Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors
Abstract
1. Introduction
2. Animal Experimental Models for Skin Flaps
2.1. Animals and Flap Designs
2.2. Skin Flap Evaluation
2.2.1. Necrosis Flap Area Analysis
2.2.2. Histopathologic Assessment
2.2.3. Inflammatory Cytokines
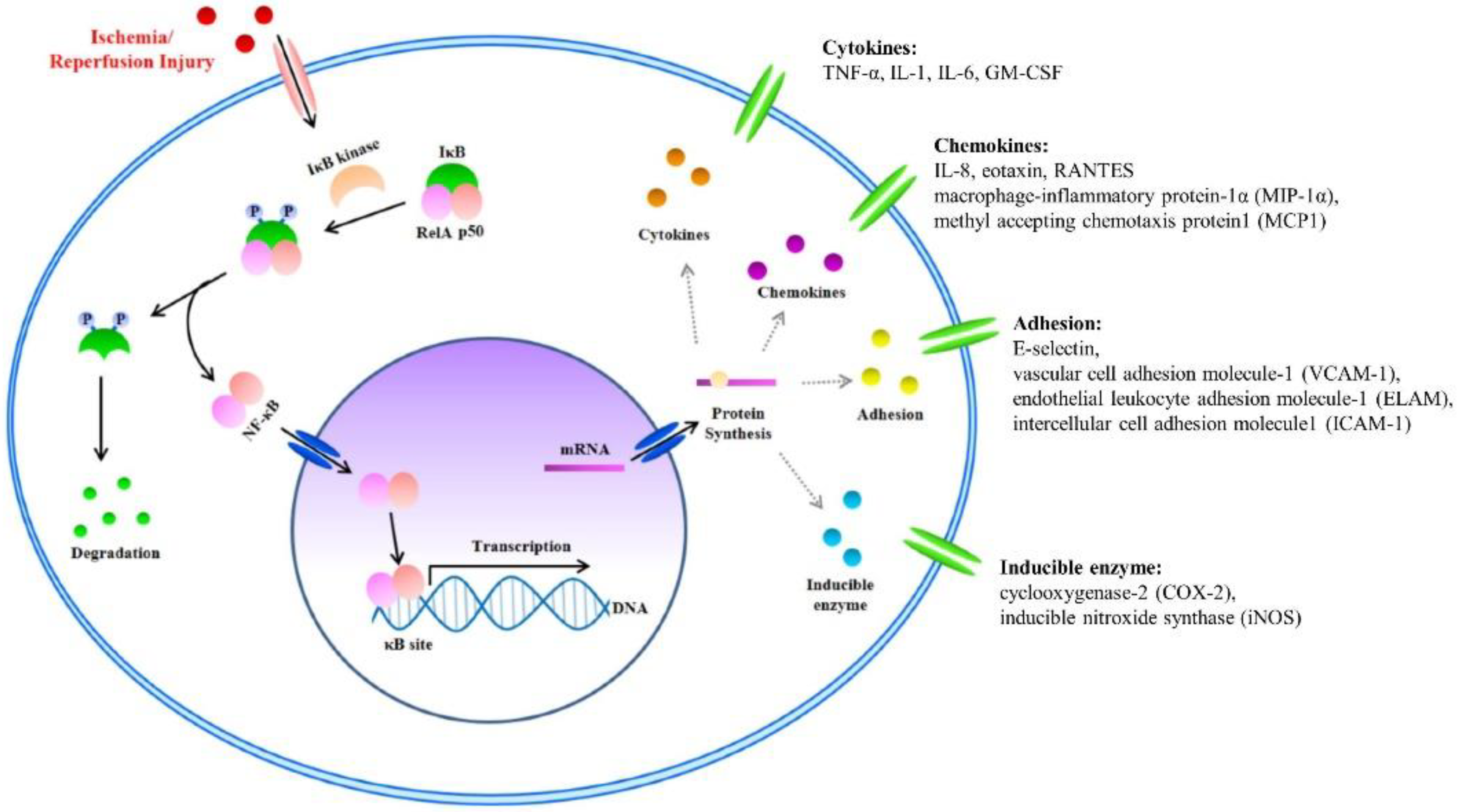
- NF-κB and IκB
- NF-κB is a known transcription factor that controls cytokine expression and cell survival in normal cells [3,4]. In addition, NF-κB regulates chemokine, adhesion, and inducible enzymes (Table 4) [58]. NF-κB dimer (RelA/p50) binds to IκB and maintains an inactive form in the cytoplasm of most resting cells. In the condition of inflammatory stimulation, IκB kinase (IKK) induces IκB phosphorylation and degradation. NF-κB separates from the NF-κB/IκB complex, and the activated NF-κB dimer (RelA/p50) translocates to the nucleus. The NF-κB dimer (RelA/p50) binds to the promoter of pro-inflammatory genes in the nuclear DNA. Finally, pro-inflammatory transcription induces the expression of inflammatory cytokines such as TNF-α, IL-1, and IL-6 (Figure 2) [4,5,6,7]. Therefore, NF-κB signal regulation is important when attempting to improve I/R injury in the skin flap.
- TNF-α, IL-1β, and IL-6
- TNF-α, IL-1β, and IL-6 play key roles as proinflammatory cytokines in I/R injury [59,60]. As described above, proinflammatory cytokines are activated by NF-κB and used as indicators of inflammation. Prior investigators have researched the potential of these cytokines to improve skin flap survival or discover novel therapeutics.
- TNF-α is a systemic inflammation cell signaling protein expressed by activated NF-κB via the PARs/p38-MAPK/NF-κB pathway [4]. It is released from activated monocytes and macrophages and can activate lymphocytes, neutrophils, eosinophils, and natural killer (NK) cells during an inflammatory response [9]. Moreover, increased TNF-α triggers additional NF-κB expressions via IKK activation [4]. Many investigators have attempted to inhibit TNF-α expression. Deheng et al. reported TNF-α presence and the inflammatory reactions were decreased by VEGF treatment, which improved skin flap survival [61].
