Ocular Ischemic Syndrome and Its Related Experimental Models
Abstract
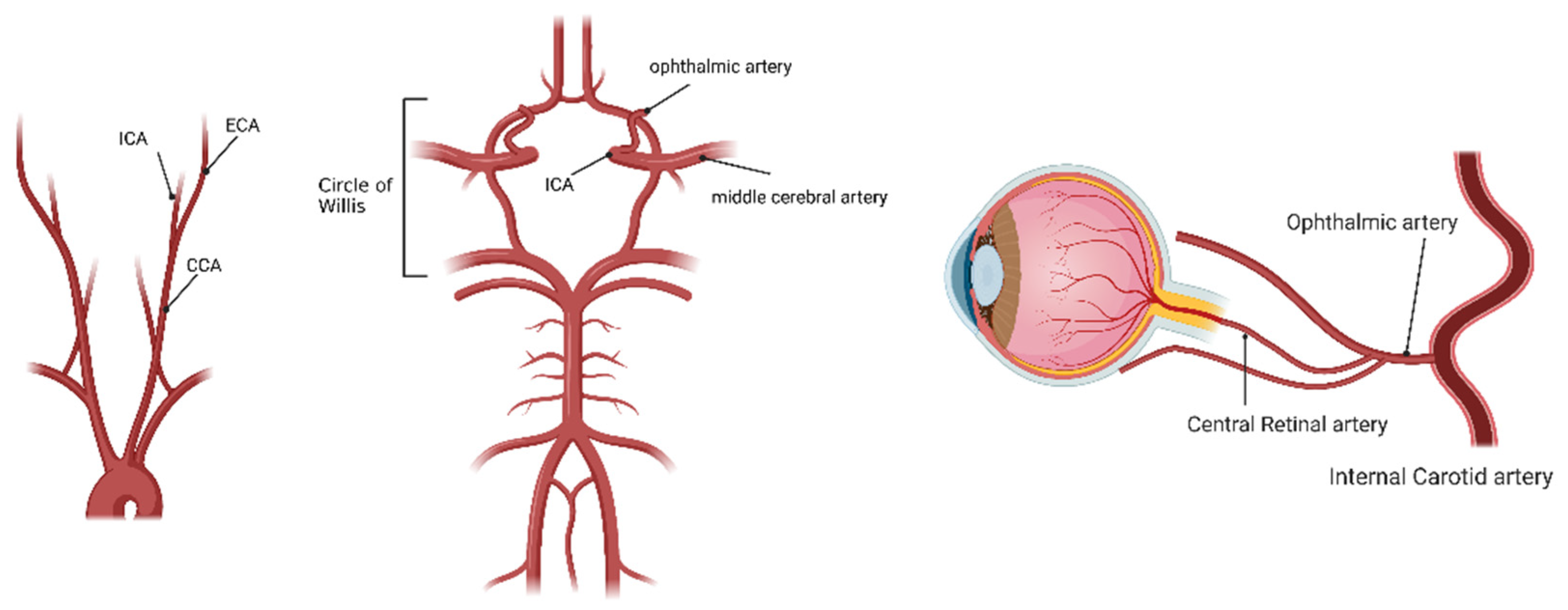
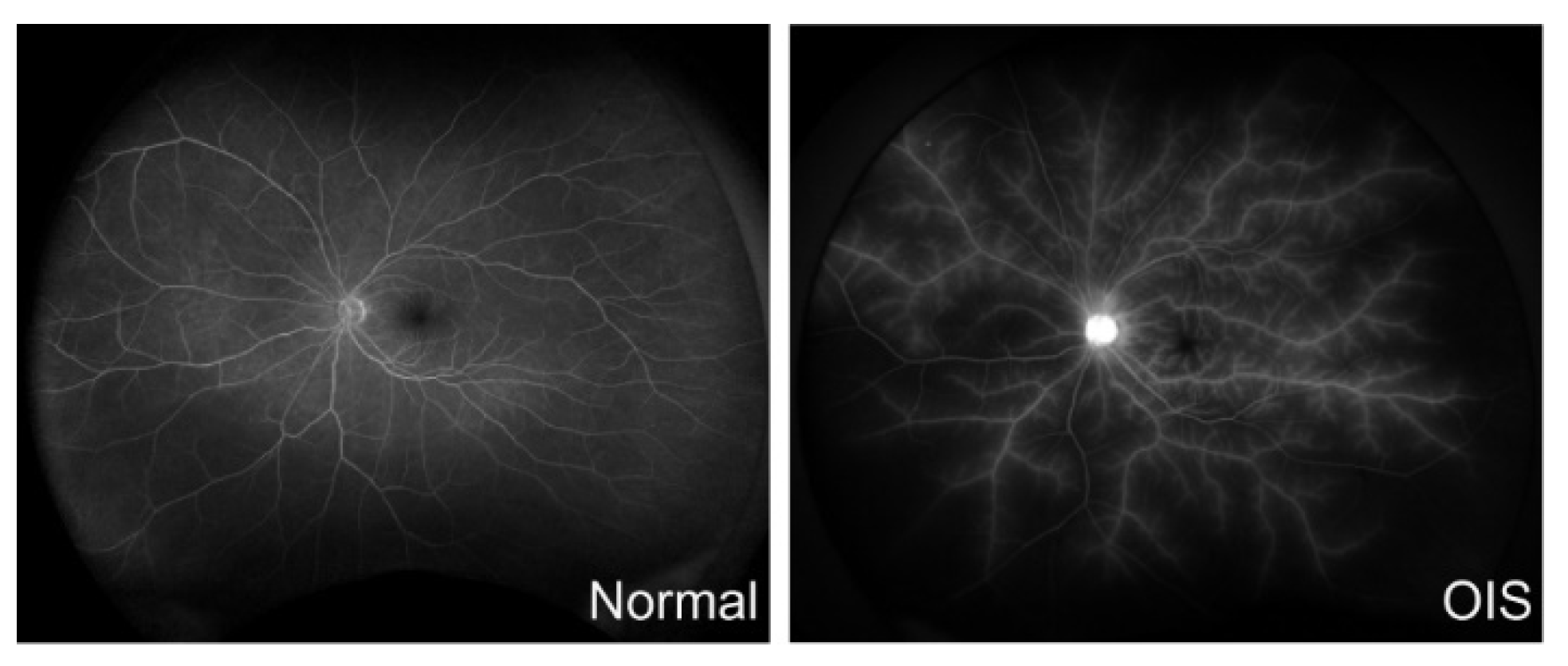
1. Ocular Ischemic Syndrome (OIS)
2. Experimental Models
2.1. Bilateral Common Carotid Artery Occlusion/Stenosis (BCCAO/BCCAS)
2.2. Unilateral Common Carotid Artery Occlusion (UCCAO)
2.3. Occlusion(s) of the Other Branches of the Carotid Artery
3. Therapeutics
3.1. Preclinical Studies
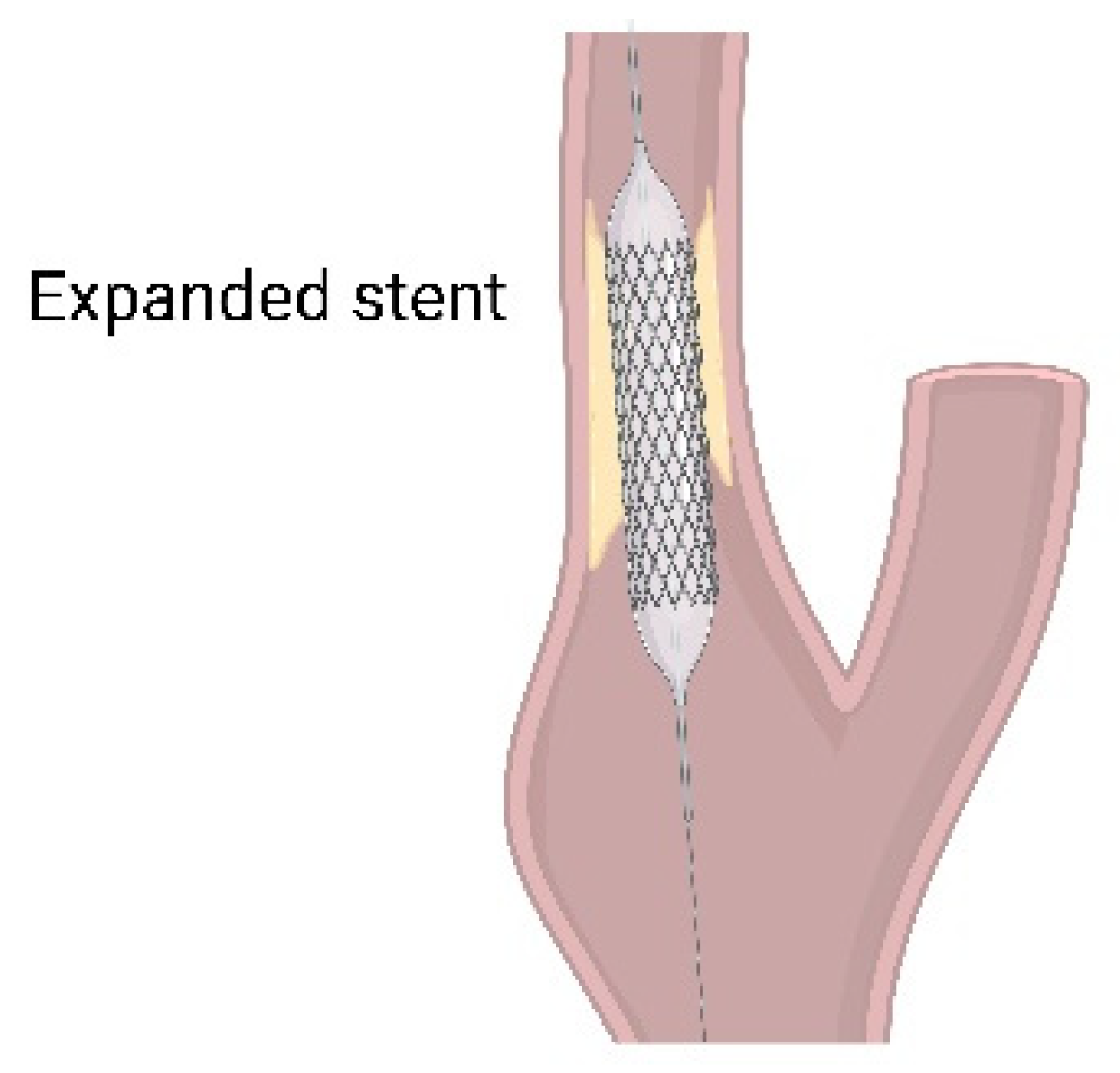
3.2. Current Clinical Treatment
3.2.1. Ocular Treatment
3.2.2. Systemic Treatment
4. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terelak-Borys, B.; Skonieczna, K.; Grabska-Liberek, I. Ocular ischemic syndrome—A systematic review. Med. Sci. Monit. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.; Stawicki, S.P. Anatomy, Head and Neck, Ophthalmic Arteries. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ros, M.A.; Magargal, L.E.; Hedges, T.R., Jr.; Simeone, F.A. Ocular ischemic syndrome: Long-term ocular complications. Ann. Ophthalmol. 1987, 19, 270–272. [Google Scholar] [PubMed]
- Hedges, T.R., Jr. Ophthalmoscopic findings in internal carotid artery occlusion. Am. J. Ophthalmol. 1963, 55, 1007–1012. [Google Scholar] [CrossRef]
- Costa, V.P.; Kuzniec, S.; Molnar, L.J.; Cerri, G.G.; Puech-Leao, P.; Carvalho, C.A. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. An investigation by color Doppler imaging. Ophthalmology 1999, 106, 306–310. [Google Scholar] [CrossRef]
- Pauk-Domanska, M.; Walasik-Szemplinska, D. Color Doppler imaging of the retrobulbar vessels in diabetic retinopathy. J. Ultrason. 2014, 14, 28–35. [Google Scholar] [CrossRef]
- Mendrinos, E.; Machinis, T.G.; Pournaras, C.J. Ocular ischemic syndrome. Surv. Ophthalmol. 2010, 55, 2–34. [Google Scholar] [CrossRef]
- Brown, G.C.; Magargal, L.E. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int. Ophthalmol. 1988, 11, 239–251. [Google Scholar] [CrossRef]
- Duker, J.S.; Belmont, J.B. Ocular ischemic syndrome secondary to carotid artery dissection. Am. J. Ophthalmol. 1988, 106, 750–752. [Google Scholar] [CrossRef]
- Hamed, L.M.; Guy, J.R.; Moster, M.L.; Bosley, T. Giant cell arteritis in the ocular ischemic syndrome. Am. J. Ophthalmol. 1992, 113, 702–705. [Google Scholar] [CrossRef]
- Sadun, A.A.; Sebag, J.; Bienfang, D.C. Complete bilateral internal carotid artery occlusion in a young man. J. Clin. Neuroophthalmol. 1983, 3, 63–66. [Google Scholar]
- Malhotra, R.; Gregory-Evans, K. Management of ocular ischaemic syndrome. Br. J. Ophthalmol. 2000, 84, 1428. [Google Scholar] [CrossRef] [PubMed]
