The Interaction of Anti-DNA Antibodies with DNA: Evidence for Unconventional Binding Mechanisms
Abstract
:1. Introduction
2. Anti-DNA Antibodies Bind Large DNA
3. Anti-DNA Antibodies Require the Fc Portion
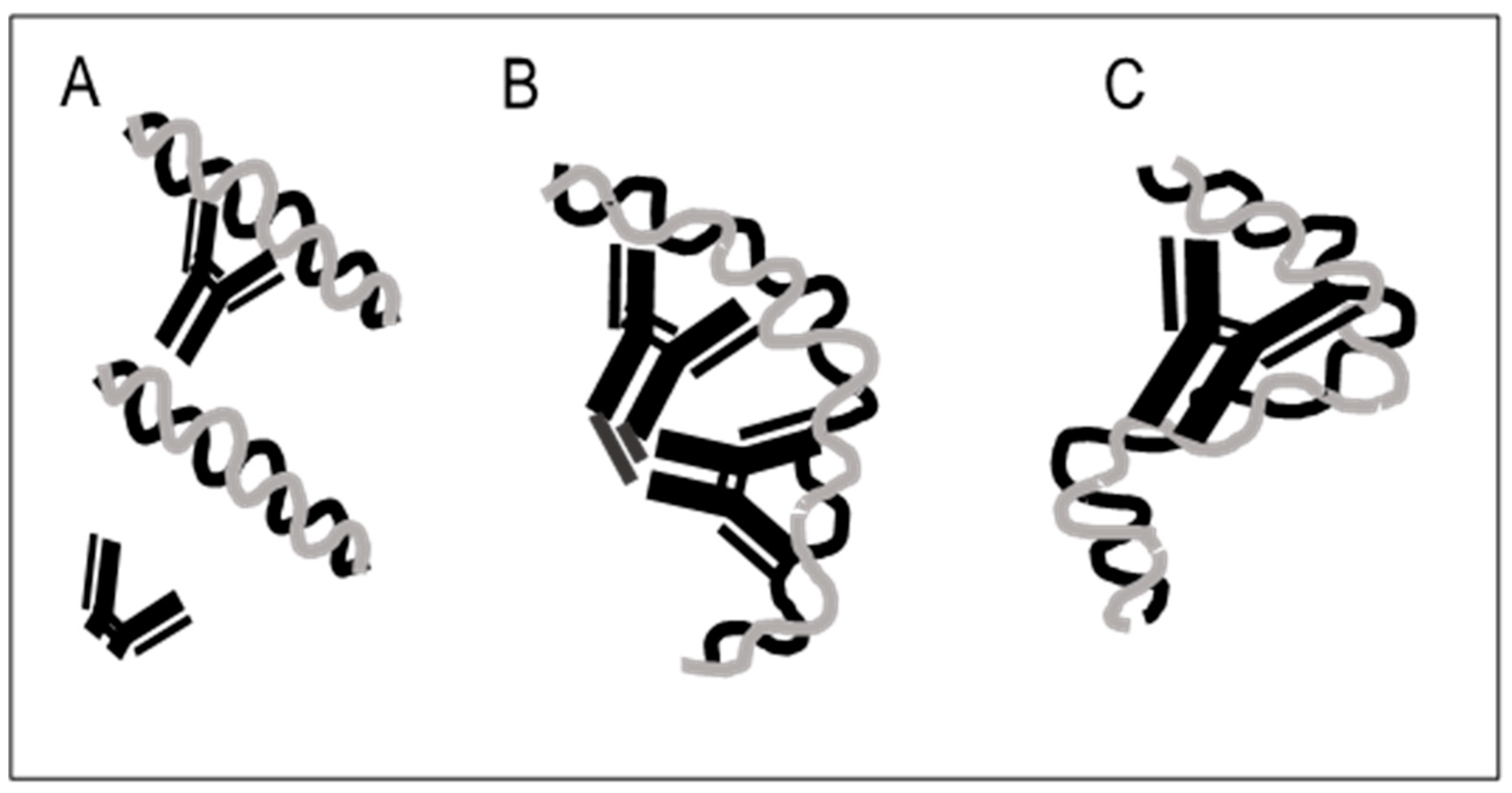
4. Antibodies to Bacterial DNA Bind by Monogamous Bivalency and Variably Require Fc
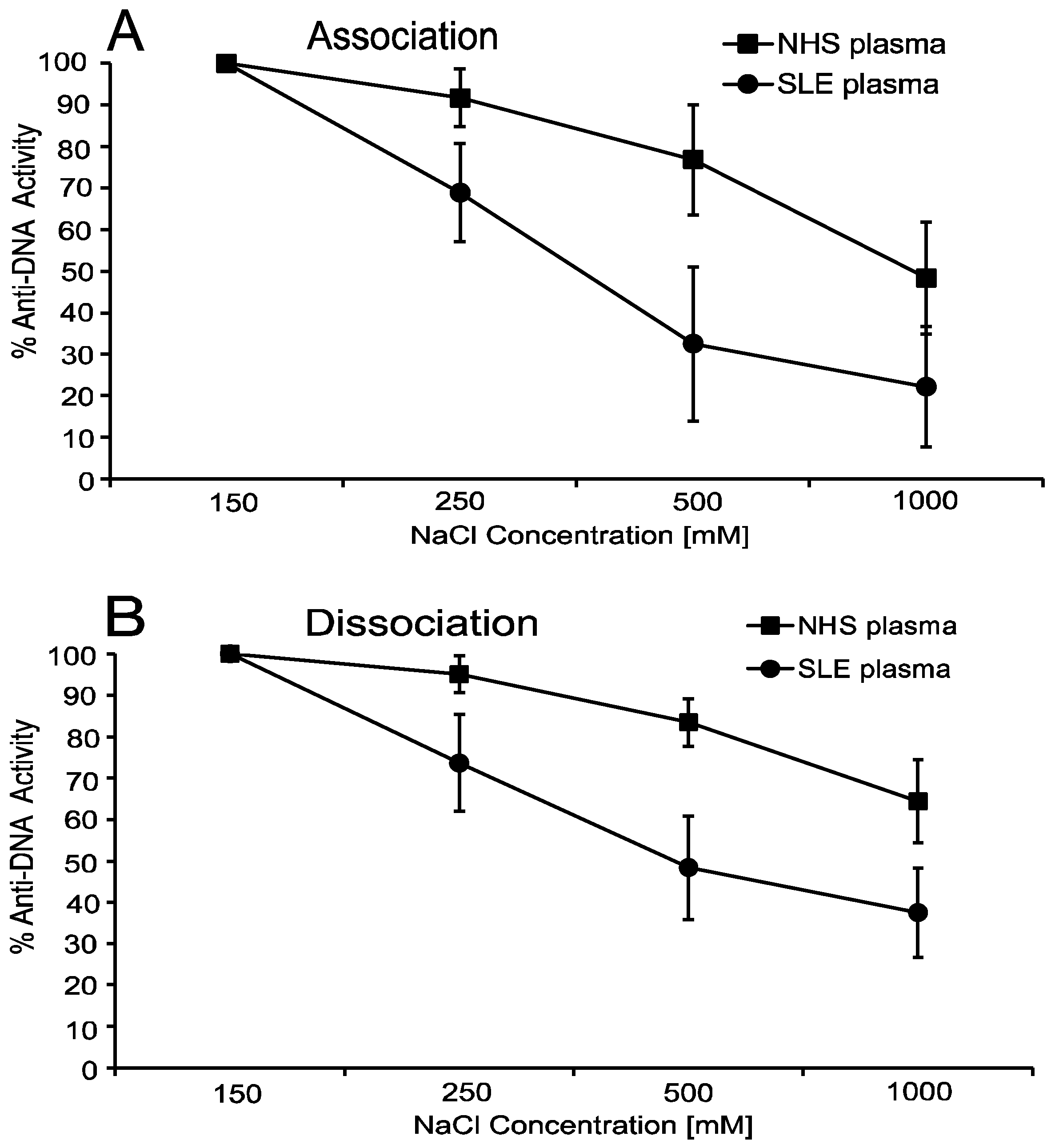
5. Anti-DNA Antibodies Depend on Electrostatic Interactions
6. Anti-DNA Antibodies Can Show Hysteresis
7. Conclusions
| Fc-dependent monogamous bivalency Requirement for a large antigenic structure for stable binding Lack of binding to small molecule representations of antigen Predominance of electrostatic interactions Hysteresis |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisetsky, D.S. Anti-DNA antibodies—Quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 2016, 12, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mummert, E.; Fritzler, M.J.; Sjöwall, C.; Bentow, C.; Mahler, M. The clinical utility of anti-double-stranded DNA antibodies and the challenges of their determination. J. Immunol. Methods 2018, 459, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rekvig, O.P. The Anti-DNA Antibodies: Their Specificities for Unique DNA Structures and Their Unresolved Clinical Impact-A System Criticism and a Hypothesis. Front. Immunol. 2021, 12, 808008. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Diaz-Gallo, L.M.; Oke, V.; Lundström, E.; Elvin, K.; Ling Wu, Y.; Eketjäll, S.; Zickert, A.; Gustafsson, J.T.; Jönsen, A.; Leonard, D.; et al. Four Systemic Lupus Erythematosus Subgroups, Defined by Autoantibodies Status, Differ Regarding HLA-DRB1 Genotype Associations and Immunological and Clinical Manifestations. ACR Open Rheumatol. 2022, 4, 27–39. [Google Scholar] [CrossRef]
- Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 2000, 37, 1141–1149. [Google Scholar] [CrossRef]
- Böröcz, K.; Simon, D.; Erdő-Bonyár, S.; Kovács, K.T.; Tuba, É.; Czirják, L.; Németh, P.; Berki, T. Relationship between natural and infection-induced antibodies in systemic autoimmune diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2021, 203, 32–40. [Google Scholar] [CrossRef]
- Abida, R.; Apsley, E.; Isenberg, D. IgG vs IgG + IgM anti-double stranded DNA measurement: A comparative report for clinical practice. Rheumatology 2021, 60, 3951–3952. [Google Scholar] [CrossRef]
