Rev-erbα Knockout Reduces Ethanol Consumption and Preference in Male and Female Mice
Abstract
1. Introduction
2. Results
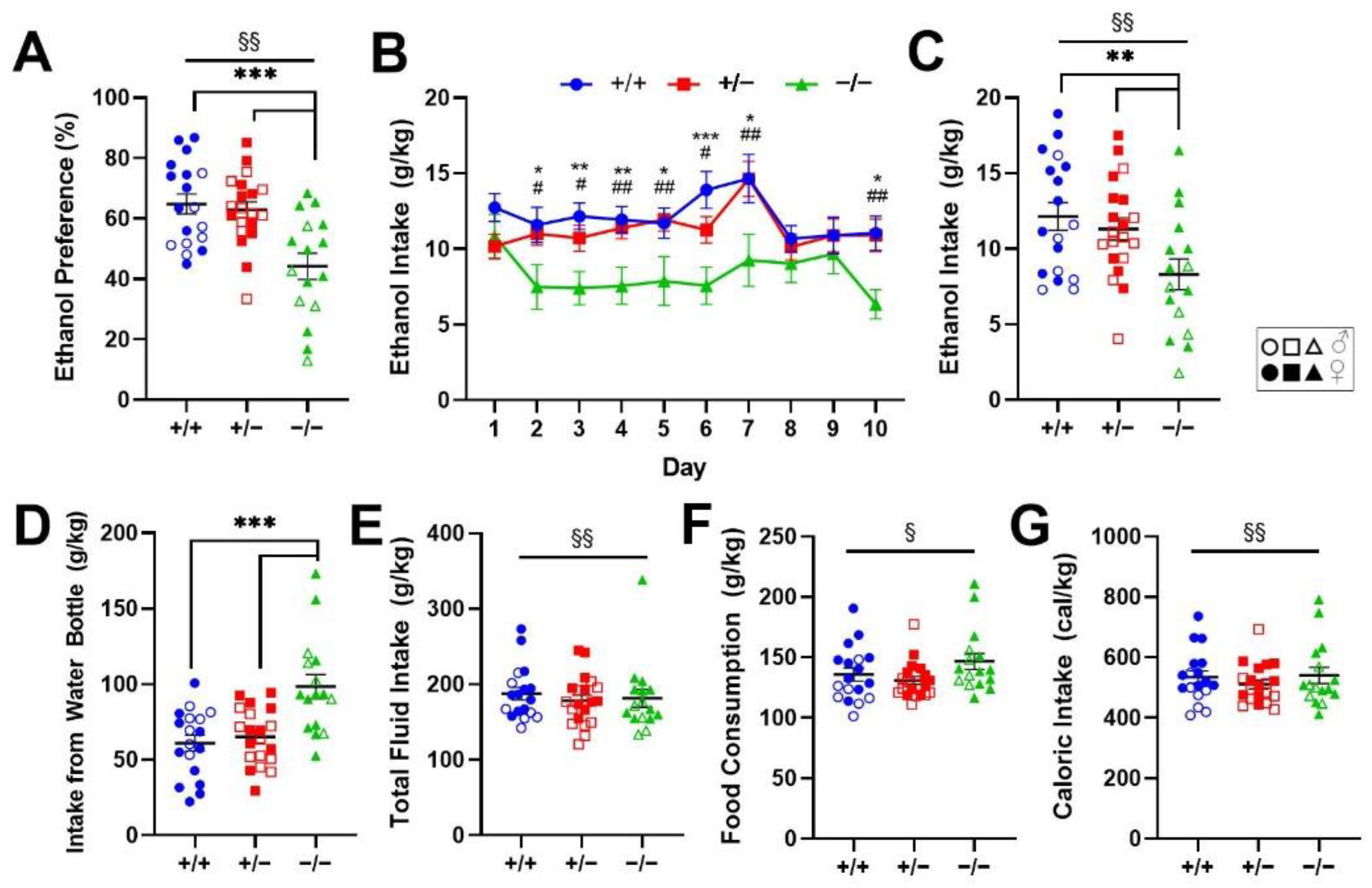
2.1. Genetic Deletion of Rev-erbα Decreases Ethanol Preference and Consumption
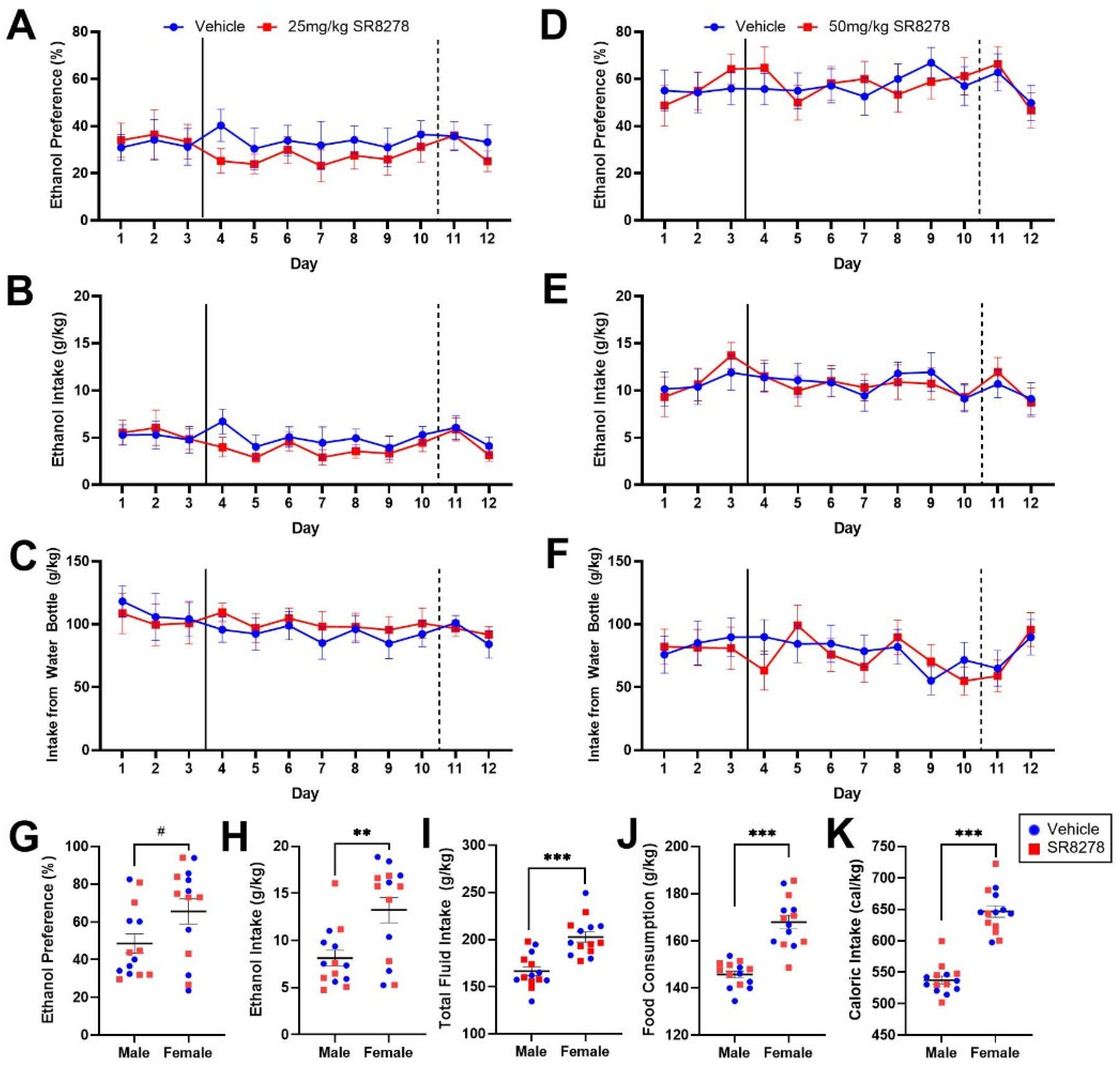
2.2. Pharmacological Inhibition of REV-ERBα/β Does Not Affect Ethanol Preference or Intake in Male and Female Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Two-Bottle Choice Test
4.3. Drug Administration
4.4. Experiment 1: Genetic Disruption
4.5. Experiment 2: Pharmacological Disruption 25 mg/kg
4.6. Experiment 3: Pharmacological Disruption, 50 mg/kg
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2020. [Google Scholar]
- Richter, K.; Peter, L.; Rodenbeck, A.; Weess, H.G.; Riedel-Heller, S.G.; Hillemacher, T. Shiftwork and Alcohol Consumption: A Systematic Review of the Literature. Eur. Addict. Res. 2021, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hasler, B.P.; Soehner, A.M.; Clark, D.B. Sleep and Circadian Contributions to Adolescent Alcohol Use Disorder. Alcohol 2015, 49, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, J.D.; Joines, R.; Cunningham-Hill, M.; Xu, B. Health and Well-Being Factors Associated with International Business Travel. J. Travel Med. 2010, 17, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.L.; Reilly, S.M. A Survey of the Health Experiences of International Business Travelers. Part One--Physiological Aspects. AAOHN J. 2002, 50, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Meyrel, M.; Rolland, B.; Geoffroy, P.A. Alterations in Circadian Rhythms Following Alcohol Use: A Systematic Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109831. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.K.; Ozburn, A.R.; McClung, C.A. Circadian Clock Genes: Effects on Dopamine, Reward and Addiction. Alcohol 2015, 49, 341–349. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Tsimakouridze, E.V.; Reitz, C.J.; Pyle, W.G.; Martino, T.A. Consequences of Circadian and Sleep Disturbances for the Cardiovascular System. Can. J. Cardiol. 2015, 31, 860–872. [Google Scholar] [CrossRef]
- Aziz, I.S.; McMahon, A.M.; Friedman, D.; Rabinovich-Nikitin, I.; Kirshenbaum, L.A.; Martino, T.A. Circadian Influence on Inflammatory Response During Cardiovascular Disease. Curr. Opin. Pharmacol. 2021, 57, 60–70. [Google Scholar] [CrossRef]
