Polysialylation in a DISC1 Mutant Mouse
Abstract
:1. Introduction
2. Results
2.1. Design of Experiment
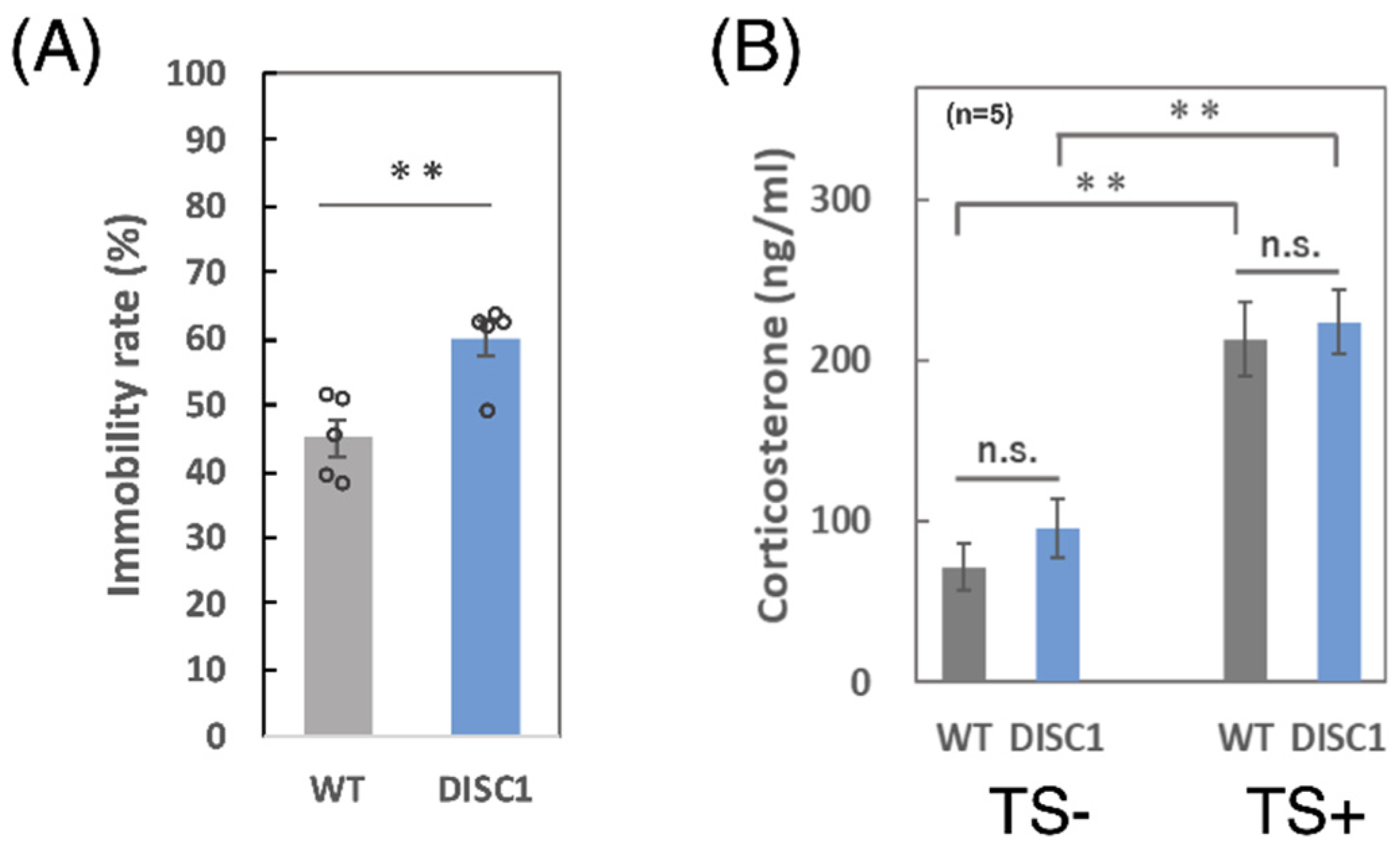
2.2. Comparison between WT and DISC1 Mutant Mice: Evaluation of the Immobility Rate and Concentration of Corticosterone in Serum
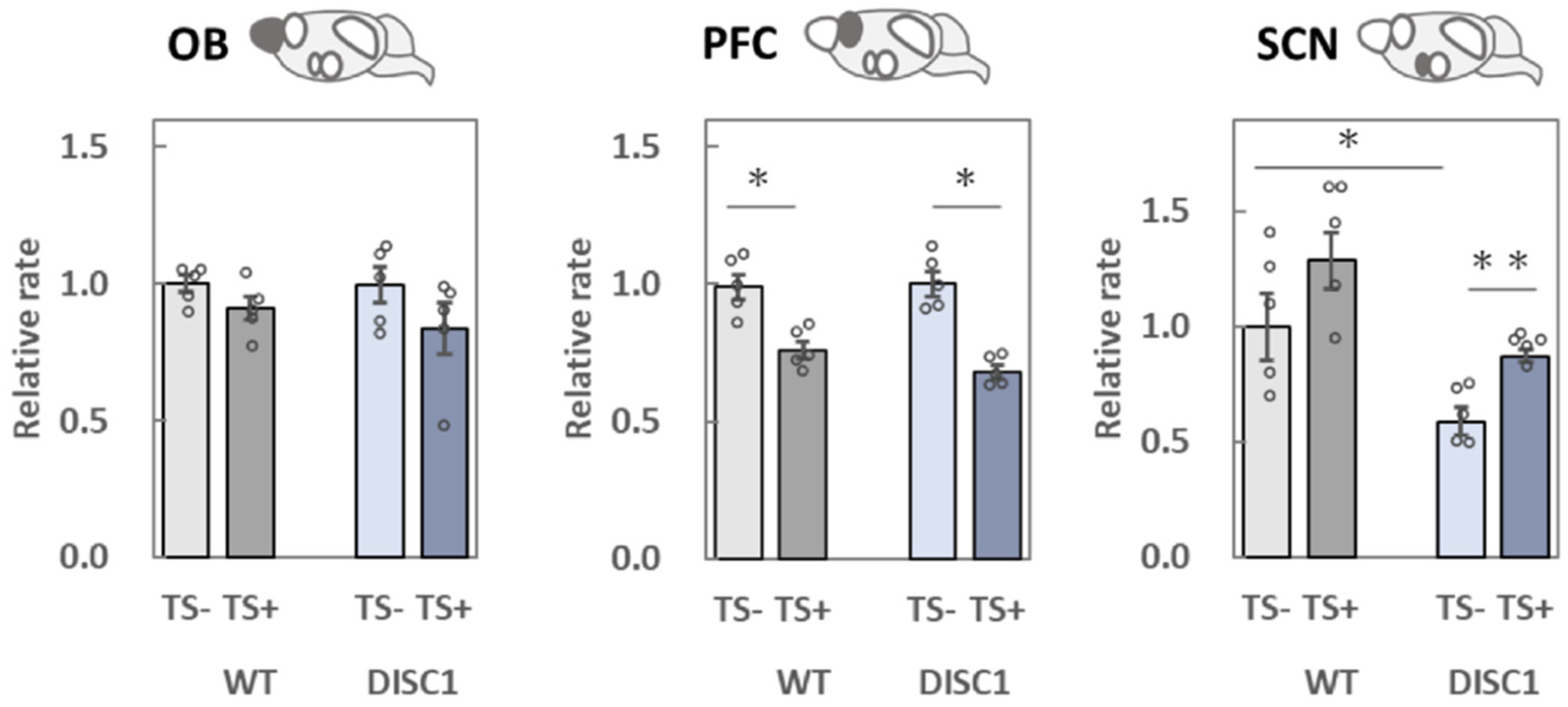
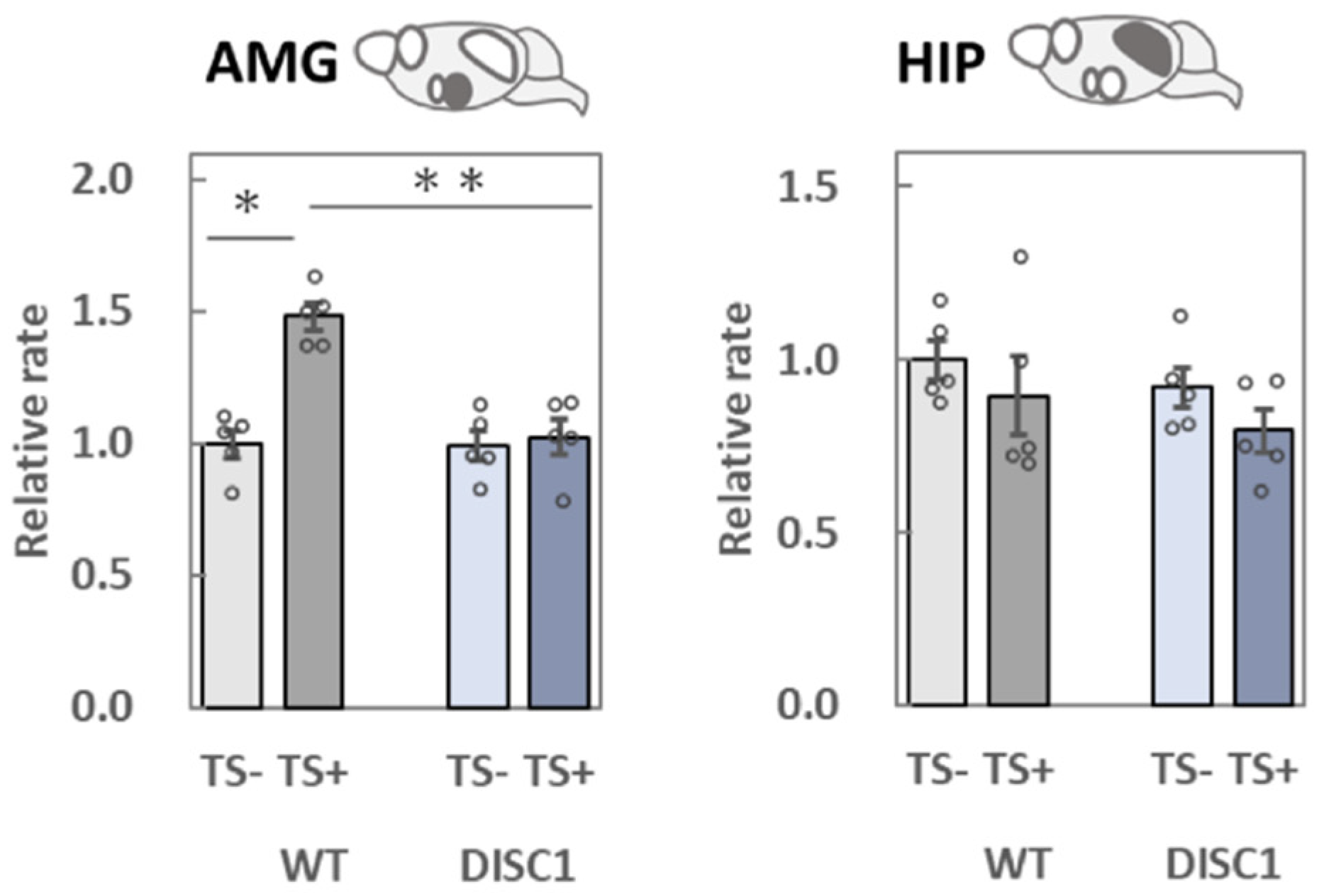
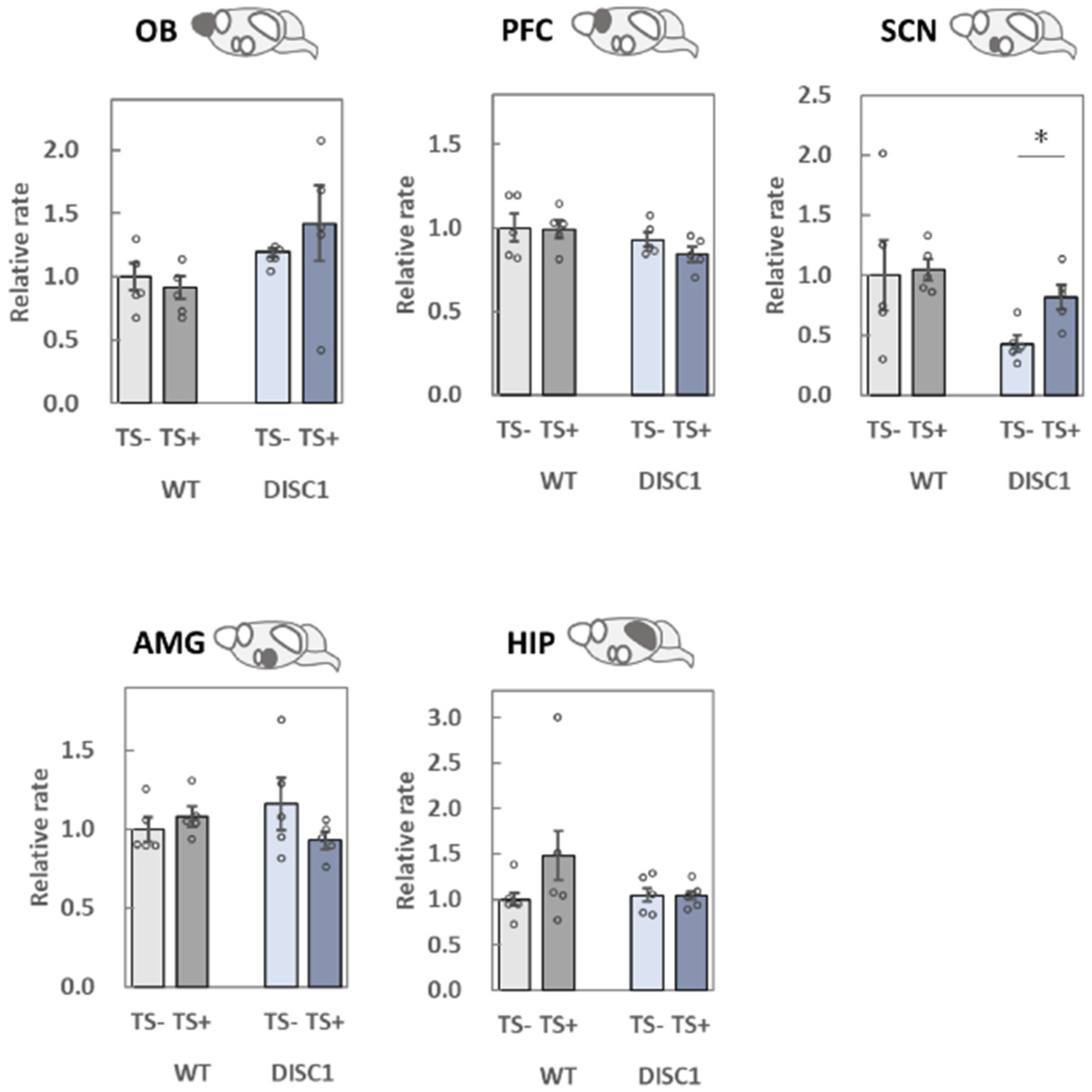
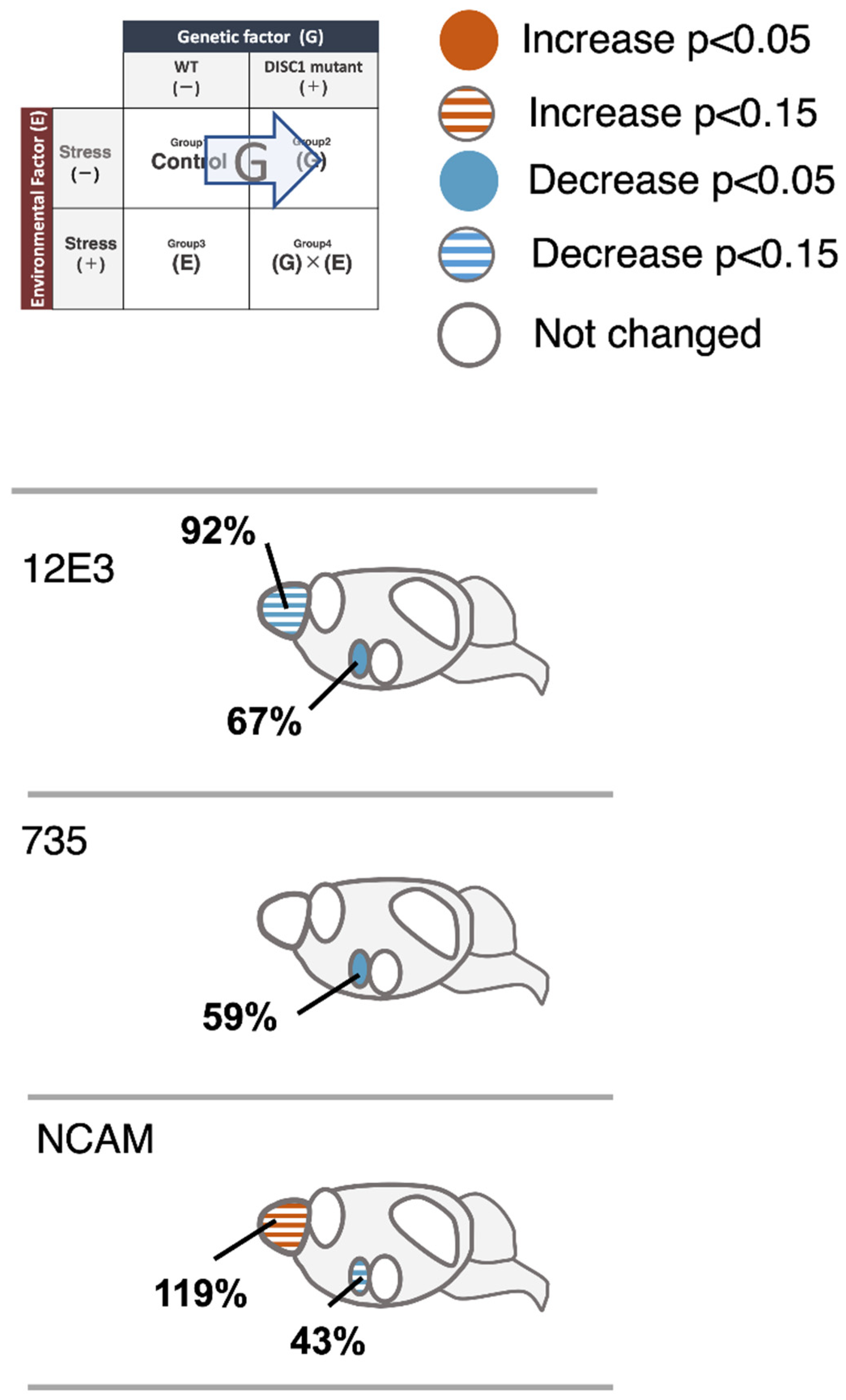
2.3. Evaluation of polySia (Glycan) Expression Based on 12E3, 735, and NCAM (Protein) Expression in the Brain
2.3.1. OB
2.3.2. PFC
2.3.3. SCN
2.3.4. AMG
2.3.5. HIP
3. Discussion
3.1. Immobility Ratio and Concentration of Corticosterone
3.2. Analysis of polySia and NCAM Expression in Five Different Brain Regions
3.2.1. OB
3.2.2. PFC
3.2.3. SCN
3.2.4. AMG
3.2.5. HIP
3.3. PolySia Expression and DISC1
4. Materials and Methods
4.1. Materials
4.2. Animals and Ethics Statement
4.3. TS Test
4.4. Sample Preparation
4.5. Corticosterone Quantification
4.6. Quantification of polySia and NCAM Using ELISA
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Koszła, O.; Targowska-Duda, K.M.; Kędzierska, E.; Kaczor, A.A. In Vitro and In Vivo Models for the Investigation of Potential Drugs Against Schizophrenia. Biomolecules 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, C.; Kitajima, K. Polysialylation and disease. Mol. Aspects Med. 2021, 79, 100892. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Kitajima, K. Disialic. oligosialic and polysialic acids: Distribution, functions and related disease. J. Biochem. 2013, 154, 115–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfanti, L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006, 80, 129–164. [Google Scholar] [CrossRef]