- Förger, F.; Matthias, T.; Oppermann, M.; Becker, H.; Helmke, K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus 2004, 13, 36–44. [Google Scholar] [CrossRef]
- Stollar, B.D. Immunochemistry of DNA. Int. Rev. Immunol. 1989, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.Z.; Weigert, M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 1994, 12, 487–520. [Google Scholar] [CrossRef] [PubMed]
- Papalian, M.; Lafer, E.; Wong, R.; Stollar, B.D. Reaction of systemic lupus erythematosus antinative DNA antibodies with native DNA fragments from 20 to 1,200 base pairs. J. Clin. Investig. 1980, 65, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Reich, C.F. The influence of DNA size on the binding of anti-DNA antibodies in the solid and fluid phase. Clin. Immunol. Immunopathol. 1994, 72, 350–356. [Google Scholar] [CrossRef]
- Kalaaji, M.; Mortensen, E.; Jorgensen, L.; Olsen, R.; Rekvig, O.P. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am. J. Pathol. 2006, 168, 1779–1792. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.; Galetti, M.; Sinico, R.A.; Moroni, G.; Bonanni, A.; Radice, A.; Tincani, A.; Pratesi, F.; Migliorini, P.; Murtas, C.; et al. Glomerular Autoimmune Multicomponents of Human Lupus Nephritis In Vivo (2): Planted Antigens. J. Am. Soc. Nephrol. 2014, 26, 1905–1924. [Google Scholar] [CrossRef] [Green Version]
- Griffith, D.M.; Jayaram, D.T.; Spencer, D.M.; Pisetsky, D.S.; Payne, C.K. DNA-nanoparticle interactions: Formation of a DNA corona and its effects on a protein corona. Biointerphases 2020, 15, 051006. [Google Scholar] [CrossRef]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The structural basis of antibody-antigen recognition. Front. Immunol. 2013, 4, 302. [Google Scholar] [CrossRef] [Green Version]
- Wilson, I.A.; Stanfield, R.L. 50 Years of structural immunology. J. Biol. Chem. 2021, 296, 100745. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [Green Version]
- Stearns, N.A.; Pisetsky, D.S. The role of monogamous bivalency and Fc interactions in the binding of anti-DNA antibodies to DNA antigen. Clin. Immunol. 2016, 166–167, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.J.; Shikhman, A.R.; Glass, D.D.; Kangisser, D.; Cunningham, M.W.; Greenspan, N.S. Role of heavy chain constant domains in antibody-antigen interaction. Apparent specificity differences among streptococcal IgG antibodies expressing identical variable domains. J. Immunol. 1993, 150, 2231–2242. [Google Scholar] [PubMed]