- Khaper, N.; Bailey, C.D.C.; Ghugre, N.R.; Reitz, C.; Awosanmi, Z.; Waines, R.; Martino, T.A. Implications of Disturbances in Circadian Rhythms for Cardiovascular Health: A New Frontier in Free Radical Biology. Free Radic. Biol. Med. 2018, 119, 85–92. [Google Scholar] [CrossRef]
- Mistry, P.; Duong, A.; Kirshenbaum, L.; Martino, T.A. Cardiac Clocks and Preclinical Translation. Heart Fail. Clin. 2017, 13, 657–672. [Google Scholar] [CrossRef]
- Gamble, K.L.; Motsinger-Reif, A.A.; Hida, A.; Borsetti, H.M.; Servick, S.V.; Ciarleglio, C.M.; Robbins, S.; Hicks, J.; Carver, K.; Hamilton, N.; et al. Shift Work in Nurses: Contribution of Phenotypes and Genotypes to Adaptation. PLoS ONE 2011, 6, e18395. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, L.K.; Kovanen, L.; Saarikoski, S.T.; Schalling, M.; Lavebratt, C.; Partonen, T. Clock Is Suggested to Associate with Comorbid Alcohol Use and Depressive Disorders. J. Circadian Rhythms. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banach, E.; Pawlak, J.; Kapelski, P.; Szczepankiewicz, A.; Rajewska-Rager, A.; Skibinska, M.; Czerski, P.; Twarowska-Hauser, J.; Dmitrzak-Weglarz, M. Clock Genes Polymorphisms in Male Bipolar Patients with Comorbid Alcohol Abuse. J. Affect. Disord. 2018, 241, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Kovanen, L.; Saarikoski, S.T.; Haukka, J.; Pirkola, S.; Aromaa, A.; Lonnqvist, J.; Partonen, T. Circadian Clock Gene Polymorphisms in Alcohol Use Disorders and Alcohol Consumption. Alcohol Alcohol 2010, 45, 303–311. [Google Scholar] [CrossRef]
- Baranger, D.A.; Ifrah, C.; Prather, A.A.; Carey, C.E.; Corral-Frias, N.S.; Drabant Conley, E.; Hariri, A.R.; Bogdan, R. Per1 Rs3027172 Genotype Interacts with Early Life Stress to Predict Problematic Alcohol Use, but Not Reward-Related Ventral Striatum Activity. Front. Psychol. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Blomeyer, D.; Buchmann, A.F.; Lascorz, J.; Zimmermann, U.S.; Esser, G.; Desrivieres, S.; Schmidt, M.H.; Banaschewski, T.; Schumann, G.; Laucht, M. Association of Per2 Genotype and Stressful Life Events with Alcohol Drinking in Young Adults. PLoS ONE 2013, 8, e59136. [Google Scholar] [CrossRef]
- Brower, K.J.; Wojnar, M.; Sliwerska, E.; Armitage, R.; Burmeister, M. Per3 Polymorphism and Insomnia Severity in Alcohol Dependence. Sleep 2012, 35, 571–577. [Google Scholar] [CrossRef]