- Bonfanti, L.; Olive, S.; Poulain, D.A.; Theodosis, D.T. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: An immunohistochemical study. Neuroscience 1992, 49, 419–436. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K. Sialic Acids in Neurology. Adv. Carbohydr. Chem. Biochem. 2019, 76, 1–64. [Google Scholar] [CrossRef]
- Sato, C.; Hane, M. Mental disorders and an acidic glycan-from the perspective of polysialic acid (PSA/polySia) and the synthesizing enzyme, ST8SIA2. Glycoconj. J. 2018, 35, 353–373. [Google Scholar] [CrossRef]
- Kröcher, T.; Malinovskaja, K.; Jürgenson, M.; Aonurm-Helm, A.; Zharkovskaya, T.; Kalda, A.; Röckle, I.; Schiff, M.; Weinhold, B.; Gerardy-Schahn, R.; et al. Schizophrenia-like phenotype of polysialyltransferase ST8SIA2-deficient mice. Brain Struct. Funct. 2015, 220, 71–83. [Google Scholar] [CrossRef]
- Barbeau, D.; Liang, J.; Robitalille, Y.; Quirion, R.; Srivastava, L. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc. Natl. Acad. Sci. USA 1995, 92, 2785–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilabert-Juan, J.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Nácher, J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci. Lett. 2012, 530, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Fukuda, M. Polysialyltransferases: Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 2003, 85, 195–206. [Google Scholar] [CrossRef]
