Small Extracellular Vesicles Containing miR-34c Derived from Bone Marrow Mesenchymal Stem Cells Regulates Epithelial Sodium Channel via Targeting MARCKS
Abstract
:1. Introduction
2. Results
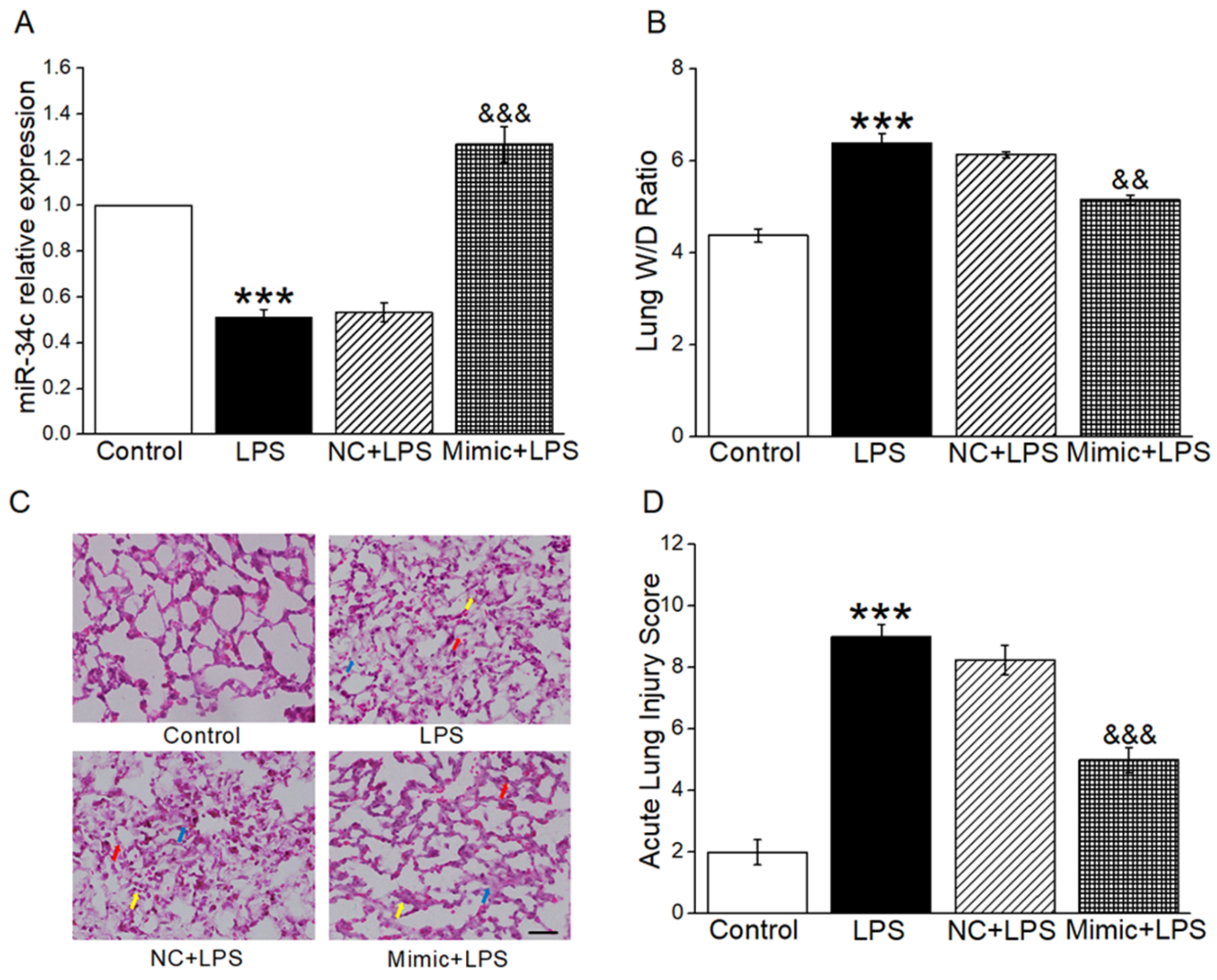
2.1. MiR-34c Attenuated Lung Edema and Histopathology Changes in ALI Mouse Model
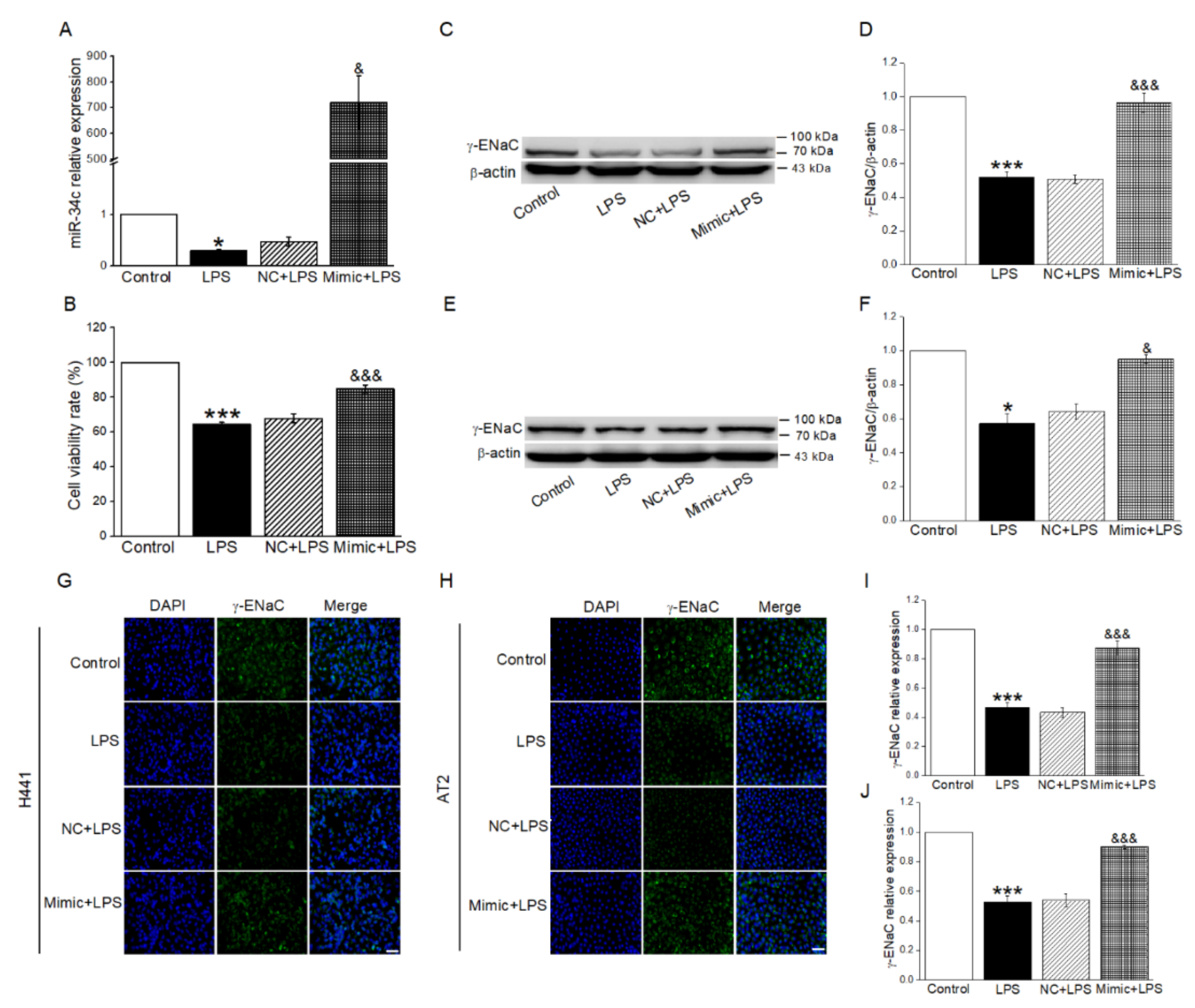
2.2. MiR-34c Upregulated LPS-Inhibited γ-ENaC Protein Expression in H441 and AT2 Cells
2.3. sEVs Derived from BMSCs Were Taken Up by Recipient Cells
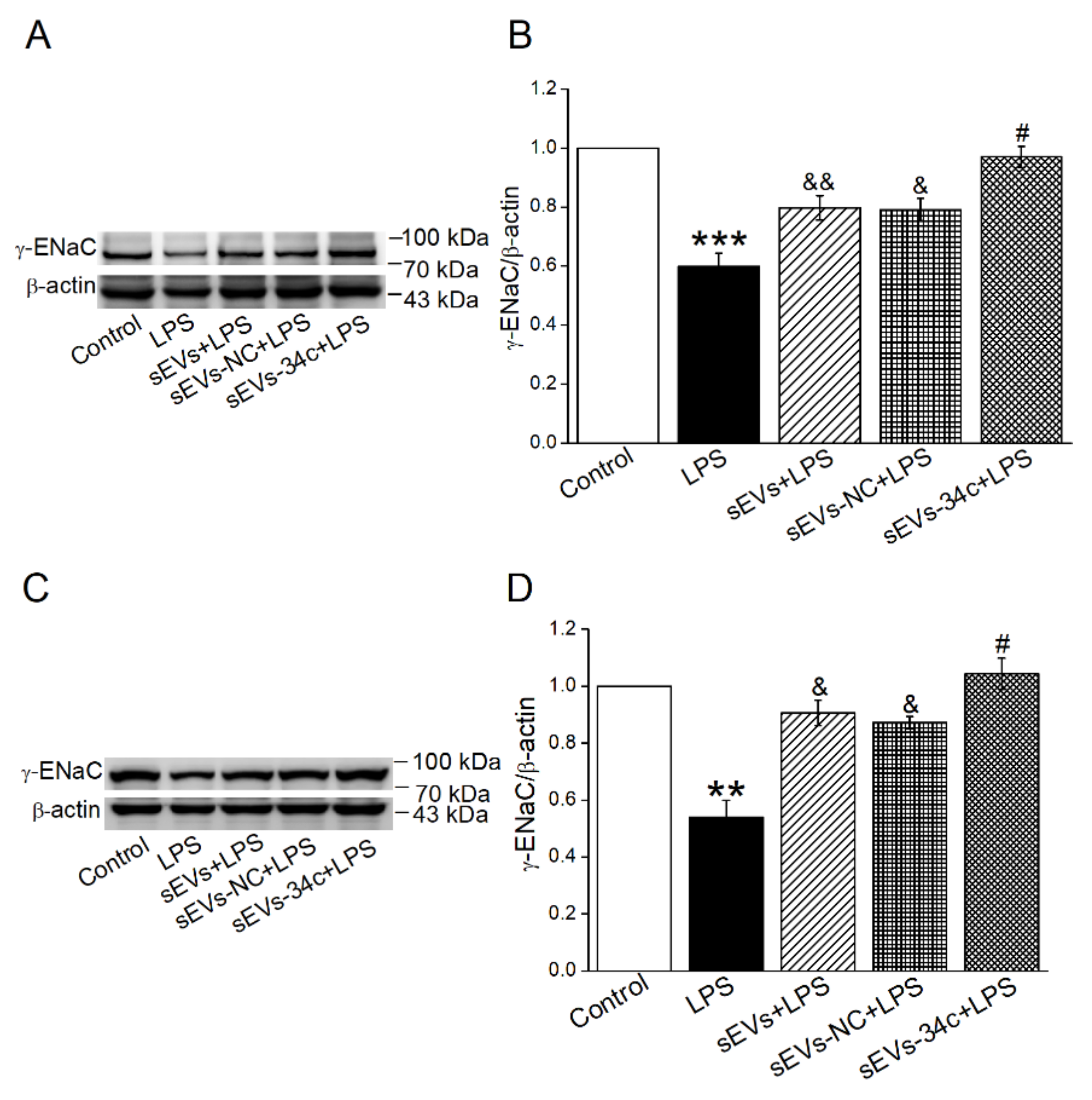
2.4. Overexpressing miR-34c in BMSC-sEVs Attenuated LPS-Inhibited γ-ENaC Expression in H441 and AT2 Cells
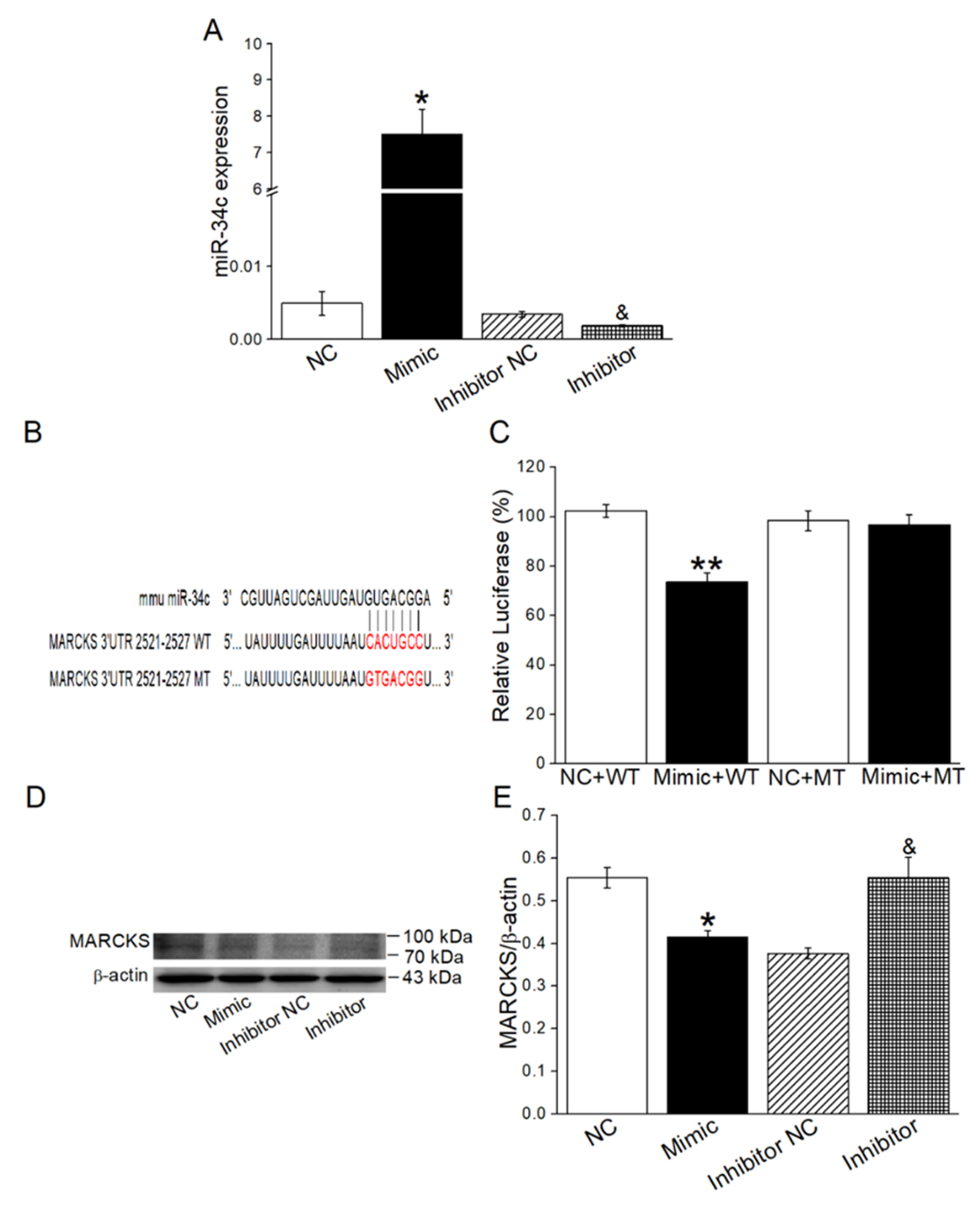
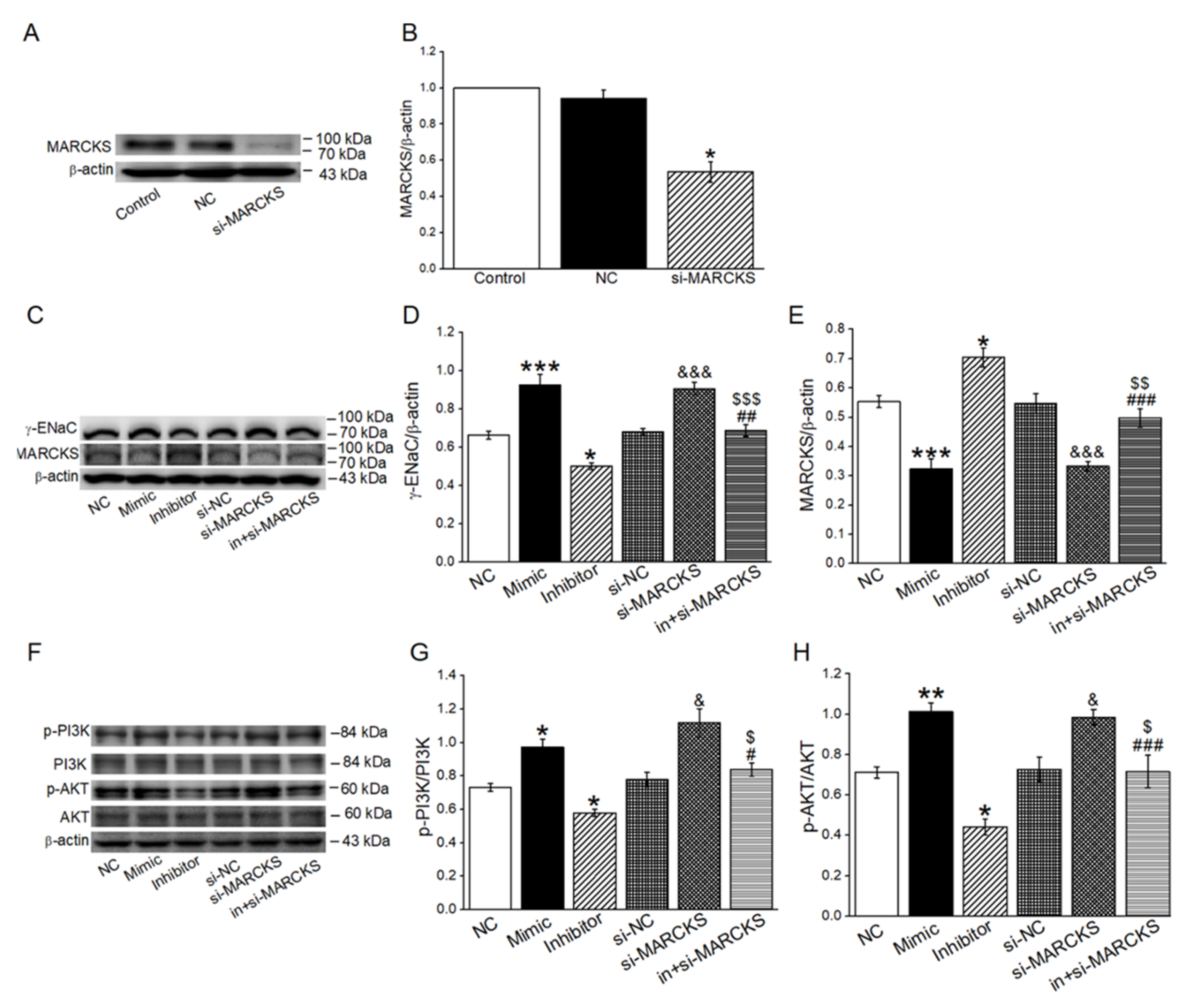
2.5. MiR-34c Increased γ-ENaC Expression by Directly Targeting MARCKS
2.6. MiR-34c Activated PI3K/AKT Signaling Pathway by Inhibiting MARCKS
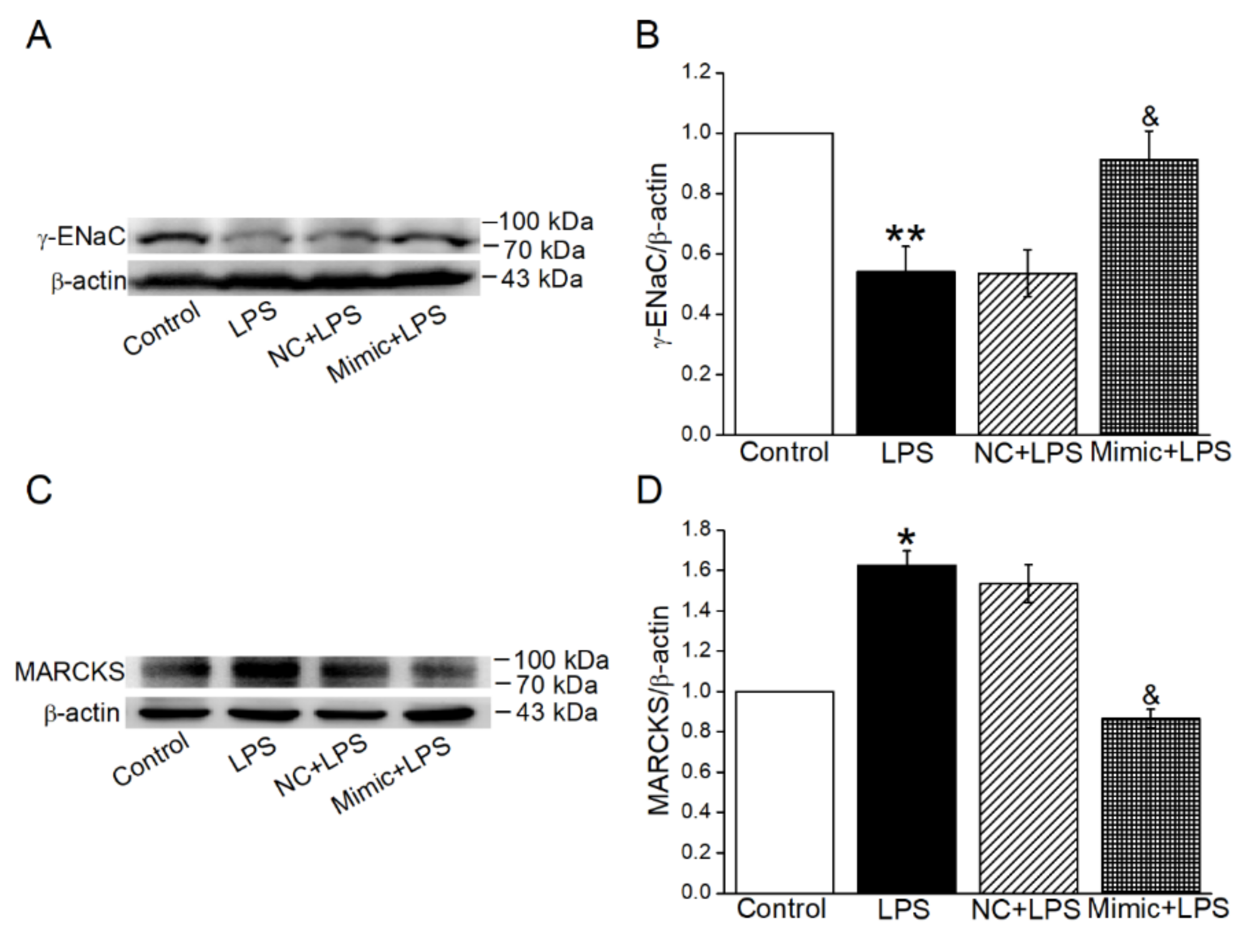
2.7. MiR-34c Activated the Inhibition of PI3K/AKT Signaling Pathway in LPS-Treated Cells
2.8. MiR-34c Could Enhance γ-ENaC Expression and Inhibit the MARCKS Expression in ALI Mouse Model
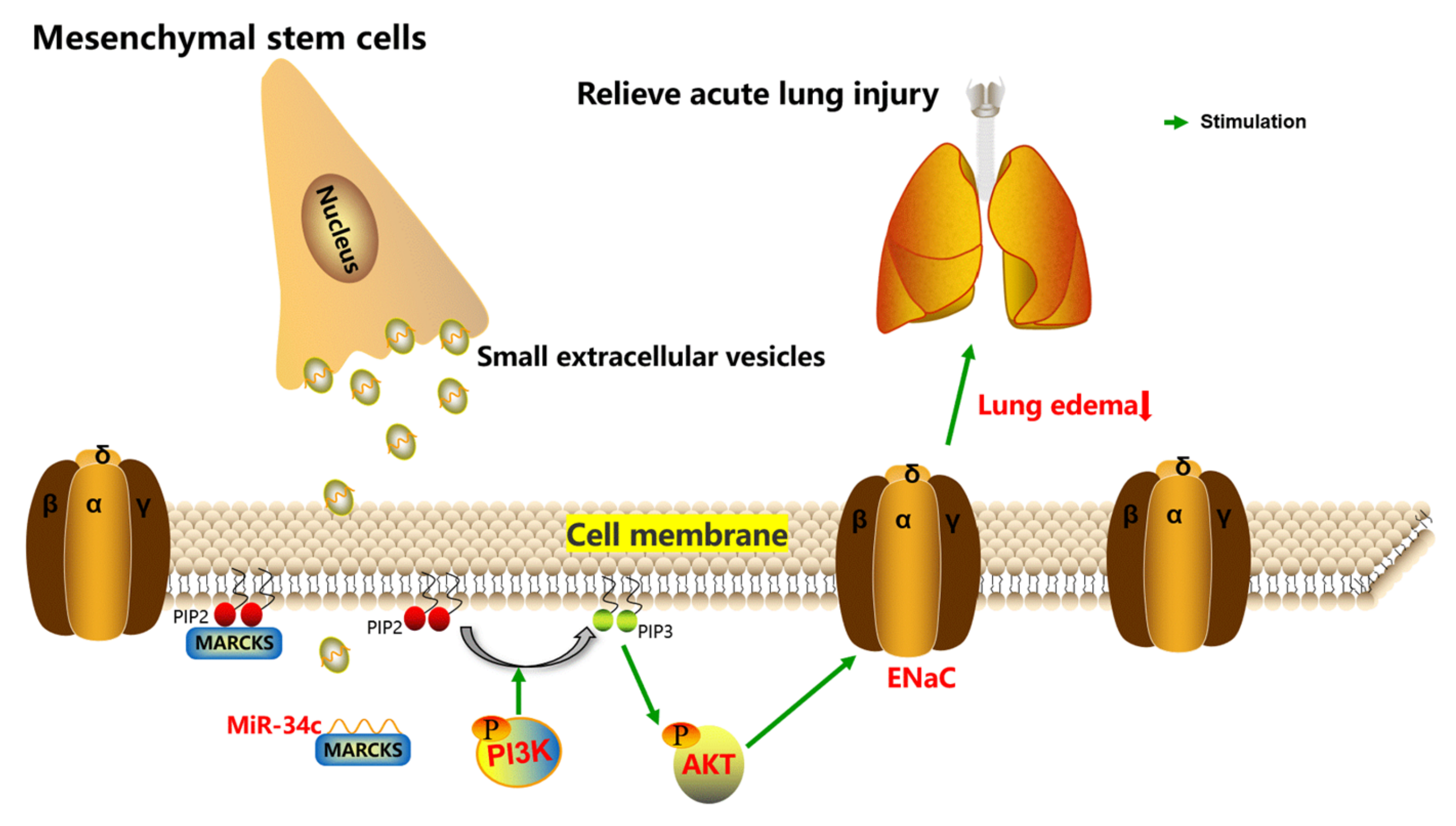
3. Discussion
4. Materials and Methods
4.1. BMSC Isolation and Culture
4.2. Mouse AT2 Cell Isolation and Culture
4.3. BMSC-sEVs’ Collection, Identification and Staining