- Comasco, E.; Nordquist, N.; Gokturk, C.; Aslund, C.; Hallman, J.; Oreland, L.; Nilsson, K.W. The Clock Gene Per2 and Sleep Problems: Association with Alcohol Consumption among Swedish Adolescents. Ups. J. Med. Sci. 2010, 115, 41–48. [Google Scholar] [CrossRef]
- Dong, L.; Bilbao, A.; Laucht, M.; Henriksson, R.; Yakovleva, T.; Ridinger, M.; Desrivieres, S.; Clarke, T.K.; Lourdusamy, A.; Smolka, M.N.; et al. Effects of the Circadian Rhythm Gene Period 1 (Per1) on Psychosocial Stress-Induced Alcohol Drinking. Am. J. Psychiatry. 2011, 168, 1090–1098. [Google Scholar] [CrossRef]
- Spanagel, R.; Pendyala, G.; Abarca, C.; Zghoul, T.; Sanchis-Segura, C.; Magnone, M.C.; Lascorz, J.; Depner, M.; Holzberg, D.; Soyka, M.; et al. The Clock Gene Per2 Influences the Glutamatergic System and Modulates Alcohol Consumption. Nat. Med. 2005, 11, 35–42. [Google Scholar] [CrossRef]
- Dalvie, S.; King, A.; Fein, G.; Ramesar, R.; Stein, D.J. Possible Involvement of the Circadian Pathway in Alcohol Use Disorder in a South African Adolescent Cohort. Metab. Brain Dis. 2016, 31, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ozburn, A.R.; Falcon, E.; Mukherjee, S.; Gillman, A.; Arey, R.; Spencer, S.; McClung, C.A. The Role of Clock in Ethanol-Related Behaviors. Neuropsychopharmacology 2013, 38, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Gamsby, J.J.; Templeton, E.L.; Bonvini, L.A.; Wang, W.; Loros, J.J.; Dunlap, J.C.; Green, A.I.; Gulick, D. The Circadian Per1 and Per2 Genes Influence Alcohol Intake, Reinforcement, and Blood Alcohol Levels. Behav. Brain Res. 2013, 249, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Brager, A.J.; Prosser, R.A.; Glass, J.D. Circadian and Acamprosate Modulation of Elevated Ethanol Drinking in Mper2 Clock Gene Mutant Mice. Chronobiol. Int. 2011, 28, 664–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perreau-Lenz, S.; Zghoul, T.; de Fonseca, F.R.; Spanagel, R.; Bilbao, A. Circadian Regulation of Central Ethanol Sensitivity by the Mper2 Gene. Addict. Biol. 2009, 14, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Puckett, H.; Kemerling, M.; Parikh, M.; Sahota, P.; Thakkar, M. Antisense-Induced Downregulation of Clock Genes in the Shell Region of the Nucleus Accumbens Reduces Binge Drinking in Mice. Alcohol Clin. Exp. Res. 2021, 45, 530–542. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, Y.; Solt, L.A.; Griffett, K.; Kazantzis, M.; Amador, A.; El-Gendy, B.M.; Huitron-Resendiz, S.; Roberts, A.J.; Shin, Y.; et al. Pharmacological Targeting of the Mammalian Clock Regulates Sleep Architecture and Emotional Behaviour. Nat. Commun 2014, 5, 5759. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.B.; Son, H.J.; Kim, K.S.; Dluzen, D.E.; Lee, I.; Hwang, O.; et al. Impact of Circadian Nuclear Receptor Rev-Erbalpha on Midbrain Dopamine Production and Mood Regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef]
- Sleipness, E.P.; Sorg, B.A.; Jansen, H.T. Diurnal Differences in Dopamine Transporter and Tyrosine Hydroxylase Levels in Rat Brain: Dependence on the Suprachiasmatic Nucleus. Brain Res. 2007, 1129, 34–42. [Google Scholar] [CrossRef]
- Jager, J.; O’Brien, W.T.; Manlove, J.; Krizman, E.N.; Fang, B.; Gerhart-Hines, Z.; Robinson, M.B.; Klein, P.S.; Lazar, M.A. Behavioral Changes and Dopaminergic Dysregulation in Mice Lacking the Nuclear Receptor Rev-Erbalpha. Mol. Endocrinol 2014, 28, 490–498. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.A.; Sidiropoulou, K.; Vitaterna, M.; Takahashi, J.S.; White, F.J.; Cooper, D.C.; Nestler, E.J. Regulation of Dopaminergic Transmission and Cocaine Reward by the Clock Gene. Proc. Natl. Acad. Sci. USA 2005, 102, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The Orphan Nuclear Receptor Rev-Erbalpha Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Kojetin, D.; Wang, Y.; Kamenecka, T.M.; Burris, T.P. Identification of Sr8278, a Synthetic Antagonist of the Nuclear Heme Receptor Rev-Erb. ACS Chem. Biol. 2011, 6, 131–134. [Google Scholar] [CrossRef]
- Middaugh, L.D.; Kelley, B.M.; Bandy, A.L.; McGroarty, K.K. Ethanol Consumption by C57bl/6 Mice: Influence of Gender and Procedural Variables. Alcohol 1999, 17, 175–183. [Google Scholar] [CrossRef]
- Yoneyama, N.; Crabbe, J.C.; Ford, M.M.; Murillo, A.; Finn, D.A. Voluntary Ethanol Consumption in 22 Inbred Mouse Strains. Alcohol 2008, 42, 149–160. [Google Scholar] [CrossRef]
- Reitz, C.J.; Alibhai, F.J.; Khatua, T.N.; Rasouli, M.; Bridle, B.W.; Burris, T.P.; Martino, T.A. Sr9009 Administered for One Day after Myocardial Ischemia-Reperfusion Prevents Heart Failure in Mice by Targeting the Cardiac Inflammasome. Commun. Biol. 2019, 2, 353. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of Circadian Behaviour and Metabolism by Synthetic Rev-Erb Agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Triqueneaux, G.; Thenot, S.; Kakizawa, T.; Antoch, M.P.; Safi, R.; Takahashi, J.S.; Delaunay, F.; Laudet, V. The Orphan Receptor Rev-Erbalpha Gene Is a Target of the Circadian Clock Pacemaker. J. Mol. Endocrinol. 2004, 33, 585–608. [Google Scholar] [CrossRef]