- Arai, M.; Yamada, K.; Toyota, T.; Obata, N.; Haga, S.; Yoshida, Y.; Nakamura, K.; Minabe, Y.; Ujike, H.; Sora, I.; et al. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol. Psychiatry 2006, 59, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Isomura, R.; Kitajima, K.; Sato, C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J. Biol. Chem. 2011, 286, 21535–21545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hane, M.; Sumida, M.; Kitajima, K.; Sato, C. Structural and functional impairments of polysialic acid (polySia)-neural cell adhesion molecule (NCAM) synthesized by a mutated polysialyltransferase of a schizophrenic patient. Pure Appl. Chem. 2012, 84, 1895–1906. [Google Scholar] [CrossRef]
- Hane, M.; Matsuoka, S.; Ono, S.; Miyata, S.; Kitajima, K.; Sato, C. Protective effects of polysialic acid on proteolytic cleavage of FGF2 and proBDNF/BDNF. Glycobiology 2015, 25, 1112–1124. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.; Hane, M.; Niimi, Y.; Kitajima, K.; Sato, C. Different properties of polysialic acids synthesized by the polysialyltransferases ST8SIA2 and ST8SIA4. Glycobiology 2017, 27, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Hane, M.; Kitajima, K.; Sato, C. Effects of intronic single nucleotide polymorphisms (iSNPs) of a polysialyltransferase, ST8SIA2 gene found in psychiatric disorders on its gene products. Biochem. Biophys. Res. Commun. 2016, 478, 1123–1129. [Google Scholar] [CrossRef]
- Varea, E.; Guirado, R.; Gilabert-Juan, J.; Martí, U.; Castillo-Gomez, E.; Blasco-Ibáñez, J.M.; Crespo, C.; Nacher, J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J. Psychiatry Res. 2012, 46, 189–197. [Google Scholar] [CrossRef]