4.4. CCK-8 Cell Viability Assay
4.5. Western Blot Assay
4.6. Quantitative Real-Time PCR
4.7. Cell Transfection
4.8. Immunofluorescent Staining
4.9. Dual Luciferase Reporter Gene Assay
4.10. ALI Mouse Model Establishment and Lung Wet-to-Dry Ratio Measurement
4.11. Hematoxylin–Eosin Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hughes, K.T.; Beasley, M.B. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch. Pathol. Lab. Med. 2017, 141, 916–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, D.; Tang, X.M.; Deng, W.; Deng, X.Y.; Zhao, Y.; Wang, D.X. Rosiglitazone promotes ENaC-mediated alveolar fluid clearance in acute lung injury through the PPARγ/SGK1 signaling pathway. Cell Mol. Biol. Lett. 2019, 24, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matalon, S.; O’Brodovich, H. Sodium channels in alveolar epithelial cells: Molecular characterization, biophysical properties, and physiological significance. Annu. Rev. Physiol. 1999, 61, 627–661. [Google Scholar] [CrossRef]
- Huppert, L.A.; Matthay, M.A. Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Front. Immunol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Bhandari, S.; Cao, F.; Wang, X.Y.; Tian, C.; Li, X.Y.; Zhang, P.H.; Liu, Y.J.; Wu, C.H.; et al. Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury. J. Cell Mol. Med. 2020, 24, 4736–4747. [Google Scholar] [CrossRef]