- Sood, G.; Siddik, A.B. Ocular Ischemic Syndrome. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sivalingam, A.; Brown, G.C.; Magargal, L.E. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int. Ophthalmol. 1991, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Sung, M.S.; Park, S.W. Clinical Features of Ocular Ischemic Syndrome and Risk Factors for Neovascular Glaucoma. Korean J. Ophthalmol. 2017, 31, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Leahy, S.; Farzad, S.; Blair, N.P.; Shahidi, M. Retinal Oxygen Delivery, Metabolism, and Extraction Fraction during Long-Term Bilateral Common Carotid Artery Occlusion in Rats. Sci. Rep. 2020, 10, 10371. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, S.; Li, J.; Wang, Y.L. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuro-Ophthalmology 2014, 38, 180–188. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, M.; Deng, T.; Luo, D.; Zi, Y.; Pan, L.; Wang, Z.; Jin, M. Functional and morphologic study of retinal hypoperfusion injury induced by bilateral common carotid artery occlusion in rats. Sci. Rep. 2019, 9, 80. [Google Scholar] [CrossRef]
- Sivilia, S.; Giuliani, A.; Fernández, M.; Turba, M.E.; Forni, M.; Massella, A.; De Sordi, N.; Giardino, L.; Calzà, L. Intravitreal NGF administration counteracts retina degeneration after permanent carotid artery occlusion in rat. BMC Neurosci. 2009, 10, 52. [Google Scholar] [CrossRef]
- Lavinsky, D.; Arterni, N.S.; Achaval, M.; Netto, C.A. Chronic bilateral common carotid artery occlusion: A model for ocular ischemic syndrome in the rat. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2006, 244, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.M.; Pappas, B.A.; Stevens, W.D.; Fortin, T.; Bennett, S.A. Chronic cerebral hypoperfusion: Loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000, 859, 96–103. [Google Scholar] [CrossRef]
- Yamamoto, H.; Schmidt-Kastner, R.; Hamasaki, D.I.; Yamamoto, H.; Parel, J.M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006, 82, 767–779. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.; Casson, R.J. Expression of inducible heat shock proteins Hsp27 and Hsp70 in the visual pathway of rats subjected to various models of retinal ganglion cell injury. PLoS ONE 2014, 9, e114838. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Holman, M.C.; Wood, J.P.; Casson, R.J. Spatiotemporal characterization of optic nerve degeneration after chronic hypoperfusion in the rat. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1483–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Y.; Zhang, L.; Wang, Y.X.; Qi, W.; Liang, W.; Wang, C.; David, T.W.Y.; Ye, C.; Sha, O. Bilateral Common Carotid Artery Occlusion in Spontaneously Hypertensive Rats: A Feasible Animal Model for Ocular Ischemic Syndrome. Anat. Rec. 2016, 299, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Holman, M.C.; Chidlow, G.; Wood, J.P.; Casson, R.J. The effect of hyperglycemia on hypoperfusion-induced injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2197–2207. [Google Scholar] [CrossRef][Green Version]
