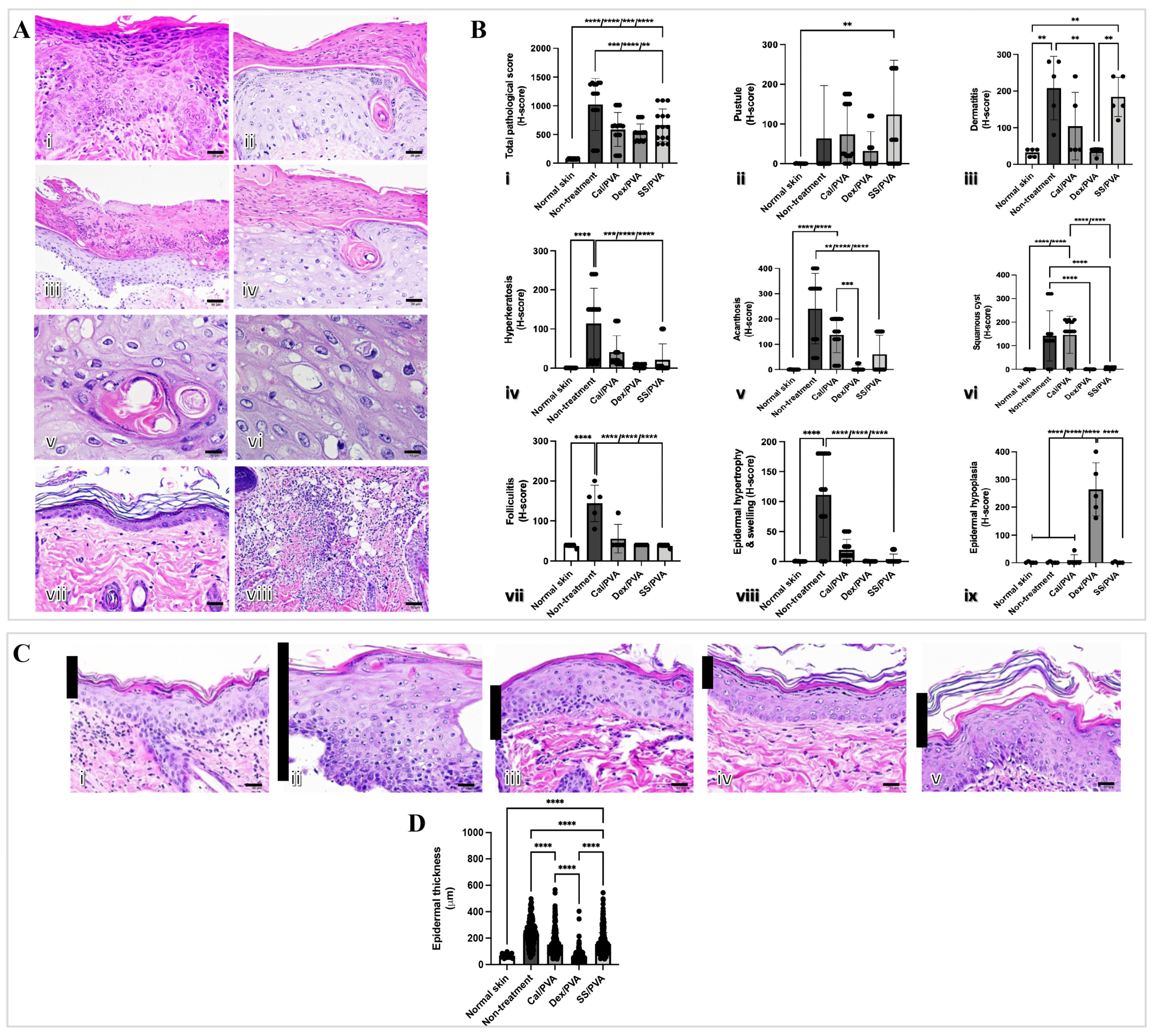
2.3. SS/PVA Reduced Epidermal Thickness and Histopathological Scores in a Psoriatic Rat Model
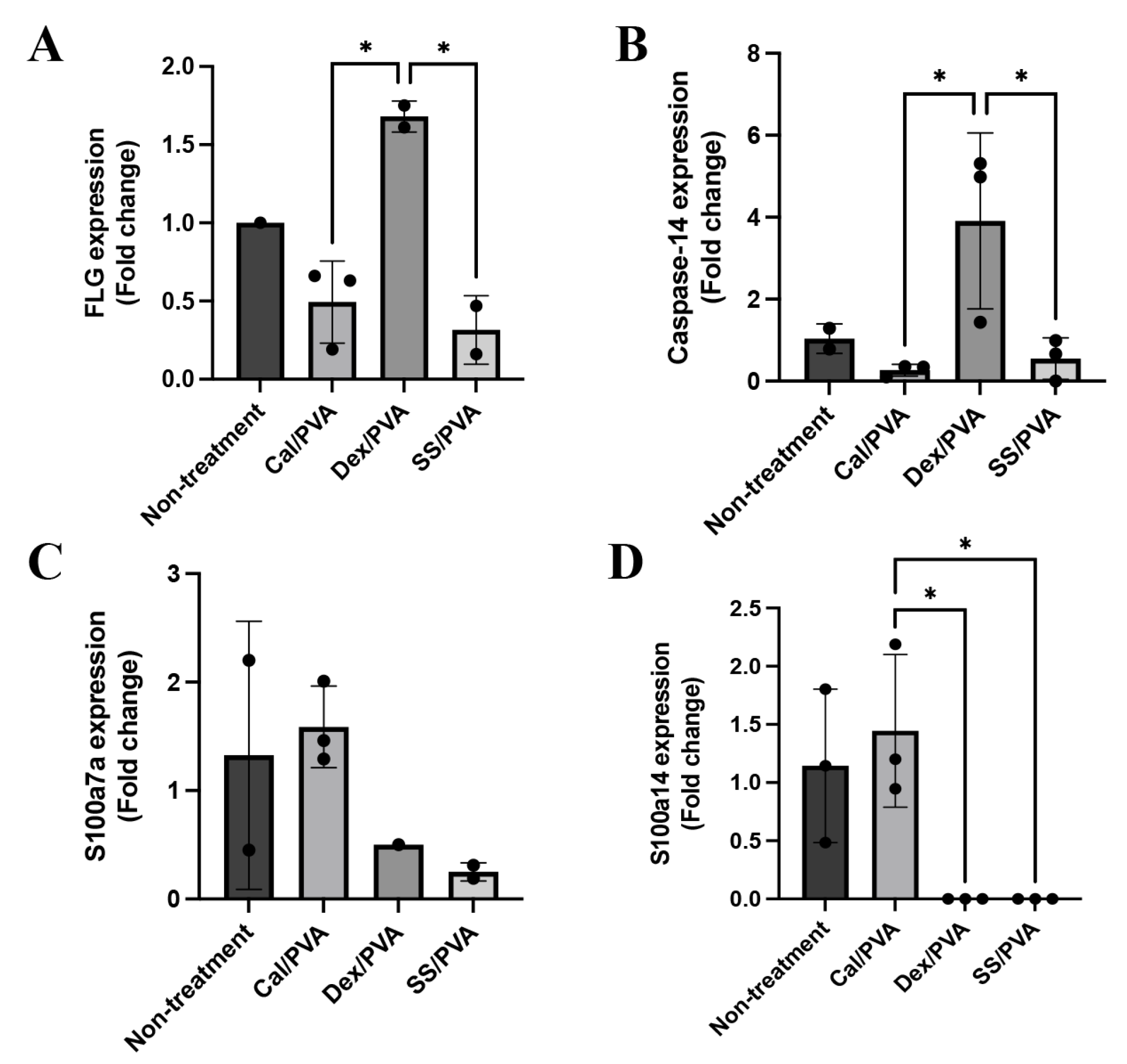
2.4. SS/PVA Modulated Inflammation, Apoptosis, and Antioxidative Properties
2.5. Effect of Sericin on the Expression Levels of Psoriatic Genes in a Rat Model
2.6. Complete Blood Count and Clinical Blood Chemistry
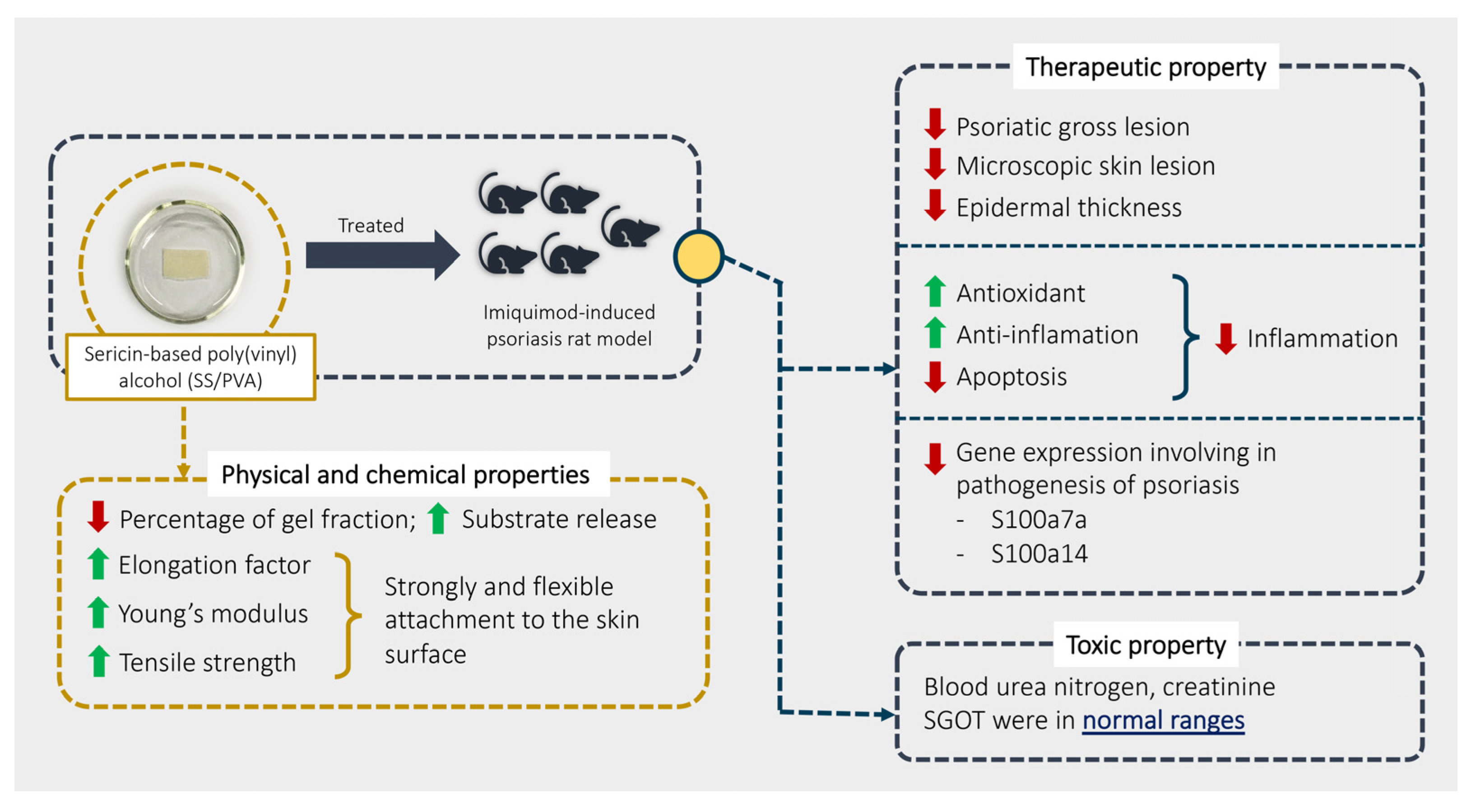
3. Discussion
4. Materials and Methods
4.1. Sericin Extraction
4.2. Hydrogel Preparation
4.3. Physical and Chemical Properties
4.4. Animal Experimental Protocol
4.4.1. Animal Ethics Statement
4.4.2. Induction of Psoriasis and Experimental Protocol
4.5. Sample Collection
4.6. Histopathological and Immunohistochemistry Studies
4.6.1. Histopathological Examination
4.6.2. Immunohistochemistry
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7.1. RNA Extraction
4.7.2. qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ampawong, S.; Kengkoom, K.; Sukphopetch, P.; Aramwit, P.; Muangkaew, W.; Kanjanapruthipong, T.; Buaban, T. Evaluating the effect of rice (Oryza sativa L.: SRNC05053-6-2) crude extract on psoriasis using in vitro and in vivo models. Sci. Rep. 2020, 10, 17618. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Lin, C.F.; Alalaiwe, A.; Yang, S.C.; Fang, J.Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef]
- Deenonpoe, R.; Prayong, P.; Thippamom, N.; Meephansan, J.; Na-Bangchang, K. Anti-inflammatory effect of naringin and sericin combination on human peripheral blood mononuclear cells (hPBMCss) from patient with psoriasis. BMC Complement. Altern. Med. 2019, 19, 168. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Bonani, W.; Yang, Y.; Motta, A.; Aramwit, P. Fibroin and Polyvinyl Alcohol Hydrogel Wound Dressing Containing Silk Sericin Prepared Using High-Pressure Carbon Dioxide. Adv. Wound. Care 2019, 8, 452–462. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhao, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm Sericin: Properties and Biomedical Applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Raj, B.J.S.J. Therapeutic applications and properties of silk proteins from Bombyx mori. Front. Life Sci. 2012, 6, 55–60. [Google Scholar]
- Wang, W.H.; Lin, W.S.; Shih, C.H.; Chen, C.Y.; Kuo, S.H.; Li, W.L.; Lin, Y.S. Functionality of Silk Cocoon (Bombyx mori L.) Sericin Extracts Obtained through High-Temperature Hydrothermal Method. Materials 2021, 14, 5431. [Google Scholar] [CrossRef]
- Rujimongkon, K.; Ampawong, S.; Reamtong, O.; Buaban, T.; Aramwit, P. The therapeutic effects of Bombyx mori sericin on rat skin psoriasis through modulated epidermal immunity and attenuated cell proliferation. J. Tradit. Complement. Med. 2021, 11, 587–597. [Google Scholar] [CrossRef]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.S.; Campos, E.V.R.; Fraceto, L.F.; Del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; de Araujo, D.R.; Fernandez-Luqueno, F.; Grillo, R.; Patra, J.K. Sericin based nanoformulations: A comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J. Nanobiotechnol. 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Pornanong Aramwit, A.S. Rungnapha Yamdech, Sericin Ameliorates the Properties of Poly(Vinyl Alcohol) Hydrogel Prepared by Simple Repeated Freeze-Thaw Process without the Use of Chemical Crosslinking. Int. J. Res. Sci. 2018, 4, 6–11. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.M.; Chaves-Rodriquez, M.I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid. Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef]
- Lisi, P. Differential diagnosis of psoriasis. Reumatismo 2007, 59 (Suppl. 1), 56–60. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef]