- Qi, D.; He, J.; Wang, D.; Deng, W.; Zhao, Y.; Ye, Y.; Feng, L. 17β-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respir. Res. 2014, 15, 159. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.G.; Gudapati, V.; Lim, H.; Lee, J.W. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin. Biol. Ther. 2016, 16, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Feng, B.; Zhu, J.; Xu, Y.; Chen, W.; Sheng, X.; Feng, X.; Shi, X.; Liu, J.; Pan, Q.; Yang, J.; et al. Immunosuppressive effects of mesenchymal stem cells on lung B cell gene expression in LPS-induced acute lung injury. Stem Cell Res. Ther. 2020, 11, 418. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Walter, J.; Ware, L.B.; Matthay, M.A. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014, 2, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes Released by Bone Marrow Mesenchymal Stem Cells Attenuate Lung Injury Induced by Intestinal Ischemia Reperfusion via the TLR4/NF-κB Pathway. Int. J. Med. Sci. 2019, 16, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Liu, J.; Chen, P.; Lin, L.; Luo, Y.; Ma, X.; Lin, J.; Shen, Y.; Zhang, L. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021, 269, 119004. [Google Scholar] [CrossRef]
- Chen, R.; Xu, X.; Tao, Y.; Qian, Z.; Yu, Y. Exosomes in hepatocellular carcinoma: A new horizon. Cell Commun. Signal. 2019, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Rizvanov, A.A. Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy. BioNanoScience 2017, 7, 240–245. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yin, Z.; Fan, J.; Zhang, S.; Yang, W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct. Target. Ther. 2019, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Militello, G.; Weirick, T.; John, D.; Döring, C.; Dimmeler, S.; Uchida, S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief. Bioinform. 2017, 18, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Shen, N.; Liu, A.; Wang, W.; Zhang, L.; Sui, Z.; Tang, Q.; Du, X.; Yang, N.; Ying, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol. Ther. 2022, 30, 763–781. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, Y.; Ding, Y.; Hou, Y.; Yu, T.; Cui, Y.; Nie, H. Conditioned Medium of Bone Marrow Mesenchymal Stem Cells Involved in Acute Lung Injury by Regulating Epithelial Sodium Channels via miR-34c. Front. Bioeng. Biotechnol. 2021, 9, 640116. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Xu, L.N.; Yin, L.H.; Qi, Y.; Peng, J.Y. MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury via targeting of myristoylated alanine-rich C-kinase substrate. Pharmacol. Res. 2020, 156, 104783. [Google Scholar] [CrossRef]