- Crespo-Garcia, S.; Reichhart, N.; Skosyrski, S.; Foddis, M.; Wu, J.; Figura, A.; Herrspiegel, C.; Füchtemeier, M.; Sassi, C.; Dirnagl, U.; et al. Individual and temporal variability of the retina after chronic bilateral common carotid artery occlusion (BCCAO). PLoS ONE 2018, 13, e019396. [Google Scholar] [CrossRef]
- Lee, B.J.; Jun, H.O.; Kim, J.H.; Kim, J.H. Astrocytic cystine/glutamate antiporter is a key regulator of erythropoietin expression in the ischemic retina. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 6045–6054. [Google Scholar] [CrossRef]
- Lee, D.; Kang, H.; Yoon, K.Y.; Chang, Y.Y.; Song, H.B. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp. Eye Res. 2020, 201, 108275. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, H.; Miwa, Y.; Shinojima, A.; Katada, Y.; Tsubota, K.; Kurihara, T. Retinal dysfunction induced in a mouse model of unilateral common carotid artery occlusion. PeerJ 2021, 9, e11665. [Google Scholar] [CrossRef]
- Lee, D.; Nakai, A.; Miwa, Y.; Tomita, Y.; Serizawa, N.; Katada, Y.; Hatanaka, Y.; Tsubota, K.; Negishi, K.; Kurihara, T. Retinal Degeneration in a Murine Model of Retinal Ischemia by Unilateral Common Carotid Artery Occlusion. BioMed Res. Int. 2021, 2021, 7727648. [Google Scholar] [CrossRef]
- Ling, Y.; Fu, Z.; Wang, Y. Surgical model for ocular ischemic syndrome in mice. Biomed. Res. 2017, 28, 6314–6318. [Google Scholar]
- Ogishima, H.; Nakamura, S.; Nakanishi, T.; Imai, S.; Kakino, M.; Ishizuka, F.; Tsuruma, K.; Shimazawa, M.; Hara, H. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9710–9720. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Kántor, O.; Schmitz, C.; Feiser, J.; Brasnjevic, I.; Korr, H.; Busto, R.; Ginsberg, M.D.; Schmidt-Kastner, R. Moderate loss of cerebellar Purkinje cells after chronic bilateral common carotid artery occlusion in rats. Acta Neuropathol. 2007, 113, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ogata, J.; Fujishima, M.; Morotomi, Y.; Omae, T. Cerebral infarction following bilateral carotid artery ligation in normotensive and spontaneously hypertensive rats: A pathological study. Stroke 1976, 7, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Howells, D.W.; Porritt, M.J.; Rewell, S.S.; O’Collins, V.; Sena, E.S.; van der Worp, H.B.; Traystman, R.J.; Macleod, M.R. Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [Google Scholar] [CrossRef]