- Rizk, A.A.; Jenkins, B.W.; Al-Sabagh, Y.; Hamidullah, S.; Reitz, C.J.; Rasouli, M.; Martino, T.A.; Khokhar, J.Y. The Impact of Sex, Circadian Disruption, and the Clockδ19/Δ19 Genotype on Alcohol Drinking. Genes 2022, 13, 701. [Google Scholar] [CrossRef]
- Adelmant, G.; Begue, A.; Stehelin, D.; Laudet, V. A Functional Rev-Erb Alpha Responsive Element Located in the Human Rev-Erb Alpha Promoter Mediates a Repressing Activity. Proc. Natl. Acad. Sci. USA 1996, 93, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Vitaterna, M.H.; King, D.P.; Chang, A.M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and Mapping of a Mouse Gene, Clock, Essential for Circadian Behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shearman, L.P.; Weaver, D.R.; Zylka, M.J.; de Vries, G.J.; Reppert, S.M. A Molecular Mechanism Regulating Rhythmic Output from the Suprachiasmatic Circadian Clock. Cell 1999, 96, 57–68. [Google Scholar] [CrossRef]
- Kume, K.; Zylka, M.J.; Sriram, S.; Shearman, L.P.; Weaver, D.R.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M. Mcry1 and Mcry2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell 1999, 98, 193–205. [Google Scholar] [CrossRef]
- Oishi, K.; Fukui, H.; Ishida, N. Rhythmic Expression of Bmal1 Mrna Is Altered in Clock Mutant Mice: Differential Regulation in the Suprachiasmatic Nucleus and Peripheral Tissues. Biochem Biophys Res. Commun 2000, 268, 164–171. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; Di Tacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of Circadian Behaviour and Metabolism by Rev-Erb-Alpha and Rev-Erb-Beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Sandu, C.; Reibel, S.; Pevet, P.; Challet, E. Rev-Erbalpha in the Brain Is Essential for Circadian Food Entrainment. Sci. Rep. 2016, 6, 29386. [Google Scholar] [CrossRef]
- Ikeda, R.; Tsuchiya, Y.; Koike, N.; Umemura, Y.; Inokawa, H.; Ono, R.; Inoue, M.; Sasawaki, Y.; Grieten, T.; Okubo, N.; et al. Rev-Erbalpha and Rev-Erbbeta Function as Key Factors Regulating Mammalian Circadian Output. Sci. Rep. 2019, 9, 10171. [Google Scholar] [CrossRef]