- Deng, W.; Li, C.Y.; Tong, J.; Zhang, W.; Wang, D.X. Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury. Respir. Res. 2012, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Canessa, C.M.; Horisberger, J.D.; Rossier, B.C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 1993, 361, 467–470. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Tong, Q.; Staruschenko, A.; Ma, H.P.; Stockand, J.D. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am. J. Physiol. Renal Physiol. 2006, 290, F949–F957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheats, M.K.; Yin, Q.; Fang, S.; Park, J.; Crews, A.L.; Parikh, I.; Dickson, B.; Adler, K.B. MARCKS and Lung Disease. Am. J. Respir. Cell Mol. Biol. 2019, 60, 16–27. [Google Scholar] [CrossRef]
- Song, C.; Yue, Q.; Moseley, A.; Al-Khalili, O.; Wynne, B.M.; Ma, H.; Wang, L.; Eaton, D.C. Myristoylated alanine-rich C kinase substrate-like protein-1 regulates epithelial sodium channel activity in renal distal convoluted tubule cells. Am. J. Physiol. Cell Physiol. 2020, 319, C589–C604. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; de Boisblanc, B.; Connors, A.F., Jr.; Hite, R.D.; Harabin, A.L. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ding, Y.; Hou, Y.; Liu, Y.; Zhou, Z.; Nie, H. Bone marrow mesenchymal stem cells derived miRNA-130b enhances epithelial sodium channel by targeting PTEN. Respir. Res. 2020, 21, 329. [Google Scholar] [CrossRef]
- Feraille, E.; Dizin, E. Coordinated Control of ENaC and Na+,K+-ATPase in Renal Collecting Duct. J. Am. Soc. Nephrol. 2016, 27, 2554–2563. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.F.; Shao, Y.; Zhang, L.; Shen, J. Lung-derived exosomes regulate the function of mesenchymal stem cells and alleviate phosgene-induced lung injury via miR-34c-3p. J. Biochem. Mol. Toxicol. 2021, 35, e22851. [Google Scholar] [CrossRef]
- Zhu, J.; Bai, J.; Wang, S.; Dong, H. Down-regulation of long non-coding RNA SNHG14 protects against acute lung injury induced by lipopolysaccharide through microRNA-34c-3p-dependent inhibition of WISP1. Respir. Res. 2019, 20, 233. [Google Scholar] [CrossRef]
- Nie, H.; Cui, Y.; Wu, S.; Ding, Y.; Li, Y. 1,25-Dihydroxyvitamin D Enhances Alveolar Fluid Clearance by Upregulating the Expression of Epithelial Sodium Channels. J. Pharm. Sci. 2016, 105, 333–338. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Yu, L.; Ghazi, Z.M.; Thai, T.L.; Al-Khalili, O.; Ma, H.P.; Eaton, D.C.; Alli, A.A. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Cell Physiol. 2017, 313, C42–C53. [Google Scholar] [CrossRef]