- Qian, B.; Rudy, R.F.; Cai, T.; Du, R. Cerebral Artery Diameter in Inbred Mice Varies as a Function of Strain. Front. Neuroanat. 2018, 12, 10. [Google Scholar] [CrossRef]
- Okuyama, S.; Okuyama, J.; Okuyama, J.; Tamatsu, Y.; Shimada, K.; Hoshi, H.; Iwai, J. The arterial circle of Willis of the mouse helps to decipher secrets of cerebral vascular accidents in the human. Med. Hypotheses 2004, 63, 997–1009. [Google Scholar] [CrossRef]
- Tamaki, M.; Kidoguchi, K.; Mizobe, T.; Koyama, J.; Kondoh, T.; Sakurai, T.; Kohmura, E.; Yokono, K.; Umetani, K. Carotid artery occlusion and collateral circulation in C57Black/6J mice detected by synchrotron radiation microangiography. Kobe J. Med. Sci. 2006, 52, 111–118. [Google Scholar]
- Lee, D.; Miwa, Y.; Jeong, H.; Ikeda, S.I.; Katada, Y.; Tsubota, K.; Kurihara, T. A Murine Model of Ischemic Retinal Injury Induced by Transient Bilateral Common Carotid Artery Occlusion. J. Vis. Exp. JoVE 2020, 165, e61865. [Google Scholar] [CrossRef]
- Kitagawa, K.; Matsumoto, M.; Yang, G.; Mabuchi, T.; Yagita, Y.; Hori, M.; Yanagihara, T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: Evaluation of the patency of the posterior communicating artery. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1998, 18, 570–579. [Google Scholar] [CrossRef]
- Yang, G.; Kitagawa, K.; Matsushita, K.; Mabuchi, T.; Yagita, Y.; Yanagihara, T.; Matsumoto, M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: Selective neuronal death in the murine transient forebrain ischemia. Brain Res. 1997, 752, 209–218. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Chen, S.D.; Zhang, X.L.; Jonas, J.B. Retinal Microglia in Glaucoma. J. Glaucoma 2016, 25, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Levatin, P. Pupillary escape in disease of the retina or optic nerve. Arch. Ophthalmol. 1959, 62, 768–779. [Google Scholar] [CrossRef]
- Hovland, P.G.; Ip, M.S. Chapter 27—Ocular Ischemic Syndrome. In Retinal Imaging; Huang, D., Kaiser, P.K., Lowder, C.Y., Traboulsi, E.I., Eds.; Mosby: Philadelphia, PA, USA, 2006; pp. 276–282. [Google Scholar] [CrossRef]
- Morris, G.P.; Wright, A.L.; Tan, R.P.; Gladbach, A.; Ittner, L.M.; Vissel, B. A Comparative Study of Variables Influencing Ischemic Injury in the Longa and Koizumi Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Mice. PLoS ONE 2016, 11, e0148503. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Models Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Hong, Y.; Won, J.; Lee, Y.; Kang, S.G.; Chang, K.T.; Hong, Y. Middle cerebral artery occlusion methods in rat versus mouse models of transient focal cerebral ischemic stroke. Neural Regen. Res. 2014, 9, 757–758. [Google Scholar] [CrossRef]