2.2.4. Apoptosis
2.2.5. Angiogenesis
3. Animal Experimental Models for Ischemic Preconditioning
3.1. Non-Invasive IPC Models
3.2. Invasive IPC Models
3.3. Molecular Factors Associated with IPC
4. Current Studies in Skin Flaps and IPC
5. Clinical Treatment for the Survival of Skin Flaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kailiang, Z.; Yihui, Z.; Dingsheng, L.; Xianyao, T. Effects of Muscone on Random Skin Flap Survival in Rats. J. Reconstr. Microsurg. 2016, 32, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chehelcheraghi, F.; Eimani, H.; Sadraie, S.H.; Torkaman, G.; Amini, A.; Shemshadi, H.; Majd, H.A. Improved viability of random pattern skin flaps with the use of bone marrow mesenchymal-derived stem cells and chicken embryo extract. Iran. J. Basic Med. Sci. 2015, 18, 764–772. [Google Scholar] [PubMed]
- Han, H.H.; Lim, Y.M.; Park, S.W.; Lee, S.J.; Rhie, J.W.; Lee, J.H. Improved skin flap survival in venous ischemia-reperfusion injury with the use of adipose-derived stem cells. Microsurgery 2015, 35, 645–652. [Google Scholar] [CrossRef]
- Peng, L.; Pan, X.; Yin, G. Natural Hirudin Increases Rat Flap Viability by Anti-Inflammation via PARs/p38/NF-kappaB Pathway. Biomed. Res. Int. 2015, 2015, 597264. [Google Scholar] [CrossRef]
- Liang, F.; Kang, N.; Liu, X.; Yang, J.; Li, Z.; Tan, J.W. Effect of HMGB1/NF-kappaB in hyperbaric oxygen treatment on decreasing injury caused by skin flap grafts in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2010–2018. [Google Scholar]
- Kang, N.; Hai, Y.; Liang, F.; Gao, C.J.; Liu, X.H. Preconditioned hyperbaric oxygenation protects skin flap grafts in rats against ischemia/reperfusion injury. Mol. Med. Rep. 2014, 9, 2124–2130. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Christman, J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Silva, J.J.; Pompeu, D.G.; Ximenes, N.C.; Duarte, A.S.; Gramosa, N.V.; Carvalho Kde, M.; Brito, G.A.; Guimaraes, S.B. Effects of Kaurenoic Acid and Arginine on Random Skin Flap Oxidative Stress, Inflammation, and Cytokines in Rats. Aesthetic Plast. Surg. 2015, 39, 971–977. [Google Scholar] [CrossRef]
- Deheng, C.; Kailiang, Z.; Weidong, W.; Haiming, J.; Daoliang, X.; Ningyu, C.; Huazi, X. Salidroside Promotes Random Skin Flap Survival in Rats by Enhancing Angiogenesis and Inhibiting Apoptosis. J. Reconstr. Microsurg. 2016, 32, 580–586. [Google Scholar] [CrossRef]
- Gersch, R.P.; Fourman, M.S.; Phillips, B.T.; Nasser, A.; McClain, S.A.; Khan, S.U.; Dagum, A.B.; Bui, D.T. AdVEGF-All6A+ Preconditioning of Murine Ischemic Skin Flaps Is Comparable to Surgical Delay. Plast. Reconstr. Surg. Glob. Open 2015, 3, e494. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, R.M.; Deyoung, G.; Henry, R.A. The Design of a Pedicle Flap in the Rat to Study Necrosis and Its Prevention. Plast. Reconstr. Surg. 1965, 35, 177–182. [Google Scholar] [CrossRef]
- Camargo, C.P.; Margarido, N.F.; Guandelini, E.; Vieira, G.A.; Jacomo, A.L.; Gemperli, R. Description of a new experimental model skin flap for studying skin viability in rats. Acta Cir. Bras. 2014, 29, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhu, K.; Cao, P.; Long, C.; Deng, Y.; Liu, T.; Yin, G.; Li, X.; Wang, Z. Ischemic preconditioning-induced protective effect for promoting angiogenesis in renal ischemia-reperfusion injury by regulating miR-376c-3p/HIF-1alpha/VEGF axis in male rats. Life Sci. 2022, 299, 120357. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Lin, Y.; Lin, D.; Cao, B. Effects of Bezafibrate on the Survival of Random Skin Flaps in Rats. J. Reconstr. Microsurg. 2016, 32, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.R.; Junior, I.E.; Masson, I.F.B.; Machado, A.F.P.; Oliveira, M.C.D.; Baldan, C.S.; Farcic, T.S.; Liebano, R.E.; Plapler, H. Photobiomodulation effect in tumoral necrosis factor-alpha(TNF-alpha) on the viability of random skin flap in rats. Lasers Med. Sci. 2022, 37, 1495–1501. [Google Scholar] [CrossRef]
- Masaoka, K.; Asato, H.; Umekawa, K.; Imanishi, M.; Suzuki, A. Value of remote ischaemic preconditioning in rat dorsal skin flaps and clamping time. J. Plast. Surg. Hand Surg. 2016, 50, 107–110. [Google Scholar] [CrossRef]
- Roh, T.S.; Jung, B.K.; Yun, I.; Lew, D.H.; Kim, Y.S. Effect of botulinum toxin A on vasoconstriction and sympathetic neurotransmitters in a murine random pattern skin flap model. Wound Repair Regen. 2017, 25, 75–85. [Google Scholar] [CrossRef]
- Dingsheng, L.; Zengbing, L.; Dong, H. Favorable effects of progesterone on skin random flap survival in rats. Iran. J. Basic Med. Sci. 2016, 19, 1166–1170. [Google Scholar]
- Lv, Q.B.; Gao, X.; Lin, D.S.; Chen, Y.; Cao, B.; Zhou, K.L. Effects of diammonium glycyrrhizinate on random skin flap survival in rats: An experimental study. Biomed. Rep. 2016, 5, 383–389. [Google Scholar] [CrossRef][Green Version]
- Chen, G.J.; Chen, Y.H.; Yang, X.Q.; Li, Z.J. Nano-microcapsule basic fibroblast growth factor combined with hypoxia-inducible factor-1 improves random skin flap survival in rats. Mol. Med. Rep. 2016, 13, 1661–1666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Li, W.; Ma, X.; He, J.; Lin, Y.; Lin, D. Rivastigmine Regulates the HIF-1alpha/VEGF Signaling Pathway to Induce Angiogenesis and Improves the Survival of Random Flaps in Rats. Front. Pharmacol 2021, 12, 818907. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Liu, Y.Y.; He, J.B.; Ma, X.Y.; Lin, Y.; Zheng, P.; Lin, D.S. Effect of paeoniflorin on distal survival of random flaps. Int. Immunopharmacol. 2022, 105, 108562. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.S.; Moon, S.Y.; Lee, Y.E.; Kang, H.J. Therapeutic Effects against Tissue Necrosis of Remote Ischemic Preconditioning Combined with Human Adipose-Derived Stem Cells in Random-Pattern Skin Flap Rat Models. J. Investig. Surg. 2021, 34, 1304–1311. [Google Scholar] [CrossRef]