- Arnold, W.P. Tar. Clin. Derm. 1997, 15, 739–744. [Google Scholar] [CrossRef]
- Sekhon, S.; Jeon, C.; Nakamura, M.; Afifi, L.; Yan, D.; Wu, J.J.; Liao, W.; Bhutani, T. Review of the mechanism of action of coal tar in psoriasis. J. Dermatol. Treat. 2018, 29, 230–232. [Google Scholar] [CrossRef]
- Weinstein, G.D.; Krueger, G.G.; Lowe, N.J.; Duvic, M.; Friedman, D.J.; Jegasothy, B.V.; Jorizzo, J.L.; Shmunes, E.; Tschen, E.H.; Lew-Kaya, D.A.; et al. Tazarotene gel, a new retinoid, for topical therapy of psoriasis: Vehicle-controlled study of safety, efficacy, and duration of therapeutic effect. J. Am. Acad. Derm. 1997, 37, 85–92. [Google Scholar] [CrossRef]
- Benezeder, T.; Painsi, C.; Patra, V.; Dey, S.; Holcmann, M.; Lange-Asschenfeldt, B.; Sibilia, M.; Wolf, P. Dithranol targets keratinocytes, their crosstalk with neutrophils and inhibits the IL-36 inflammatory loop in psoriasis. eLife 2020, 9, e56991. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef] [PubMed]
- Hansel, A.; Gunther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schakel, K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.e1-9. [Google Scholar] [CrossRef] [PubMed]
- Schadler, E.D.; Ortel, B.; Mehlis, S.L. Biologics for the primary care physician: Review and treatment of psoriasis. Dis. Mon. 2019, 65, 51–90. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, F.; Bertino, L.; Pioggia, G.; Casciaro, M.; Gangemi, S. Therapies with Antioxidant Potential in Psoriasis, Vitiligo, and Lichen Planus. Antioxidants 2021, 10, 1087. [Google Scholar] [CrossRef]
- Guarneri, F.; Sapienza, D.; Papaianni, V.; Marafioti, I.; Guarneri, C.; Mondello, C.; Roccuzzo, S.; Asmundo, A.; Cannavò, S.P. Association between genetic polymorphisms of glutathione S-transferase M1/T1 and psoriasis in a population from the area of the strict of messina (Southern Italy). Free Radic. Res. 2020, 54, 57–63. [Google Scholar] [CrossRef]