- Lelong, D.C.; Bieche, I.; Perez, E.; Bigot, K.; Leemput, J.; Laurendeau, I.; Vidaud, M.; Jais, J.P.; Menasche, M.; Abitbol, M. Novel mouse model of monocular amaurosis fugax. Stroke 2007, 38, 3237–3244. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Sun, B.; Guo, J.; Zhou, G. Intranasal injection of recombinant human erythropoietin improves cognitive and visual impairments in chronic cerebral ischemia rats. Biomed. Rep. 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Meng, Z.Y.; Wang, J.L.; Wang, Y.L. Efficacy of Osthole in Management of Hypoperfused Retina. J. Ophthalmol. 2018, 2018, 6178347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A Review on Its Bioactivities, Pharmacological Properties, and Potential as Alternative Medicine. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 919616. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ding, D.; Zhang, M. Neuroprotection of Osthole against Cerebral Ischemia/Reperfusion Injury through an Anti-apoptotic Pathway in Rats. Biol. Pharm. Bull. 2016, 39, 336–342. [Google Scholar] [CrossRef]
- Wang, X.Y.; Dong, W.P.; Bi, S.H.; Pan, Z.G.; Yu, H.; Wang, X.W.; Ma, T.; Wang, J.; Zhang, W.D. Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. Int. J. Mol. Med. 2013, 32, 365–372. [Google Scholar] [CrossRef]
- Guan, J.; Wei, X.; Qu, S.; Lv, T.; Fu, Q.; Yuan, Y. Osthole prevents cerebral ischemia-reperfusion injury via the Notch signaling pathway. Biochem. Cell Biol. Biochim. Biol. Cell. 2017, 95, 459–467. [Google Scholar] [CrossRef]
- Lebonvallet, N.; Pennec, J.P.; Le Gall, C.; Pereira, U.; Boulais, N.; Cheret, J.; Jeanmaire, C.; Danoux, L.; Pauly, G.; Misery, L. Effect of human skin explants on the neurite growth of the PC12 cell line. Exp. Dermatol. 2013, 22, 224–225. [Google Scholar] [CrossRef]
- Maffei, L.; Berardi, N.; Domenici, L.; Parisi, V.; Pizzorusso, T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 4651–4662. [Google Scholar] [CrossRef]
- Carmignoto, G.; Comelli, M.C.; Candeo, P.; Cavicchioli, L.; Yan, Q.; Merighi, A.; Maffei, L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp. Neurol. 1991, 111, 302–311. [Google Scholar] [CrossRef]
- Chan, J.R.; Watkins, T.A.; Cosgaya, J.M.; Zhang, C.; Chen, L.; Reichardt, L.F.; Shooter, E.M.; Barres, B.A. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 2004, 43, 183–191. [Google Scholar] [CrossRef]
- Szabadfi, K.; Atlasz, T.; Reglodi, D.; Kiss, P.; Dányádi, B.; Fekete, E.M.; Zorrilla, E.P.; Tamás, A.; Szabó, K.; Gábriel, R. Urocortin 2 protects against retinal degeneration following bilateral common carotid artery occlusion in the rat. Neurosci. Lett. 2009, 455, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Reglodi, D.; Kiss, P.; Toth, G.; Szabadfi, K.; Tamas, A.; Biro, Z.; Atlasz, T. Investigation of PACAP Fragments and Related Peptides in Chronic Retinal Hypoperfusion. J. Ophthalmol. 2014, 2014, 563812. [Google Scholar] [CrossRef]