- Fan, W.; Liu, Z.; Chen, J.; Liu, S.; Chen, T.; Li, Z.; Lin, D. Effect of memantine on the survival of an ischemic random skin flap and the underlying mechanism. Biomed. Pharmacother. 2021, 143, 112163. [Google Scholar] [CrossRef]
- Jaleel, Z.; Blasberg, E.; Troiano, C.; Montanaro, P.; Mazzilli, S.; Gertje, H.P.; Crossland, N.A.; Platt, M.; Spiegel, J. Association of vaping with decreased vascular endothelial growth factor expression and decreased microvessel density in cutaneous wound healing tissue in rats. Wound Repair Regen. 2021, 29, 1024–1034. [Google Scholar] [CrossRef]
- Huang, T.; Shi, J.; Sang, K.; Yu, C.; Xie, Y.; Chen, H.; Jin, Z.; Yan, H.; Zhao, B. The effect of different modes of microneedling technique on random flap survival in rats. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2768–2775. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Liu, Y.; Li, W.; He, J.; Fang, M.; Lin, D. Effects of Apigenin Treatment on Random Skin Flap Survival in Rats. Front. Pharmacol. 2021, 12, 625733. [Google Scholar] [CrossRef]
- Luo, Z.; Bian, Y.; Zheng, G.; Wang, H.; Yan, B.; Su, W.; Dong, W.; Hu, Z.; Ding, J.; Wang, A.; et al. Chemically Modified SDF-1alpha mRNA Promotes Random Flap Survival by Activating the SDF-1alpha/CXCR4 Axis in Rats. Front. Cell Dev. Biol. 2021, 9, 623959. [Google Scholar] [CrossRef]
- Pan, X.Y.; Peng, L.; Han, Z.Q.; Yin, G.Q.; Song, Y.K.; Huang, J. Hirudin promotes angiogenesis by modulating the cross-talk between p38 MAPK and ERK in rat ischemic skin flap tissue. Tissue Cell 2015, 47, 301–310. [Google Scholar] [CrossRef]
- Koh, K.S.; Park, S.W.; Oh, T.S.; Choi, J.W. Flap preconditioning by pressure-controlled cupping in a rat model. J. Surg. Res. 2016, 204, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, Y.; Ohura, N.; Kurita, M.; Takushima, A.; Harii, K. Examination of tissue oxygen saturation (StO2) changes associated with vascular pedicle occlusion in a rat Island flap model using near-Infrared spectroscopy. Microsurgery 2015, 35, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wald, G.; Van, Y.V.; Towne, W.; Otterburn, D.M. The Effect of Topical Tacrolimus on Pedicled Flap Survival: A Histological Analysis. Ann. Plast. Surg. 2021, 87, S57–S59. [Google Scholar] [CrossRef]
- Khavanin, N.; Darrach, H.; Kraenzlin, F.; Yesantharao, P.S.; Sacks, J.M. The Intra.Ox Near-Infrared Spectrometer Measures Variations in Flap Oxygenation That Correlate to Flap Necrosis in a Preclinical Rodent Model. Plast. Reconstr. Surg. 2021, 147, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.; Metin Guler, E.; Kocyigit, A.; Bayindir, N.; Esrefoglu, M.; Mirasoglu, B.O.; Yenigun, A.; Ozturan, O. Comparison of the efficacy of multiple antioxidant and hyperbaric oxygen treatments in the prevention of ischemia and necrosis of local random McFarlane skin flap. J. Tissue Viability 2021, 30, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Yotsuyanagi, T.; Radtke, C.; Kocsis, J.D.; Honmou, O. Intravenous Infusion of Mesenchymal Stem Cells Promotes the Survival of Random Pattern Flaps in Rats. Plast. Reconstr. Surg. 2021, 148, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Park, T.H.; Park, Y.J. The Effect of Botulinum Toxin A on Ischemia-Reperfusion Injury in a Rat Model. Biomed. Res. Int. 2017, 2017, 1074178. [Google Scholar] [CrossRef]
- Kanayama, K.; Mineda, K.; Mashiko, T.; Wu, S.H.; Feng, J.; Kinoshita, K.; Sunaga, A.; Yoshimura, K. Blood Congestion Can Be Rescued by Hemodilution in a Random-Pattern Skin Flap. Plast. Reconstr. Surg. 2017, 139, 365–374. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Y.; Yin, D.; Zhang, M.; Wang, Y.; Ma, X.; Liu, Y.; Zhao, P. Inhibition of c-Jun N-terminal kinase signaling suppresses skin flap apoptosis in a rat ischemia and/or reperfusion model. J. Surg. Res. 2016, 206, 337–346. [Google Scholar] [CrossRef]
- Hartrampf, C.R.; Scheflan, M.; Black, P.W. Breast reconstruction with a transverse abdominal island flap. Plast. Reconstr. Surg. 1982, 69, 216–225. [Google Scholar] [CrossRef]
- Song, K.; Zhang, M.; Hu, J.; Liu, Y.; Liu, Y.; Wang, Y.; Ma, X. Methane-rich saline attenuates ischemia/reperfusion injury of abdominal skin flaps in rats via regulating apoptosis level. BMC Surg. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.S.; Zeng, L.R.; Quan, R.F.; Tang, Y.H.; Zheng, W.J.; Qu, G.; Xu, C.D.; Zhu, F.B.; Huang, Z.M. 4Phenylbutyrate protects rat skin flaps against ischemiareperfusion injury and apoptosis by inhibiting endoplasmic reticulum stress. Mol. Med. Rep. 2016, 13, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Odake, K.; Tsujii, M.; Iino, T.; Chiba, K.; Kataoka, T.; Sudo, A. Febuxostat treatment attenuates oxidative stress and inflammation due to ischemia-reperfusion injury through the necrotic pathway in skin flap of animal model. Free Radic. Biol. Med. 2021, 177, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Izawa-Ishizawa, Y.; Horinouchi, Y.; Sairyo, E.; Ikeda, Y.; Ishizawa, K.; Tsuchiya, K.; Abe, Y.; Hashimoto, I.; Tamaki, T. Topical application of nitrosonifedipine, a novel radical scavenger, ameliorates ischemic skin flap necrosis in a mouse model. Wound Repair Regen. 2017, 25, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Chung, P.S.; Ahn, J.C.; Leproux, A. Human adipose-derived stem cell spheroid treated with photobiomodulation irradiation accelerates tissue regeneration in mouse model of skin flap ischemia. Lasers Med. Sci. 2017, 32, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Rah, D.K.; Min, H.J.; Kim, Y.W.; Cheon, Y.W. Effect of Platelet-Rich Plasma on Ischemia-Reperfusion Injury in a Skin Flap Mouse Model. Int. J. Med. Sci. 2017, 14, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.H.; Pennington, L.A.; Scordino, J.W.; Alexander, J.S.; Lian, T. Dynamics of early stem cell recruitment in skin flaps subjected to ischemia reperfusion injury. Pathophysiology 2016, 23, 221–228. [Google Scholar] [CrossRef]