- Abe, C.; Nishimura, S.; Mori, A.; Niimi, Y.; Yang, Y.; Hane, M.; Kitajima, K.; Sato, C. Chlorpromazine Increases the Expression of Polysialic Acid (PolySia) in Human Neuroblastoma Cells and Mouse Prefrontal Cortex. Int. J. Mol. Sci. 2017, 18, 1123. [Google Scholar] [CrossRef] [Green Version]
- Abe, C.; Yi, Y.; Hane, M.; Kitajima, K.; Sato, C. Acute stress-induced change in polysialic acid levels mediated by sialidase in mouse brain. Sci. Rep. 2019, 9, 9950. [Google Scholar] [CrossRef]
- Devon, R.; Anderson, S.; Teague, P.; Burgess, P.; Kipari, T.; Semple, C.; Millar, J.; Muir, W.; Murray, V.; Pelosi, A.; et al. Identification of polymorphisms within Disrupted in Schizophrenia 1 and Disrupted in Schizophrenia 2, and an investigation of their association with schizophrenia and bipolar affective disorder. Psychiatry Genet. 2001, 11, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Clapcote, S.J.; Lipina, T.V.; Millar, J.K.; Mackie, S.; Christie, S.; Ogawa, F.; Lerch, J.P.; Trimble, K.; Uchiyama, M.; Sakuraba, Y.; et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 2007, 54, 387–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomoda, T.; Sumitomo, A.; Jaaro-Peled, H.; Sawa, A. Utility and validity of DISC1 mouse models in biological psychiatry. Neuroscience 2016, 321, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K.; Inoue, S.; Seki, T.; Troy, F.A.; Inoue, Y. Characterization of the antigenic specificity of four different anti-(alpha 2-->8-linked polysialic acid) antibodies using lipid-conjugated oligo/polysialic acids. J. Biol. Chem. 1995, 270, 18923–18928. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.; Yang, Y.; Takahashi, Y.; Hane, M.; Kitajima, K.; Chihiro, S. Combinational Analyses with Multiple Methods Reveal the Existence of Several Forms of Polysialylated Neural Cell Adhesion Molecule in Mouse Developing Brains. Int. J. Mol. Sci. 2020, 21, 5892. [Google Scholar] [CrossRef]
- Sato, C.; Fukuoka, H.; Ohta, K.; Matsuda, T.; Koshino, R.; Kobayashi, K.; Troy, F.A.; Kitajima, K. Frequent occurrence of pre-existing alpha 2-->8-linked disialic and oligosialic acids with chain lengths up to 7 Sia residues in mammalian brain glycoproteins. Prevalence revealed by highly sensitive chemical methods and anti-di-, oligo-, and poly-Sia antibodies specific for defined chain lengths. J. Biol. Chem. 2000, 275, 15422–15431. [Google Scholar]
- Armario, A. The hypothalamic-pituitary-adrenal axis: What can it tell us about stressors? CNS Neurol Disord Drug Targets 2006, 5, 485–501. [Google Scholar] [CrossRef]
- Whitnall, M.H. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 1993, 40, 573–629. [Google Scholar] [CrossRef]
- Eachus, H.; Bright, C.; Cunliffe, V.T.; Placzek, M.; Wood, J.D.; Watt, P.J. Disrupted-in-Schizophrenia-1 is essential for normal hypothalamic-pituitary-interrenal (HPI) axis function. Hum. Mol. Genet. 2017, 26, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Wrynn, A.S.; Leonard, B.E. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef]