- Lee, D.; Tomita, Y.; Miwa, Y.; Jeong, H.; Mori, K.; Tsubota, K.; Kurihara, T. Fenofibrate Protects against Retinal Dysfunction in a Murine Model of Common Carotid Artery Occlusion-Induced Ocular Ischemia. Pharmaceuticals 2021, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Tomita, Y.; Jeong, H.; Miwa, Y.; Tsubota, K.; Negishi, K.; Kurihara, T. Pemafibrate Prevents Retinal Dysfunction in a Mouse Model of Unilateral Common Carotid Artery Occlusion. Int. J. Mol. Sci. 2021, 22, 9408. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Miwa, Y.; Jiang, X.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Protects Against Retinal Dysfunction in a Murine Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 6243. [Google Scholar] [CrossRef]
- Wang, H.W.; Jiang, X.; Zhang, Y.; Wang, J.; Xie, J.; Wang, Y.Q.; Li, Y.H. FGF21 Protects Against Hypoxia Injury Through Inducing HSP72 in Cerebral Microvascular Endothelial Cells. Front. Pharmacol. 2019, 10, 101. [Google Scholar] [CrossRef]
- Kuroda, M.; Muramatsu, R.; Maedera, N.; Koyama, Y.; Hamaguchi, M.; Fujimura, H.; Yoshida, M.; Konishi, M.; Itoh, N.; Mochizuki, H.; et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Investig. 2017, 127, 3496–3509. [Google Scholar] [CrossRef]
- Chen, J.; Hu, J.; Liu, H.; Xiong, Y.; Zou, Y.; Huang, W.; Shao, M.; Wu, J.; Yu, L.; Wang, X.; et al. FGF21 Protects the Blood-Brain Barrier by Upregulating PPARγ via FGFR1/β-klotho after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2091–2103. [Google Scholar] [CrossRef]
- Greenhill, C. Mechanism for the effects of FGF21. Nat. Rev. Endocrinol. 2020, 16, 472. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, E.; Landers, M.B., 3rd; Wolbarsht, M.L. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans. Am. Ophthalmol. Soc. 1981, 79, 307–334. [Google Scholar] [PubMed]
- Saito, Y.; Higashide, T.; Takeda, H.; Ohkubo, S.; Sugiyama, K. Beneficial effects of preoperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta Ophthalmol. 2010, 88, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, J.J.; Aburahma, A.; Ascher, E.; Eskandari, M.; Faries, P.; Lal, B.K.; Society for Vascular, S. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary. J. Vasc. Surg. 2011, 54, 832–836. [Google Scholar] [CrossRef]
- Cohn, E.J., Jr.; Sandager, G.P.; Benjamin, M.E.; Lilly, M.P.; Hanna, D.J.; Flinn, W.R. Assessment of ocular perfusion after carotid endarterectomy with color-flow duplex scanning. J. Vasc. Surg. 1999, 29, 665–671. [Google Scholar] [CrossRef][Green Version]