- Huang, L. What happened if various kinds of postconditioning working on the preconditioned ischemic skin flaps. PLoS ONE 2013, 8, e72818. [Google Scholar] [CrossRef]
- Prasetyono, T.O.; Adianto, S. The Relationship between Oxygen Saturation and Color Alteration of a Compromised Skin Flap: Experimental Study on the Rabbit. Arch. Plast. Surg. 2013, 40, 505–509. [Google Scholar] [CrossRef]
- Yan, H.; He, Z.; Li, Z.; Lin, K.; Lv, L.; Li, Z.; Chen, X.; Gao, W. Large prefabricated skin flaps based on the venous system in rabbits: A preliminary study. Plast. Reconstr. Surg. 2013, 132, 372e–380e. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, I.; Goishi, K.; Kashiwagi, K.; Yamano, M.; Nakanishi, H. Transcutaneous PCO2 Measurement at Low Temperature for Reliable and Continuous Free Flap Monitoring: Experimental and Clinical Study. Plast. Reconstr. Surg. Glob. Open 2013, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, J.H.; Han, Y.S.; Kim, H. The effect of platelet-rich plasma on flap survival in random extension of an axial pattern flap in rabbits. Plast. Reconstr. Surg. 2013, 132, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yang, M.; Liu, C. An Islanded Rabbit Auricular Skin Flap Model of Hyaluronic Acid Injection-Induced Embolism. Aesthetic. Plast. Surg. 2016, 40, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Geng, Q.; Hu, J.; Shao, J.; Ruan, J.; Zheng, J. Platelet-rich plasma reduces skin flap inflammatory cells infiltration and improves survival rates through induction of angiogenesis: An experiment in rabbits. J. Plast. Surg. Hand Surg. 2016, 50, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Menevse, G.T.; TeomanTellioglu, A.; Altuntas, N.; Comert, A.; Tekdemir, I. Polidocanol injection for chemical delay and its effect on the survival of rat dorsal skin flaps. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 851–856. [Google Scholar] [CrossRef]
- Miyawaki, T.; Jackson, I.T.; Elmazar, H.; Bier, U.C.; Barakat, K.; Andrus, L.; Williams, F. The effect of low-molecular-weight heparin in the survival of a rabbit congested skin flap. Plast. Reconstr. Surg. 2002, 109, 1994–1999. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, E.C.; Topp, S.; Lei, M.; Chen, W.; Lineaweaver, W.C. Proinflammatory cytokines gene expression in skin flaps with arterial and venous ischemia in rats. J. Reconstr. Microsurg. 2006, 22, 641–647. [Google Scholar] [CrossRef]
- Wu, X.; Yu, M.; Li, A. Protective effect of a nuclear factor-kappaB inhibitor on ischemia-reperfusion injury in a rat epigastric flap model. J. Reconstr. Microsurg. 2008, 24, 351–359. [Google Scholar] [CrossRef]
- Bennett, N.T.; Schultz, G.S. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am. J. Surg. 1993, 165, 728–737. [Google Scholar] [CrossRef]
- Gailit, J.; Clark, R.A. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 1994, 6, 717–725. [Google Scholar] [CrossRef]
- Fang, T.; Lineaweaver, W.C.; Chen, M.B.; Kisner, C.; Zhang, F. Effects of vascular endothelial growth factor on survival of surgical flaps: A review of experimental studies. J. Reconstr. Microsurg. 2014, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.C.; Wong, C.X.; Micaroni, M.; Venturato, J.; Khromykh, T.; Stow, J.L.; Lacy, P. The Rho GTPase Rac1 is required for recycling endosome-mediated secretion of TNF in macrophages. Immunol. Cell Biol. 2014, 92, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.G.; Oliveira, R.J.; Dourado, D.M.; Filho, E.A.; Fernandes, W.S.; Souza, A.S.; Araujo, F.H. Morphological study of rat skin flaps treated with subcutaneous dimethyl sulfoxide combined with hyperbaric oxygen therapy. Genet. Mol. Res. 2015, 14, 18160–18171. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 2019, 71, 461–473. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Tu, Q.; Liu, S.; Chen, T.; Li, Z.; Lin, D. Effects of adiponectin on random pattern skin flap survival in rats. Int. Immunopharmacol. 2019, 76, 105875. [Google Scholar] [CrossRef]
- Yu, W.Y.; Sun, W.; Yu, D.J.; Zhao, T.L.; Wu, L.J.; Zhuang, H.R. Adipose-derived stem cells improve neovascularization in ischemic flaps in diabetic mellitus through HIF-1alpha/VEGF pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 10–16. [Google Scholar] [CrossRef]
- Vourtsis, S.A.; Spyriounis, P.K.; Agrogiannis, G.D.; Ionac, M.; Papalois, A.E. VEGF application on rat skin flap survival. J. Investig. Surg. 2012, 25, 14–19. [Google Scholar] [CrossRef]
- Huang, N.; Khan, A.; Ashrafpour, H.; Neligan, P.C.; Forrest, C.R.; Kontos, C.D.; Pang, C.Y. Efficacy and mechanism of adenovirus-mediated VEGF-165 gene therapy for augmentation of skin flap viability. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H127–H137. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Liu, W.T.; Wang, G.Y.; Shi, X.J. Impact of combined ischemic preconditioning and remote ischemic perconditioning on ischemia-reperfusion injury after liver transplantation. Sci. Rep. 2018, 8, 17979. [Google Scholar] [CrossRef] [PubMed]