- Elango, T.; Dayalan, H.; Gnanaraj, P.; Malligarjunan, H.; Subramanian, S. Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin. Exp. Med. 2014, 14, 431–437. [Google Scholar] [CrossRef]
- Dujic, J.; Kippenberger, S.; Hoffmann, S.; Ramirez-Bosca, A.; Miquel, J.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J. Investig. Derm. 2007, 127, 1992–2000. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Cai, Q.; Ren, Q.; Wei, L. Red Light Combined with Blue Light Irradiation Regulates Proliferation and Apoptosis in Skin Keratinocytes in Combination with Low Concentrations of Curcumin. PLoS ONE 2015, 10, e0138754. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar]
- Kastelan, M.; Prpic-Massari, L.; Brajac, I. Apoptosis in psoriasis. Acta Derm. Croat. 2009, 17, 182–186. [Google Scholar]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Bebars, S.M.M.; Al-Sharaky, D.R.; Gaber, M.A.; Afify, D.R. Immunohistochemical Expression of Caspase-3 in Psoriasis. J. Clin. Diagn Res. 2017, 11, EC01–EC05. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.; Crane, J.S. Histology, Mast Cells. StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kubo, M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Nilsson, G.; Suttle, M.-M.; Naukkarinen, A. Is there a role for mast cells in psoriasis? Arch. Dermatol. Res. 2008, 300, 461–478. [Google Scholar] [CrossRef]
- Chiang, C.C.; Cheng, W.J.; Korinek, M.; Lin, C.Y.; Hwang, T.L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Schon, M.P.; Broekaert, S.M.; Erpenbeck, L. Sexy again: The renaissance of neutrophils in psoriasis. Exp. Derm. 2017, 26, 305–311. [Google Scholar] [CrossRef]