- Narins, C.R.; Illig, K.A. Patient selection for carotid stenting versus endarterectomy: A systematic review. J. Vasc. Surg. 2006, 44, 661–672. [Google Scholar] [CrossRef]
- Mekonnen, Z.K.; Everett, L.A.; Hetts, S.W.; Afshar, A.R. Retinal emboli after cervicopetrous junction internal carotid artery pseudoaneurysm stenting. Am. J. Ophthalmol. Case Rep. 2021, 23, 101164. [Google Scholar] [CrossRef]

| Publication | Strain | Method | Main Outcome |
|---|---|---|---|
| Leahy et al., 2020 [16] | Long–Evans rats | BCCAO | Abnormal changes in artery/vein diameter, vein velocity, total retinal blood flow, and oxygen delivery and metabolism |
| Huang et al., 2014 [17] | Clean-grade Wister rats | BCCAO | Longer artery filling time; decreases in ocular blood flow (the pupil, iris, and total eye) |
| Qin et al., 2019 [18] | Sprague–Dawley rats | BCCAO | Decreases in amplitudes of scotopic a-wave and b-wave; decreases in retinal thickness; disorder and damage in retinal ganglion cells (especially karyopyknosis, chromatic agglutination, and decreased or swelling organelles); disorder and damage in photoreceptor cells |
| Sivilia et al., 2009 [19] | Sprague–Dawley rats | BCCAO | PLR loss; decreases in the outer plexiform layer and the inner plexiform and ganglion cell layers without affecting the outer nuclear and inner nuclear layers |
| Lavinsky et al., 2006 [20] | Wistar rats | BCCAO | PLR loss; more impairment in retinal thickness and ganglion cell density in BCCAO-operated rats with PLR loss |
| Davidson et al., 2000 [21] | Sprague–Dawley rats | BCCAO | PLR loss; general retinal damage and visual loss |
| Yamamoto et al., 2006 [22] | Wistar rats | BCCAO | Molecular alterations in various pro- and antiapoptotic factors and retinal cell markers such as cleaved caspase-3, ubiquitin, COX-2, HSP70, calbindin, BRN3, microtubule-associated protein 2, and synaptophysin |
| Chidlow et al., 2014 [23] | Sprague–Dawley rats | BCCAO | Increases in HSP27 protein expression in the retina and optic nerve (especially, in the ganglion cell and inner plexiform layers) |
| Chidlow et al., 2010 [24] | Sprague–Dawley rats | BCCAO | Gradual increases in HSP27 and αB-crystallin expressions and gradual decreases in NFL and β3-tubulin protein expressions found in the proximal optic nerve; deposition of extracellular matrix components (collagen I, collagen VI, and laminin) |
| Wang et al., 2016 [25] | Hypertensive and normotensive Wistar–Kyoto rats | BCCAO | Higher dysfunction rates in the PLR under hypertension; extensive avascular areas of blood vessels; dramatic decreases in retinal thickness |