- Cremer, H.; Lange, R.; Christoph, A.; Plomann, M.; Vopper, G.; Roes, J.; Brown, R.; Baldwin, S.; Kraemer, P.; Scheff, S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994, 367, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, B.I.; Moberg, P.J.; Yousem, D.M.; Doty, R.L.; Arnold, S.E.; Gur, R.E. Reduced olfactory bulb volume in patients with schizophrenia. Am. J. Psychiatry 2000, 157, 828–830. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Shenton, M.E.; Levitt, J.J. Olfactory dysfunction in schizophrenia: A review of neuroanatomy and psychophysiological measurements. Harv. Rev. Psychiatry 2010, 18, 279–292. [Google Scholar] [CrossRef]
- Ruggiero, R.N.; Rossignoli, M.T.; Marques, D.B.; de Sousa, B.M.; Romcy-Pereira, R.N.; Lopes-Aguiar, C.; Leite, J.P. Neuromodulation of Hippocampal-Prefrontal Cortical Synaptic Plasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders. Front. Cell Neurosci. 2021, 15, 732360. [Google Scholar] [CrossRef]
- Okamura, H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Wulff, K.; Gatti, S.; Wettstein, J.G.; Foster, R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010, 11, 589–599. [Google Scholar] [CrossRef]
- Monti, J.M.; BaHammam, A.S.; Pandi-Perumal, S.R.; Bromundt, V.; Spence, D.W.; Cardinali, D.P.; Brown, G.M. Sleep and circadian rhythm dysregulation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 209–216. [Google Scholar] [CrossRef]
- Dzirasa, K.; Ribeiro, S.; Costa, R.; Santos, L.M.; Lin, S.C.; Grosmark, A.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A. Dopaminergic control of sleep-wake states. J. Neurosci. 