- Filaretova, L.; Komkova, O.; Sudalina, M.; Yarushkina, N. Non-Invasive Remote Ischemic Preconditioning May Protect the Gastric Mucosa Against Ischemia-Reperfusion-Induced Injury Through Involvement of Glucocorticoids. Front. Pharmacol. 2021, 12, 682643. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.L.; Lautenschlager, I.; Hummitzsch, L.; Zitta, K.; Cossais, F.; Wedel, T.; Rusch, R.; Berndt, R.; Gruenewald, M.; Weiler, N.; et al. Effects of different ischemic preconditioning strategies on physiological and cellular mechanisms of intestinal ischemia/reperfusion injury: Implication from an isolated perfused rat small intestine model. PLoS ONE 2021, 16, e0256957. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Iliodromitis, E.K.; Lazou, A.; Kremastinos, D.T. Ischemic preconditioning: Protection against myocardial necrosis and apoptosis. Vasc. Health Risk Manag. 2007, 3, 629–637. [Google Scholar]
- Kloner, R.A.; Shi, J.; Dai, W.; Carreno, J.; Zhao, L. Remote Ischemic Conditioning in Acute Myocardial Infarction and Shock States. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 103–109. [Google Scholar] [CrossRef]
- Hao, Y.; Xin, M.; Feng, L.; Wang, X.; Wang, X.; Ma, D.; Feng, J. Review Cerebral Ischemic Tolerance and Preconditioning: Methods, Mechanisms, Clinical Applications, and Challenges. Front. Neurol. 2020, 11, 812. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Pedone, C.; Pinto, A.; Di Raimondo, D.; Fernandez, P.; Di Sciacca, R.; Licata, G. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int. J. Cardiol. 2008, 125, 391–396. [Google Scholar] [CrossRef]
- Della Corte, V.; Tuttolomondo, A.; Pecoraro, R.; Di Raimondo, D.; Vassallo, V.; Pinto, A. Inflammation, Endothelial Dysfunction and Arterial Stiffness as Therapeutic Targets in Cardiovascular Medicine. Curr. Pharm. Des. 2016, 22, 4658–4668. [Google Scholar] [CrossRef]
- Albanese, A.; Tuttolomondo, A.; Anile, C.; Sabatino, G.; Pompucci, A.; Pinto, A.; Licata, G.; Mangiola, A. Spontaneous chronic subdural hematomas in young adults with a deficiency in coagulation factor XIII. Report of three cases. J. Neurosurg. 2005, 102, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Torregroza, C.; Gnaegy, L.; Raupach, A.; Stroethoff, M.; Feige, K.; Heinen, A.; Hollmann, M.W.; Huhn, R. Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC). Int. J. Mol. Sci. 2021, 22, 8880. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.W.; Dykes, A.; Jeffers, M.S.; Carter, A.; Nevins, R.; Ripley, A.; Silasi, G.; Corbett, D. Remote Ischemic Conditioning and Stroke Recovery. Neurorehabil. Neural. Repair. 2021, 35, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, M.; Wu, J.; Jiang, Q.; Zheng, X. Exosomes isolated from the plasma of remote ischemic conditioning rats improved cardiac function and angiogenesis after myocardial infarction through targeting Hsp70. Aging 2020, 12, 3682–3693. [Google Scholar] [CrossRef]
- Schmidt, M.R.; Stottrup, N.B.; Michelsen, M.M.; Contractor, H.; Sorensen, K.E.; Kharbanda, R.K.; Redington, A.N.; Botker, H.E. Remote ischemic preconditioning impairs ventricular function and increases infarct size after prolonged ischemia in the isolated neonatal rabbit heart. J. Thorac. Cardiovasc. Surg. 2014, 147, 1049–1055. [Google Scholar] [CrossRef]
- Galan-Arriola, C.; Villena-Gutierrez, R.; Higuero-Verdejo, M.I.; Diaz-Rengifo, I.A.; Pizarro, G.; Lopez, G.J.; Molina-Iracheta, A.; Perez-Martinez, C.; Garcia, R.D.; Gonzalez-Calle, D.; et al. Remote ischaemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc. Res. 2021, 117, 1132–1143. [Google Scholar] [CrossRef]
- Skyschally, A.; Kleinbongard, P.; Lieder, H.; Gedik, N.; Stoian, L.; Amanakis, G.; Elbers, E.; Heusch, G. Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H159–H172. [Google Scholar] [CrossRef]
- Herajarvi, J.; Anttila, T.; Dimova, E.Y.; Laukka, T.; Myllymaki, M.; Haapanen, H.; Olenchock, B.A.; Tuominen, H.; Puistola, U.; Karihtala, P.; et al. Exploring effects of remote ischemic preconditioning in a pig model of hypothermic circulatory arrest. Scand. Cardiovasc. J. 2017, 51, 233–241. [Google Scholar] [CrossRef]
- Nizari, S.; Basalay, M.; Chapman, P.; Korte, N.; Korsak, A.; Christie, I.N.; Theparambil, S.M.; Davidson, S.M.; Reimann, F.; Trapp, S.; et al. Glucagon-like peptide-1 (GLP-1) receptor activation dilates cerebral arterioles, increases cerebral blood flow, and mediates remote (pre)conditioning neuroprotection against ischaemic stroke. Basic Res. Cardiol. 2021, 116, 32. [Google Scholar] [CrossRef]
- Li, H.; An, C. Exploring the role of neurogenic pathway-linked cholecystokinin release in remote preconditioning-induced cardioprotection. Acta Cir. Bras. 2020, 35, e202000906. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Jiang, L.; Zhang, J.; Chen, S.; Wang, L.; Zhou, Y.; Xie, H.; Zhou, L.; Zheng, S. Protective effect of remote limb ischemic perconditioning on the liver grafts of rats with a novel model. PLoS ONE 2015, 10, e0121972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merlocco, A.C.; Redington, K.L.; Disenhouse, T.; Strantzas, S.C.; Gladstone, R.; Wei, C.; Tropak, M.B.; Manlhiot, C.; Li, J.; Redington, A.N. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: In vitro validation in an animal model and first human observations. Basic Res. Cardiol. 2014, 109, 406. [Google Scholar] [CrossRef] [PubMed]
- Chalidis, B.E.; Kalivas, E.; Parziali, M.; Christodoulou, A.G.; Dimitriou, C.G. Cuff width increases the serum biochemical markers of tourniquet-induced skeletal muscle ischemia in rabbits. Orthopedics 2012, 35, e1245–e1250. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.S.; Welham, S.J.; Dunford, L.J.; McCulloch, T.A.; Hodi, Z.; Sleeman, P.; O’Sullivan, S.; Devonald, M.A. Remote conditioning or erythropoietin before surgery primes kidneys to clear ischemia-reperfusion-damaged cells: A renoprotective mechanism? Am. J. Physiol. Renal Physiol. 2014, 306, F873–F884. [Google Scholar] [CrossRef]