- Tung, S.L.; Huang, W.C.; Hsu, F.C.; Yang, Z.P.; Jang, T.H.; Chang, J.W.; Chuang, C.M.; Lai, C.R.; Wang, L.H. miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis 2017, 6, e326. [Google Scholar] [CrossRef] [Green Version]
- Bickeböller, M.; Tagscherer, K.E.; Kloor, M.; Jansen, L.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M.; Toth, C.; Schirmacher, P.; Roth, W.; et al. Functional characterization of the tumor-suppressor MARCKS in colorectal cancer and its association with survival. Oncogene 2015, 34, 1150–1159. [Google Scholar] [CrossRef]
- Liu, H.; Su, P.; Zhi, L.; Zhao, K. miR-34c-3p acts as a tumor suppressor gene in osteosarcoma by targeting MARCKS. Mol. Med. Rep. 2017, 15, 1204–1210. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Zhou, Z.; Liu, H.; Zhang, H.; Ding, Y.; Cui, Y.; Nie, H. Mesenchymal Stem Cell-Conditioned Medium Rescues LPS-Impaired ENaC Activity in Mouse Trachea via WNK4 Pathway. Curr. Pharm. Des. 2020, 26, 3601–3607. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhuo, X.J.; Lin, J.; Luo, L.C.; Ying, W.Y.; Xie, X.; Zhang, H.W.; Yang, J.X.; Li, D.; Gao Smith, F.; et al. Maresin1 stimulates alveolar fluid clearance through the alveolar epithelial sodium channel Na,K-ATPase via the ALX/PI3K/Nedd4-2 pathway. Lab. Investig. 