- Vanderlinden, L.A.; Saba, L.M.; Bennett, B.; Hoffman, P.L.; Tabakoff, B. Influence of Sex on Genetic Regulation of “Drinking in the Dark” Alcohol Consumption. Mamm. Genome. 2015, 26, 43–56. [Google Scholar] [CrossRef]
- de Zavalia, N.; Schoettner, K.; Goldsmith, J.A.; Solis, P.; Ferraro, S.; Parent, G.; Amir, S. Bmal1 in the Striatum Influences Alcohol Intake in a Sexually Dimorphic Manner. Commun. Biol. 2021, 4, 1227. [Google Scholar] [CrossRef]
- Bass, C.E.; Grinevich, V.P.; Gioia, D.; Day-Brown, J.D.; Bonin, K.D.; Stuber, G.D.; Weiner, J.L.; Budygin, E.A. Optogenetic Stimulation of Vta Dopamine Neurons Reveals that Tonic but Not Phasic Patterns of Dopamine Transmission Reduce Ethanol Self-Administration. Front. Behav. Neurosci. 2013, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Budygin, E.A.; Bass, C.E.; Grinevich, V.P.; Deal, A.L.; Bonin, K.D.; Weiner, J.L. Opposite Consequences of Tonic and Phasic Increases in Accumbal Dopamine on Alcohol-Seeking Behavior. iScience 2020, 23, 100877. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Varodayan, F.P. Synaptic Targets: Chronic Alcohol Actions. Neuropharmacology 2017, 122, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Li, X.; Hou, X.; Zhou, W.; Gong, Y.; Liu, F.; He, Y.; Song, J.; Wang, J.; Basil, P.; et al. Rev-Erb in Gabaergic Neurons Controls Diurnal Hepatic Insulin Sensitivity. Nature 2021, 592, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, F.; Xu, H.; Chen, M.; Chen, X.; Guo, L.; Zhou, C.; Xu, Y.; Wang, F.; Yu, J.; et al. Dysregulation of Rev-Erbalpha Impairs Gabaergic Function and Promotes Epileptic Seizures in Preclinical Models. Nat. Commun. 2021, 12, 1216. [Google Scholar] [CrossRef]
- Frie, J.A.; Khokhar, J.Y. An Open Source Automated Two-Bottle Choice Test Apparatus for Rats. HardwareX 2019, 5, e00061. [Google Scholar] [CrossRef]
- Feillet, C.A.; Bainier, C.; Mateo, M.; Blancas-Velazquez, A.; Salaberry, N.L.; Ripperger, J.A.; Albrecht, U.; Mendoza, J. Rev-Erbalpha Modulates the Hypothalamic Orexinergic System to Influence Pleasurable Feeding Behaviour in Mice. Addict. Biol. 2017, 22, 411–422. [Google Scholar] [CrossRef]
- Zhao, C.; Gammie, S.C. The Circadian Gene Nr1d1 in the Mouse Nucleus Accumbens Modulates Sociability and Anxiety-Related Behavior. Eur. J. Neurosci. 2018, 48, 1924–1943. [Google Scholar] [CrossRef]