- Lieder, H.R.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Plasma from remotely conditioned pigs reduces infarct size when given before or after ischemia to isolated perfused rat hearts. Pflugers Arch. 2019, 471, 1371–1379. [Google Scholar] [CrossRef]
- Waldow, T.; Alexiou, K.; Witt, W.; Albrecht, S.; Wagner, F.; Knaut, M.; Matschke, K. Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl. Int. 2005, 18, 198–205. [Google Scholar] [CrossRef]
- Kuntscher, M.V.; Hartmann, B.; Germann, G. Remote ischemic preconditioning of flaps: A review. Microsurgery 2005, 25, 346–352. [Google Scholar] [CrossRef]
- Halim, A.S.; Wan Ahmad Kamal, W.S.; Noor, N.M.; Abdullah, S. Effects of ischemic preconditioning of different intraoperative ischemic times of vascularized bone graft rabbit models. Arch. Plast. Surg. 2013, 40, 687–696. [Google Scholar] [CrossRef]
- Harkin, D.W.; Barros D’Sa, A.A.; McCallion, K.; Hoper, M.; Campbell, F.C. Ischemic preconditioning before lower limb ischemia--reperfusion protects against acute lung injury. J. Vasc. Surg. 2002, 35, 1264–1273. [Google Scholar] [CrossRef]
- Przyklenk, K.; Whittaker, P. Remote ischemic preconditioning: Current knowledge, unresolved questions, and future priorities. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 255–259. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. Gadolinium and ruthenium red attenuate remote hind limb preconditioning-induced cardioprotection: Possible role of TRP and especially TRPV channels. Naunyn Schmiedebergs Arch. Pharm. 2016, 389, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Nezami, B.G.; Rahimpour, S.; Sadeghi, M.; Sianati, S.; Anaraki, D.K.; Ebrahimi, F.; Ghasemi, M.; Dehpour, A.R. Chronic lithium impairs skin tolerance to ischemia in random-pattern skin flap of rats. J. Surg. Res. 2011, 171, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Afraz, S.; Kamran, A.; Moazzami, K.; Nezami, B.G.; Dehpour, A.R. Protective effect of pharmacologic preconditioning with pioglitazone on random-pattern skin flap in rat is mediated by nitric oxide system. J. Surg. Res. 2012, 176, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, M.V.; Loiola, R.T.; Rocha, E.L.; Simao, A.F.; Gomes, A.S.; Souza, M.H.; Ribeiro, R.A. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz J. Med. Biol Res. 2009, 42, 921–929. [Google Scholar] [CrossRef]
- Konstantinov, I.E.; Arab, S.; Li, J.; Coles, J.G.; Boscarino, C.; Mori, A.; Cukerman, E.; Dawood, F.; Cheung, M.M.; Shimizu, M.; et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J. Thorac. Cardiovasc. Surg. 2005, 130, 1326–1332. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Invest. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Z.; Wu, M.; Sun, M.; Xia, Y.; Wang, Y. Comparison of Proangiogenic Effects of Adipose-Derived Stem Cells and Foreskin Fibroblast Exosomes on Artificial Dermis Prefabricated Flaps. Stem Cells Int. 2020, 2020, 5293850. [Google Scholar] [CrossRef]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef]
- Peng, W.; Peng, Z.; Tang, P.; Sun, H.; Lei, H.; Li, Z.; Hui, D.; Du, C.; Zhou, C.; Wang, Y. Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology. Materials 2020, 13, 4108. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Bayat, A. Microarchitectural analysis of decellularised unscarred and scarred dermis provides insight into the organisation and ultrastructure of the human skin with implications for future dermal substitute scaffold design. J. Tissue Eng. 2019, 10, 2041731419843710. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.V.; Francis, L.; Somasundaram, M.; Greco, G.; English, N.R.; Roether, J.A.; Boccaccini, A.R.; Sibbons, P.; Ansari, T. Characterisation of porcine dermis scaffolds decellularised using a novel non-enzymatic method for biomedical applications. J. Biomater. Appl. 2015, 30, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.S.; Heo, C.Y.; Shin, J.; Moon, S.Y.; Cho, S.W.; Kang, H.J. Effects of a Catechol-Functionalized Hyaluronic Acid Patch Combined with Human Adipose-Derived Stem Cells in Diabetic Wound Healing. Int. J. Mol. Sci. 2021, 22, 2632. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Liao, H.; Ye, J.; Huang, J.; Li, S.; Peng, H.; Yu, X.; Wen, H.; Wang, X. Soluble microneedle patch with photothermal and NO-release properties for painless and precise treatment of ischemic perforator flaps. J. Mater. Chem. B 2021, 9, 7725–7733. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, L.; Chen, L.; Xu, Y.; Chen, Y.; Li, Z.; Liu, X.; Wu, J.; Qi, S. Prevascularized mesenchymal stem cell-sheets increase survival of random skin flaps in a nude mouse model. Am. J. Transl. Res. 2019, 11, 1403–1416. [Google Scholar]
- Cai, Z.; Saiding, Q.; Cheng, L.; Zhang, L.; Wang, Z.; Wang, F.; Chen, X.; Chen, G.; Deng, L.; Cui, W. Capturing dynamic biological signals via bio-mimicking hydrogel for precise remodeling of soft tissue. Bioact. Mater. 2021, 6, 4506–4516. [Google Scholar] [CrossRef]
- Feige, K.; Torregroza, C.; Gude, M.; Maddison, P.; Stroethoff, M.; Roth, S.; Lurati Buse, G.; Hollmann, M.W.; Huhn, R. Cardioprotective Properties of Humoral Factors Released after Remote Ischemic Preconditioning in CABG Patients with Propofol-Free Anesthesia-A Translational Approach from Bedside to Bench. J. Clin. Med. 2022, 11, 1450. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.L.; Van der Merwe, L.; Lou, D.H.; Lin, C. Preclinical efficacy of stem cell therapy for skin flap: A systematic review and meta-analysis. Stem Cell Res. Ther. 2021, 12, 28. [Google Scholar] [CrossRef]