2006, 26, 10577–10589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaaro-Peled, H.; Altimus, C.; LeGates, T.; Cash-Padgett, T.; Zoubovsky, S.; Hikida, T.; Ishizuka, K.; Hattar, S.; Mongrain, V.; Sawa, A. Abnormal wake/sleep pattern in a novel gain-of-function model of DISC1. Neurosci. Res. 2016, 112, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Watanabe, M.; Tomasiewicz, H.; Glass, J. Genetic deletions of NCAM and PSA impair circadian function in the mouse. Physiol. Behav. 2001, 73, 185–193. [Google Scholar] [CrossRef]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, S.N.; Friedman, A.R.; Kaufer, D.; Sakhai, S.A. Glucocorticoids and the Brain: Neural Mechanisms Regulating the Stress Response. Adv. Exp. Med. Biol. 2015, 872, 235–252. [Google Scholar] [CrossRef]
- Sandi, C.; Merino, J.; Cordero, M.; Touyarot, K.; Venero, C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 2001, 102, 329–339. [Google Scholar] [CrossRef]
- Pham, K.; Nacher, J.; Hof, P.R.; McEwen, B.S. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003, 17, 879–886. [Google Scholar] [CrossRef]
- Millar, J.; Wilson-Annan, J.; Anderson, S.; Christie, S.; Taylor, M.; Semple, C.; Devon, R.; Clair, D.S.; Muir, W.; Blackwood, D.; et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 2000, 9, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Camargo, L.; Collura, V.; Rain, J.; Mizuguchi, K.; Hermjakob, H.; Kerrien, S.; Bonnert, T.; Whiting, P.; Brandon, N. Disrupted in Schizophrenia 1 Interactome: Evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol. Psychiatry 2007, 12, 74–86. [Google Scholar] [CrossRef]