| Holman et al., 2010 [26] | Sprague–Dawley rats with streptozotocin | BCCAO | Retinal protection (such as preservation of retinal thickness and survival of BRN3-, Islet-1-, PGP 9.5-, and calbindin-positive retinal cells); pathological gliosis (such as activation of astrocytes, Müller cells, and microglia) reduction by short-term hyperglycemia |
| Crespo-Garcia et al., 2018 [27] | C57BL/6J mice | BCCAO/BCCAS | Retinal vein dilatation; mobilization and accumulation of mononuclear phagocytes in surrounding veins; decreases in amplitudes in scotopic a-wave, b-wave, and oscillatory potentials; synaptic degeneration (vesicular glutamate transporter 1, C-terminal-binding protein 2, protein kinase C-α, and calbindin-D28k) |
| Lee et al., 2019 [28] | C57BL/6 mice | UCCAO | Retinal HIF-2α stabilization; increases in Epo mRNA expression; decreases in total retinal thickness |
| Lee and Kang et al., 2020 [29] | C57BL/6 mice | UCCAO | Abnormal retinal blood perfusion; eyelid drooping; retinal HIF-1α stabilization; acute GFAP-positive gliosis; chronic retinal thinning |
| Lee and Jeong et al., 2021 [30] | C57BL/6 mice | UCCAO | Decreases in amplitudes of scotopic b-wave; chronic retinal gliosis; transient retinal cell death |
| Lee et al., 2021 [31] | C57BL/6 mice | UCCAO | No change in PLR or IOP; acute reversible cataract development; visual evoked potential reduction; NeuN-positive cell loss; chronic retinal inflammation |
| Ling et al., 2017 [32] | C57BL/6 mice | BICAO | Abnormal ocular blood flow; alterations in retinal thickness |
| Ogishima et al., 2011 [33] | ddY mice | Occlusion of the PPA and ECA | Decreases in ocular blood flow; functional and histologic damage in the inner retina |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Tomita, Y.; Yang, L.; Negishi, K.; Kurihara, T. Ocular Ischemic Syndrome and Its Related Experimental Models. Int. J. Mol. Sci. 2022, 23, 5249. https://doi.org/10.3390/ijms23095249
Lee D, Tomita Y, Yang L, Negishi K, Kurihara T. Ocular Ischemic Syndrome and Its Related Experimental Models. International Journal of Molecular Sciences. 2022; 23(9):5249. https://doi.org/10.3390/ijms23095249
Chicago/Turabian StyleLee, Deokho, Yohei Tomita, Lizhu Yang, Kazuno Negishi, and Toshihide Kurihara. 2022. "Ocular Ischemic Syndrome and Its Related Experimental Models" International Journal of Molecular Sciences 23, no. 9: 5249. https://doi.org/10.3390/ijms23095249
APA StyleLee, D., Tomita, Y., Yang, L., Negishi, K., & Kurihara, T. (2022). Ocular Ischemic Syndrome and Its Related Experimental Models. International Journal of Molecular Sciences, 23(9), 5249. https://doi.org/10.3390/ijms23095249