- Hamilton, K.; Wolfswinkel, E.M.; Weathers, W.M.; Xue, A.S.; Hatef, D.A.; Izaddoost, S.; Hollier, L.H., Jr. The Delay Phenomenon: A Compilation of Knowledge across Specialties. Craniomaxillofac Trauma Reconstr. 2014, 7, 112–118. [Google Scholar] [CrossRef]
- Xu, H.; Steinberger, Z.; Wo, Y.; Li, K.; Kuo, C.; Tong, Y.; Zhang, Y. Supercharging Strategies for Prefabricated Flaps in a Rat Model. J. Reconstr. Microsurg. 2019, 35, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Baynosa, R.C. Hyperbaric Oxygen Therapy for the Compromised Graft or Flap. Adv. Wound Care (New Rochelle) 2017, 6, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ashjian, P.; Disa, J.J.; Cordeiro, P.G.; Pusic, A.L.; Mehrara, B.J. Is the use of intraoperative heparin safe? Plast. Reconstr. Surg. 2008, 121, 49e–53e. [Google Scholar] [CrossRef] [PubMed]
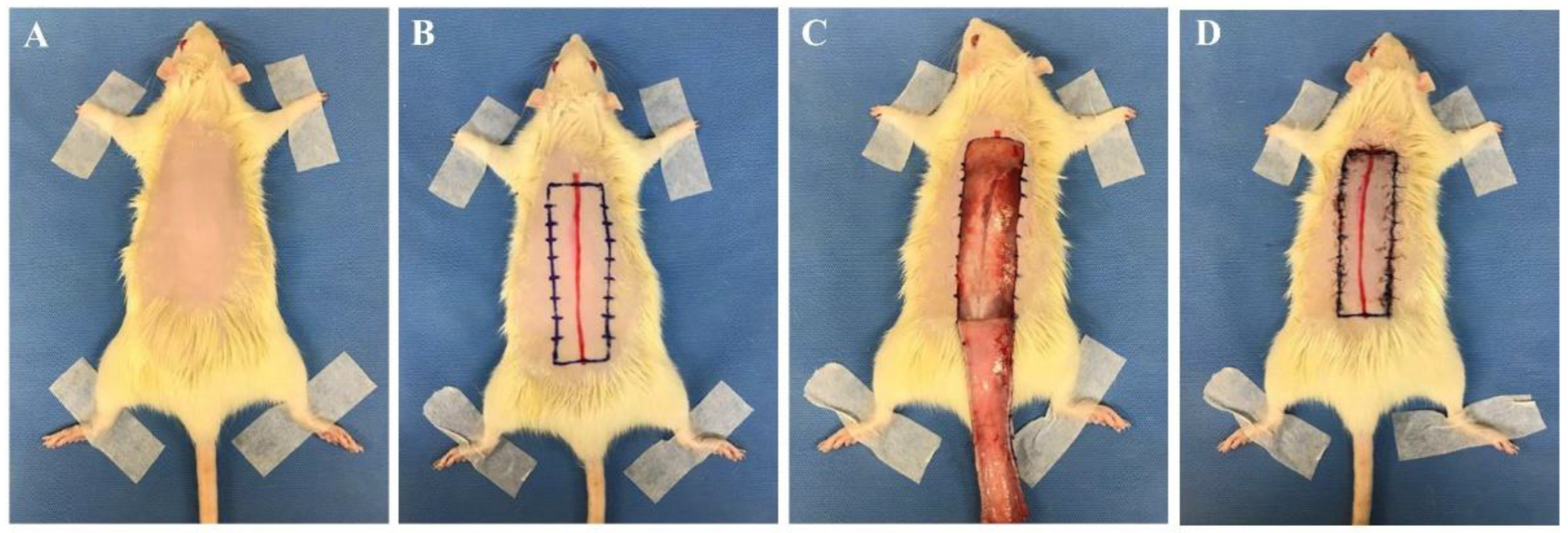
| Flap Size | Animal Type | Suture | Author | Flap Type |
|---|---|---|---|---|
| 1.5 cm × 7.5 cm (Two flaps) | Sprague-Dawley (250~300 g) | unknown | Pan XY | Dorsal flap |
| Wistar (250~350 g) | 4-0 nylon | Habibi M | McFarlane flap (Dorsal flap) | |
| 1.5 cm × 6 cm 1.5 cm × 6 cm (Two flaps) | Sprague-Dawley (240~280 g) | 4-0 vicryl (1) 6-0 nylon (1) | Park TH, Offodile AC 2nd | McFarlane flap (Dorsal flap) |
| Wistar (277~305 g) | unknown | Kanayama K | McFarlane flap (Dorsal flap) | |
| 2 cm × 8 cm | Sprague-Dawley (250~350 g) | 4-0 nylon (1) unknown (3) | Fayazzadeh E, Koh KS, Burusapat C, Doğan F | McFarlane flap (Dorsal flap) |
| Wistar (243~310 g) | 4-0 silk (2) unknown (1) | İnce B, Aryannejad A, Tabary M | McFarlane flap (Dorsal flap) | |
| Norvegicus albinus (280~320 g) | 4-0 nylon | Rech FV | McFarlane flap (Dorsal flap) | |
| 2 cm × 9 cm | Sprague-Dawley (200~300 g) | unknown | Kashimura T | Dorsal flap |
| 2.5 cm × 5 cm | Wistar (300~350 g) | 4-0 polypropylene | Nacak U | TRAM flap * |
| 2.5 cm × 8 cm | Wistar (170~285 g) | 5-0 nylon | Silva JJ | McFarlane flap |
| 2.5 cm × 11 cm | Sprague-Dawley (250~300 g) | 4-0 silk (2) | Wang L, Gao ZM | McFarlane flap (Dorsal flap) |
| Wistar (424~545 g) | 5-0 nylon | Kagaya Y | Island flap (epigastric vessels) | |
| 3 cm × 3 cm | Sprague-Dawley (16 weeks) | unknown | Zhu C | Island flap (epigastric vessels) |
| 3 cm × 5 cm | Sprague-Dawley (250~300 g) | unknown | Kim SY | DIEP flap ** |
| 3 cm × 6 cm | Wistar (300~350 g) | unknown | Yue ZS | Abdominal island skin flap |
| 3 cm × 8 cm | Sprague-Dawley (200~350 g) | 4–0 monofilament (1) unknown (3) | Qing L, Acartürk TO, Karimi AA, Ma Y | McFarlane flap (Dorsal flap) |
| Wistar (200~330 g) | 4-0 nylon (2) | Chehelcheraghi F, Nakagawa T | McFarlane flap (Dorsal flap) | |
| 3 cm × 9 cm | Sprague-Dawley (180~430 g) | 4-0 silk (7) 4-0 nylon (5) 4-0 prolene (1) 5-0 prolene (1) unknown (6) | Wang LR, Rau AS, Xu L, Roh TS, Dingsheng L, Lv QB, Deheng C, Lin B, Chen GJ, Kailiang Z, Lin Y, Xie XG, Liu Y, Li WJ, Pak CS, Fan W, Jaleel Z, Huang T, Ma X, Luo Z | McFarlane flap (Dorsal flap) |
| Wistar (161~350 g) | 4-0 vicryl (1) unknown (2) | Orhan E, Masaoka K, Öksüz M, | McFarlane flap (Dorsal flap) | |
| 3 cm × 10 cm | Sprague-Dawley (179~300 g) | 4-0 nylon (3) 4-0 prolene (1) 4–0 polydioxanone (1) unknown (2) | Jia YC, Peng L, Dölen UC, Hasdemir M, Wald G, Khavanin N, Dogan R | McFarlane flap (Dorsal flap) |
| Wistar (200~330 g) | 4-0 nylon (2) 4-0 silk (1) 5-0 nylon (1) | António NN, Görgülü T, Ghanbarzadeh K, Camargo CP | McFarlane flap (Dorsal flap) | |
| Lewis (~350 g) | unknown | Stone R | McFarlane flap (Dorsal flap) | |
| 3 cm × 11 cm | Wistar (250~300 g) | 3-0 propylene | Güner MH | McFarlane flap (Dorsal flap) |
| 3 cm × 12 cm | Sprague-Dawley (450~550 g) | unknown | Zheng J | McFarlane flap (Dorsal flap) |
| Fischer 344 (16 weeks) | unknown | Kira T | McFarlane flap (Dorsal flap) | |
| 3.6 cm × 7.2 cm | Sprague-Dawley (270~300 g) | 4-0 polypropylene | Hsueh YY | McFarlane flap (Dorsal flap) |
| 4 cm × 5 cm | Sprague-Dawley (290~350 g) | unknown | Zhang Y | Island flap (epigastric vessels) |
| 4 cm × 6 cm | Sprague-Dawley (275~300 g) | unknown | Aksakal İA | Island flap (epigastric artery) |
| Wistar (225~300 g) | unknown | Han HH | Island flap (epigastric artery) | |
| 4 cm × 7 cm | Wistar (280~320 g) | 6–0 monofilament | Fichter AM | Dorsal flap |
| 4-0 silk suture | Bagdas D | Island flap (epigastric artery) | ||
| 4 cm × 10 cm | Wistar (250~350 g) | 4–0 nylon | Can A | McFarlane flap (Dorsal flap) |
| Wistar EPM-1 (292~381 g) | 4–0 nylon (2) | Baldan CS, Esteves GR | McFarlane flap (Dorsal flap) | |
| 5 cm × 5 cm | Sprague-Dawley (220~270 g) | 4–0 silk | Lee YK | ventral abdomen |
| 5 cm × 13 cm | Sprague-Dawley | unknown | Gersch RP | Dorsal flap |
| 6 cm × 6 cm | Sprague-Dawley (250~350 g) | unknown | Akcal A | Island flap (epigastric vessels) |
| 6 cm × 9 cm | Sprague-Dawley (280~320 g) | Unknown (4) | Bai M | Abdomen Flap |
| Song K, Xiao YD, Odake K | Island flap (epigastric artery) |
| Flap Size | Animal Type | Suture | Author | Flap Type |
|---|---|---|---|---|
| Mouse | ||||
| 1 cm × 2 cm | C57Bl/6J (12 weeks) | - | Tang YH | Island flap (epigastric vessels) |
| 1 cm × 3 cm | C57Bl/6 (9~10 weeks) | 4-0 nylon | Fukunaga Y | Dorsal skin flap |
| 1 cm × 4 cm | C57Bl/6J | - | Pu CM | Pectoral skin flap |
| 1.25 cm × 2.25 cm | ICR (CD1) (8~12 weeks) | 6-0 prolene | Rednam CK | Dorsal skin flap |
| 1.5 cm × 3 cm | ICR (8 weeks) | - | Moon JH | Dorsal skin flap |
| SKH-1 | - | Chin MS | Dorsal skin flap | |