- Brandon, N.; Millar, J.; Korth, C.; Sive, H.; Singh, K.; Sawa, A. Understanding the role of DISC1 in psychiatric disease and during normal development. J. Neurosci. 2009, 29, 12768–12775. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, K.; Asanuma, M.; Miyazaki, I.; Diaz-Corrales, F.; Katayama, T.; Tohyama, M.; Ogawa, N. DISC1 localizes to the centrosome by binding to kendrin. Biochem. Biophys. Res. Commun. 2004, 317, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chang, J.; Ge, S.; Faulkner, R.; Kim, J.; Kitabatake, Y.; Liu, X.; Yang, C.; Jordan, J.; Ma, D.; et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 2007, 130, 1146–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angata, K.; Huckaby, V.; Ranscht, B.; Terskikh, A.; Marth, J.; Fukuda, M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol. Cell Biol. 2007, 27, 6659–6668. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Abe, C.; Hane, M.; Wu, D.; Kitajima, K.; Sato, C. Polysialylation in a DISC1 Mutant Mouse. Int. J. Mol. Sci. 2022, 23, 5207. https://doi.org/10.3390/ijms23095207
Takahashi Y, Abe C, Hane M, Wu D, Kitajima K, Sato C. Polysialylation in a DISC1 Mutant Mouse. International Journal of Molecular Sciences. 2022; 23(9):5207. https://doi.org/10.3390/ijms23095207
Chicago/Turabian StyleTakahashi, Yuka, Chikara Abe, Masaya Hane, Di Wu, Ken Kitajima, and Chihiro Sato. 2022. "Polysialylation in a DISC1 Mutant Mouse" International Journal of Molecular Sciences 23, no. 9: 5207. https://doi.org/10.3390/ijms23095207
APA StyleTakahashi, Y., Abe, C., Hane, M., Wu, D., Kitajima, K., & Sato, C. (2022). Polysialylation in a DISC1 Mutant Mouse. International Journal of Molecular Sciences, 23(9), 5207. https://doi.org/10.3390/ijms23095207