- McLean, G.R.; Torres, M.; Elguezabal, N.; Nakouzi, A.; Casadevall, A. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 2002, 169, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Fernandez-Fuentes, N.; Fiser, A.; Casadevall, A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 2007, 282, 13917–13927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Janda, A.; Eryilmaz, E.; Casadevall, A.; Putterman, C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol. Immunol. 2013, 56, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janda, A.; Bowen, A.; Greenspan, N.S.; Casadevall, A. Ig Constant Region Effects on Variable Region Structure and Function. Front. Microbiol. 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Czaja, A.J.; Morshed, S.A.; Parveen, S.; Nishioka, M. Antibodies to single-stranded and double-stranded DNA in antinuclear antibody-positive type 1-autoimmune hepatitis. Hepatology 1997, 26, 567–572. [Google Scholar] [CrossRef]
- Muratori, P.; Granito, A.; Pappas, G.; Pendino, G.M.; Quarneti, C.; Cicola, R.; Menichella, R.; Ferri, S.; Cassani, F.; Bianchi, F.B.; et al. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am. J. Gastroenterol. 2009, 104, 1420–1425. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Tovoli, F.; Muratori, P. Diagnostic role of anti-dsDNA antibodies: Do not forget autoimmune hepatitis. Nat. Rev. Rheumatol. 2021, 17, 244. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Lipsky, P.E. Reply to: Diagnostic role of anti-dsDNA antibodies: Do not forget autoimmune hepatitis. Nat. Rev. Rheumatol. 2021, 17, 245. [Google Scholar] [CrossRef]
- Karounos, D.G.; Grudier, J.P.; Pisetsky, D.S. Spontaneous expression of antibodies to DNA of various species origin in sera of normal subjects and patients with systemic lupus erythematosus. J. Immunol. 1988, 140, 451–455. [Google Scholar] [PubMed]