- Hu, S.C.; Yu, H.S.; Yen, F.L.; Lin, C.L.; Chen, G.S.; Lan, C.C. Neutrophil extracellular trap formation is increased in psoriasis and induces human beta-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Penn, L.; Brinster, N.K. Eosinophils Among the Histological Features of Psoriasis. Am. J. Dermatopathol. 2019, 41, 347–349. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Folds, J.D.; Evans, D.L. Dexamethasone effects on numbers of cells in lymphocyte subpopulations: Changes associated with major depression and DST nonsuppression. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Crish, J.F.; Efimova, T.; Dashti, S.R.; Deucher, A.; Bone, F.; Adhikary, G.; Huang, G.; Gopalakrishnan, R.; Balasubramanian, S. Regulation of involucrin gene expression. J. Investig. Derm. 2004, 123, 13–22. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Ski. Pharm. Physiol 2015, 28, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Man, X.Y.; Li, W.; Zhou, J.; Landeck, L.; Cai, S.Q.; Zheng, M. Regulation of involucrin in psoriatic epidermal keratinocytes: The roles of ERK1/2 and GSK-3beta. Cell Biochem. Biophys. 2013, 66, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Rungnapa Yamdej, K.P. Teerapol Srichana, and Pornanong Aramwit, Superior physicochemical and biological properties of poly(vinyl alcohol)/sericin hydrogels fabricated by a non-toxic gamma-irradiation technique. J. Bioact. Compat. Polym. 2017, 32, 32–44. [Google Scholar] [CrossRef]
- Lin, Y.K.; Yang, S.H.; Chen, C.C.; Kao, H.C.; Fang, J.Y. Using Imiquimod-Induced Psoriasis-Like Skin as a Model to Measure the Skin Penetration of Anti-Psoriatic Drugs. PLoS ONE 2015, 10, e0137890. [Google Scholar] [CrossRef]


| Parameter | Complete Blood Count and Blood Chemical Chemistry (Mean ± S.D) | ||||
|---|---|---|---|---|---|
| Standard | Hydrogel | Calcitriol | Dexamethasone | Sericin | |
| WBC (106/µL) | 4.23 ± 0.72 | 9.19 ± 4.30 | 8.25 ± 1.51 * | 2.88 ± 1.64 * | 7.71 ± 3.80 |
| RBC (106/µL) | 9.27 ± 0.63 | 7.27 ± 0.25 * | 7.52 ± 0.50 | 7.92 ± 0.25 * | 7.31 ± 0.36 |