| 1.5 cm × 3.5 cm | C57BL/6N (8 weeks) | 4-0 polyglactin | Rah DK | Island flap (thoracic artery) |
| CD-1(ICR) (8~10 weeks) | unknown | Yin Z | Island flap (thoracic artery) | |
| FVB/NJNarl (8 weeks) | - | Tsai TC | Dorsal skin flap | |
| 1.5 cm × 4 cm | ICR (6 weeks) | - | Lee MS | Dorsal skin flap |
| 2 cm × 4 cm | BALB/c (7 weeks) | - | Salvador DRN, Park IS | Dorsal skin flap |
| 4 cm × 4 cm | ICR (30~40 g) | 6-0 nylon | Cao Minh T | Island flap (dorsal bipedicle) |
| Rabbit | ||||
| Two 2 cm × 8 cm | rabbit | 5-0 nylon | Wang B | Dorsal skin flap |
| 2.5 cm × 6 cm | New Zealand (3.0~3.5 kg) | 7-0 prolene | Zhuang Y | Dorsal skin flap |
| 4 cm × 5 cm | New Zealand (2.0~2.5 kg) | - | Prasetyono TO | Island flap (fasciocutaneous) |
| 5 cm × 17 cm | Japanese white (3.0~3.5 kg) | - | Abe Y | Island flap (epigastric vessels) |
| 10 cm × 10 cm | New Zealand (2.5~3.0 kg) | - | Kim HY | Island flap (fasciocutaneous) |
| 12 cm × 13 cm | Japanese white (3.5~4.0 kg) | - | Yan H | Island flap (artery graft) |
| 15 cm × 19 cm | New Zealand (4.0~5.0 kg) | 6-0 polypropylene | Huang L | Island flap (abdominal) |
| Pig | ||||
| Two 3 cm × 15 cm | Mini pigs (23 kg) | - | Tang Y | Rectangular skin flap |
| 4 cm × 14 cm (Three/Six) | Chinese Bama minipigs (9~10 kg) | - | Yin GQ, Zhao H | Rectangular skin flap |
| Four 4 cm × 16 cm | Yorkshire cross adult pigs (50~80 kg) | - | Zellner S | Rectangular skin flap |
| Four 5 cm × 15 cm | Yorkshire pigs (31~37 kg) | - | Elgharably H | Rectangular skin flap |
| 10 cm × 25 cm | Yorkshire pigs (10 kg) | - | Minqiang X | Rectangular skin flap |
| Score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Edema | Normal | Mild | Moderate | Marked | Extensive |
| Inflammation | None | Some | Moderate | Effusive | Severe |
| Congestion | None | Mild | Moderate | Marked | Extensive |
| Regulating Factors | Factors Associated NF-κB |
|---|---|
| Cytokines | TNF-α, IL-1, IL-6, GM-CSF |
| Chemokines | IL-8, macrophage-inflammatory protein-1α (MIP-1α), methyl accepting chemotaxis protein1 (MCP1), RANTES, eotaxin |
| Adhesion molecules | E-selectin, vascular cell adhesion molecule-1 (VCAM-1), endothelial leukocyte adhesion molecule-1 (ELAM), intercellular cell adhesion molecule1 (ICAM-1) |
| Inducible enzyme | cyclooxygenase-2 (COX-2), inducible nitroxide synthase (iNOS) |
| Author (Year) | Animals | Ischemic Preconditioning | IPC Tools |
|---|---|---|---|
| Torregroza C et al. (2021) | Wistar rats (2–3 months) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuffs > 200 mmHg |
| McDonald MW et al. (2021) | Sprague-Dawley rats (250–275 g) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuffs > 170 mmHg |
| Nizari S et al. (2021) | Sprague–Dawley rats (220–250 g) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuffs > 200 mmHg |
| Li H et al. (2020) | Wistar albino rats (210–240 g) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuffs > 150 mmHg |
| Chen Q et al. (2020) | Wistar rats (280–300 g) | 10 cycles 2 min occlusion/2 min reperfusion | tourniquet |
| Pak CS et al. (2021) | Sprague-Dawley rats (240–260 g) | 3 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Danková M et al. (2021) | New Zealand white rabbits (2.5–3 kg) | 3 cycles 2 min occlusion/2 min reperfusion | tourniquet |
| Merlocco AC et al. (2014) | White rabbits (3–3.5 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Schmidt MR et al. (2014) | New Zealand white rabbits (3 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Surendra H et al. (2013) | New Zealand White rabbits (3–3.5 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Shimizu M et al. (2009) | New Zealand white rabbits | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Galán-Arriola C et al. (2021) | Large-White male pigs (3 months) | 3 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Lieder HR et al. (2019) | Göttingen minipigs (34.6 ± 5.4 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Skyschally A et al. (2018) | Göttingen minipigs (30.9 ± 2.1 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet |
| Herajärvi J et al. (2017) | Pigs (7–8 weeks) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuffs > 250 mmHg |
| Haapanen H et al. (2016) | Pigs (19–22 kg) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuff > 250 mmHg |
| Gardner DS et.al (2014) | Pig (58 ± 4.6 kg) | 3 cycles 5 min occlusion/5 min reperfusion | sphygmomanometer cuff > 200 mmHg |
| Yannopoulos FS et.al (2010) | Pig (8 weeks) | 4 cycles 5 min occlusion/5 min reperfusion | blood pressure cuff > 230 mmHg |
| Zhao JL et al. (2009) | mini-pigs (30.3 ± 3.0 kg) | 4 cycles 5 min occlusion/5 min reperfusion | tourniquet cuff |
| Shimizu M et al. (2007) | Yorkshire pig | 3 cycles 5 min occlusion/5 min reperfusion | tourniquet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; You, H.-J.; Lee, T.-Y.; Kang, H.J. Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors. Int. J. Mol. Sci. 2022, 23, 5234. https://doi.org/10.3390/ijms23095234
Lee J-H, You H-J, Lee T-Y, Kang HJ. Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors. International Journal of Molecular Sciences. 2022; 23(9):5234. https://doi.org/10.3390/ijms23095234
Chicago/Turabian StyleLee, Ju-Hee, Hi-Jin You, Tae-Yul Lee, and Hyo Jin Kang. 2022. "Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors" International Journal of Molecular Sciences 23, no. 9: 5234. https://doi.org/10.3390/ijms23095234
APA StyleLee, J.-H., You, H.-J., Lee, T.-Y., & Kang, H. J. (2022). Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors. International Journal of Molecular Sciences, 23(9), 5234. https://doi.org/10.3390/ijms23095234