- Wu, Z.Q.; Drayton, D.; Pisetsky, D.S. Specificity and immunochemical properties of antibodies to bacterial DNA in sera of normal human subjects and patients with systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 1997, 109, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.R.; Gilkeson, G.S.; Ward, M.M.; Pisetsky, D.S. Patterns of heavy and light chain utilization in the antibody response to single-stranded bacterial DNA in normal human subjects and patients with systemic lupus erythematosus. Clin. Immunol. Immunopathol. 1992, 62, 25–32. [Google Scholar] [CrossRef]
- Suurmond, J.; Calise, J.; Malkiel, S.; Diamond, B. DNA-reactive B cells in lupus. Curr. Opin. Immunol. 2016, 43, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belina, M.E.; Spencer, D.M.; Pisetsky, D.S. The Binding Mechanisms of Antibodies to DNA from Healthy Subjects and Patients with Systemic Lupus Erythematosus: The Role of Monogamous Bivalency and Fc Dependence. Immunohorizons 2021, 5, 792–801. [Google Scholar] [CrossRef]
- Robertson, C.R.; Pisetsky, D.S. Specificity analysis of antibodies to single-stranded micrococcal DNA in the sera of normal human subjects and patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 1992, 10, 589–594. [Google Scholar] [PubMed]
- Pisetsky, D.S.; Shaffer, R.; Armstrong, D.D.; Spencer, D.M. The interaction of anti-DNA antibodies with DNA antigen: Evidence for hysteresis for high avidity binding. Clin. Immunol. 2021, 231, 108848. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisetsky, D.S.; Garza Reyna, A.; Belina, M.E.; Spencer, D.M. The Interaction of Anti-DNA Antibodies with DNA: Evidence for Unconventional Binding Mechanisms. Int. J. Mol. Sci. 2022, 23, 5227. https://doi.org/10.3390/ijms23095227
Pisetsky DS, Garza Reyna A, Belina ME, Spencer DM. The Interaction of Anti-DNA Antibodies with DNA: Evidence for Unconventional Binding Mechanisms. International Journal of Molecular Sciences. 2022; 23(9):5227. https://doi.org/10.3390/ijms23095227
Chicago/Turabian StylePisetsky, David S., Angel Garza Reyna, Morgan E. Belina, and Diane M. Spencer. 2022. "The Interaction of Anti-DNA Antibodies with DNA: Evidence for Unconventional Binding Mechanisms" International Journal of Molecular Sciences 23, no. 9: 5227. https://doi.org/10.3390/ijms23095227
APA StylePisetsky, D. S., Garza Reyna, A., Belina, M. E., & Spencer, D. M. (2022). The Interaction of Anti-DNA Antibodies with DNA: Evidence for Unconventional Binding Mechanisms. International Journal of Molecular Sciences, 23(9), 5227. https://doi.org/10.3390/ijms23095227