| HGB (g/dL) | 17.78 ± 1.02 | 13.76 ± 0.58 | 14.18 ±0.86 | 14.56 ± 0.55* | 13.58 ± 0.61 * |
| HCT (%) | 56.45 ± 3.80 | 41.40 ± 1.81 | 42.30 ± 2.70 | 44.40 ± 2.41 | 40.92 ± 1.48 |
| MCV (fl) | 60.93 ± 1.75 | 56.94 ± 1.65 | 56.26 ± 2.26 | 56.00 ± 2.07 | 55.98 ± 1.04 |
| MCH (pg) | 19.20 ± 0.51 | 18.94 ± 0.49 | 18.86 ±0.58 | 18.88 ± 0.43 | 18.58 ± 0.33 |
| MCHC (g/dL) | 31.50 ± 0.38 | 33.24 ± 0.55 | 33.50 ± 0.51 | 33.72 ± 0.71 | 33.18 ± 0.31 |
| PLT (103/µL) | 804.50 ± 136.88 | 642.00 ± 54.12 * | 728.60 ± 123.32 | 473.00 ± 90.12 * | 631.40 ± 195.39 |
| RDW (%) | 17.79 ± 1.94 | 29.30 ± 1.14 | 27.82 ± 1.53 | 30.66 ± 1.52 | 28.26 ± 0.72 |
| PDW (fl) | 8.41 ± 0.45 | 8.62 ± 0.31 | 8.88 ± 0.33 | 8.26 ± 0.82 | 8.72 ± 0.48 |
| RET (K/µL) | 232.88 ± 53.78 | 411.04 ± 33.64 | 450.92 ± 119.98 | 327.36 ± 75.09 | 404.38 ± 40.20 |
| MPV (fl) | 7.63 ± 0.69 | 8.96 ± 0.29 | 8.74 ± 0.58 | 8.44 ± 1.03 | 8.84 ± 0.54 |
| PCT (%) | 0.61 ± 0.08 | 0.57 ± 0.06 * | 0.62 ± 0.14 | 0.39 ± 0.08 * | 0.56 ± 0.20 |
| Neutrophils (%) | 11.73 ± 6.98 | 15.90 ± 9.53 | 10.90 ± 11.06 | 13.00 ± 18.58 | 16.90 ± 5.51 |
| Lymphocytes (%) | 79.88 ± 7.03 | 72.42 ± 6.16 | 74.82 ± 15.05 | 60.60 ± 14.83 | 72.68 ± 5.50 |
| Eosinophils (%) | 1.17 ± 0.33 | 10.00 ± 6.30 | 12.08 ± 7.16 | 25.22 ± 8.14 * | 8.32 ± 1.74 * |
| Basophils (%) | 0.35 ± 0.38 | 1.62 ± 0.97 | 2.04 ± 1.35 | 1.02 ± 0.60 | 2.02 ± 0.82 |
| Monocytes (%) | 6.87 ± 1.49 | 0.06 ± 0.05 | 0.16 ± 0.25 | 0.16 ± 0.23 | 0.08 ± 0.08 |
| Blood urea nitrogen (mg/dL) | 20.73 ± 2.51 | 10.98 ± 5.74 | 15.84 ± 5.50 | 22.28 ± 5.36 | 23.34 ± 5.99 |
| Creatinine (mg/dL) | 0.67 ± 0.04 | 0.07 ± 0.07 | 0.14 ± 0.08 | 0.16 ± 0.05 | 0.21 ± 0.06 |
| SGPT (U/L) | 53.18 ± 10.15 | 81.86 ± 34.63 | 265.56 ± 329.42 | 475.98 ± 688.57 | 202.12 ± 166.64 |
| SGOT (U/L) | 93.73 ± 11.96 | 21.06 ± 14.07 | 49.58 ± 35.48 | 55.20 ± 46.14 | 47.30 ± 16.67 |
| Gene | Primers | |
|---|---|---|