2017, 97, 543–554. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.; Han, A.; Yu, T.; Hou, Y.; Ding, Y.; Nie, H. Small Extracellular Vesicles Containing miR-34c Derived from Bone Marrow Mesenchymal Stem Cells Regulates Epithelial Sodium Channel via Targeting MARCKS. Int. J. Mol. Sci. 2022, 23, 5196. https://doi.org/10.3390/ijms23095196
Hua Y, Han A, Yu T, Hou Y, Ding Y, Nie H. Small Extracellular Vesicles Containing miR-34c Derived from Bone Marrow Mesenchymal Stem Cells Regulates Epithelial Sodium Channel via Targeting MARCKS. International Journal of Molecular Sciences. 2022; 23(9):5196. https://doi.org/10.3390/ijms23095196
Chicago/Turabian StyleHua, Yu, Aixin Han, Tong Yu, Yapeng Hou, Yan Ding, and Hongguang Nie. 2022. "Small Extracellular Vesicles Containing miR-34c Derived from Bone Marrow Mesenchymal Stem Cells Regulates Epithelial Sodium Channel via Targeting MARCKS" International Journal of Molecular Sciences 23, no. 9: 5196. https://doi.org/10.3390/ijms23095196
APA StyleHua, Y., Han, A., Yu, T., Hou, Y., Ding, Y., & Nie, H. (2022). Small Extracellular Vesicles Containing miR-34c Derived from Bone Marrow Mesenchymal Stem Cells Regulates Epithelial Sodium Channel via Targeting MARCKS. International Journal of Molecular Sciences, 23(9), 5196. https://doi.org/10.3390/ijms23095196