- Castillo-Carniglia, A.; Keyes, K.M.; Hasin, D.S.; Cerda, M. Psychiatric Comorbidities in Alcohol Use Disorder. Lancet. Psychiatry 2019, 6, 1068–1080. [Google Scholar] [CrossRef]
- Ruffolo, J.; Frie, J.A.; Thorpe, H.H.A.; Talhat, M.A.; Khokhar, J.Y. Alcohol and Vaporized Nicotine Co-Exposure During Adolescence Contribute Differentially to Sex-Specific Behavioral Effects in Adulthood. Nicotine. Tob. Res. 2021, ntab250. [Google Scholar] [CrossRef]
- Hamidullah, S.; Lutelmowski, C.D.; Creighton, S.D.; Luciani, K.R.; Frie, J.A.; Winters, B.D.; Khokhar, J.Y. Effects of Vapourized Thc and Voluntary Alcohol Drinking During Adolescence on Cognition, Reward, and Anxiety-Like Behaviours in Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110141. [Google Scholar] [CrossRef] [PubMed]
- Lesscher, H.M.; Spoelder, M.; Rotte, M.D.; Janssen, M.J.; Hesseling, P.; Lozeman-van’t Klooster, J.G.; Baars, A.M.; Vanderschuren, L.J. Early Social Isolation Augments Alcohol Consumption in Rats. Behav. Pharmacol. 2015, 26, 673–680. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sabagh, Y.; Thorpe, H.H.A.; Jenkins, B.W.; Hamidullah, S.; Talhat, M.A.; Suggett, C.B.; Reitz, C.J.; Rasouli, M.; Martino, T.A.; Khokhar, J.Y. Rev-erbα Knockout Reduces Ethanol Consumption and Preference in Male and Female Mice. Int. J. Mol. Sci. 2022, 23, 5197. https://doi.org/10.3390/ijms23095197
Al-Sabagh Y, Thorpe HHA, Jenkins BW, Hamidullah S, Talhat MA, Suggett CB, Reitz CJ, Rasouli M, Martino TA, Khokhar JY. Rev-erbα Knockout Reduces Ethanol Consumption and Preference in Male and Female Mice. International Journal of Molecular Sciences. 2022; 23(9):5197. https://doi.org/10.3390/ijms23095197
Chicago/Turabian StyleAl-Sabagh, Yasmine, Hayley Hope Allyssa Thorpe, Bryan William Jenkins, Shahnaza Hamidullah, Malik Asfandyaar Talhat, Cara Beth Suggett, Cristine Joelle Reitz, Mina Rasouli, Tami Avril Martino, and Jibran Younis Khokhar. 2022. "Rev-erbα Knockout Reduces Ethanol Consumption and Preference in Male and Female Mice" International Journal of Molecular Sciences 23, no. 9: 5197. https://doi.org/10.3390/ijms23095197
APA StyleAl-Sabagh, Y., Thorpe, H. H. A., Jenkins, B. W., Hamidullah, S., Talhat, M. A., Suggett, C. B., Reitz, C. J., Rasouli, M., Martino, T. A., & Khokhar, J. Y. (2022). Rev-erbα Knockout Reduces Ethanol Consumption and Preference in Male and Female Mice. International Journal of Molecular Sciences, 23(9), 5197. https://doi.org/10.3390/ijms23095197