| FLG | F | 5′AGATGTGGACCACGATGACAA3′ |
| R | 5′TAGTGCTGGATCCTCGTCTTTT3′ | |
| β-Actin | F | 5′CACTATCGGCAATGAGCGGTTCC3′ |
| R | 5′AGCACTGTGTTGGCATAGAGGTC3′ | |
| Caspase-14 | F | 5′CAGACCCTGACGGATGTGTTC3′ |
| R | 5′GCGAGGGTGCTTTGGATTTCGG3′ | |
| Involucrin | F | 5′TGTAGGGGTTTGCTGCGTAAG3′ |
| R | 5′AGTCACTGGCACTGTGTGTTG3′ | |
| S100a7a | F | 5′TAGTGTGCCTCGCTTCATGGAC3′ |
| R | 5′CACAACTGCCGGTGAAACTGA3′ | |
| S100a14 | F | 5′AACAATGGGACAGTGTCGGTC3′ |
| R | 5′ACTGCTGGGTAACCAGGTCTC3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuentam, K.; Aramwit, P.; Reamtong, O.; Supasai, S.; Chaisri, U.; Fongsodsri, K.; Yamdech, R.; Tirawanchai, N.; Sukphopetch, P.; Ampawong, S. Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area. Int. J. Mol. Sci. 2023, 24, 145. https://doi.org/10.3390/ijms24010145
Tuentam K, Aramwit P, Reamtong O, Supasai S, Chaisri U, Fongsodsri K, Yamdech R, Tirawanchai N, Sukphopetch P, Ampawong S. Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area. International Journal of Molecular Sciences. 2023; 24(1):145. https://doi.org/10.3390/ijms24010145
Chicago/Turabian StyleTuentam, Khwanchanok, Pornanong Aramwit, Onrapak Reamtong, Suangsuda Supasai, Urai Chaisri, Kamonpan Fongsodsri, Rungnapha Yamdech, Napatara Tirawanchai, Passanesh Sukphopetch, and Sumate Ampawong. 2023. "Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area" International Journal of Molecular Sciences 24, no. 1: 145. https://doi.org/10.3390/ijms24010145
APA StyleTuentam, K., Aramwit, P., Reamtong, O., Supasai, S., Chaisri, U., Fongsodsri, K., Yamdech, R., Tirawanchai, N., Sukphopetch, P., & Ampawong, S. (2023). Sericin-Based Poly(Vinyl) Alcohol Relieves Plaque and Epidermal Lesions in Psoriasis; a Chance for Dressing Development in a Specific Area. International Journal of Molecular Sciences, 24(1), 145. https://doi.org/10.3390/ijms24010145






