Non-Photochemical Quenching under Drought and Fluctuating Light
Abstract
:1. Introduction
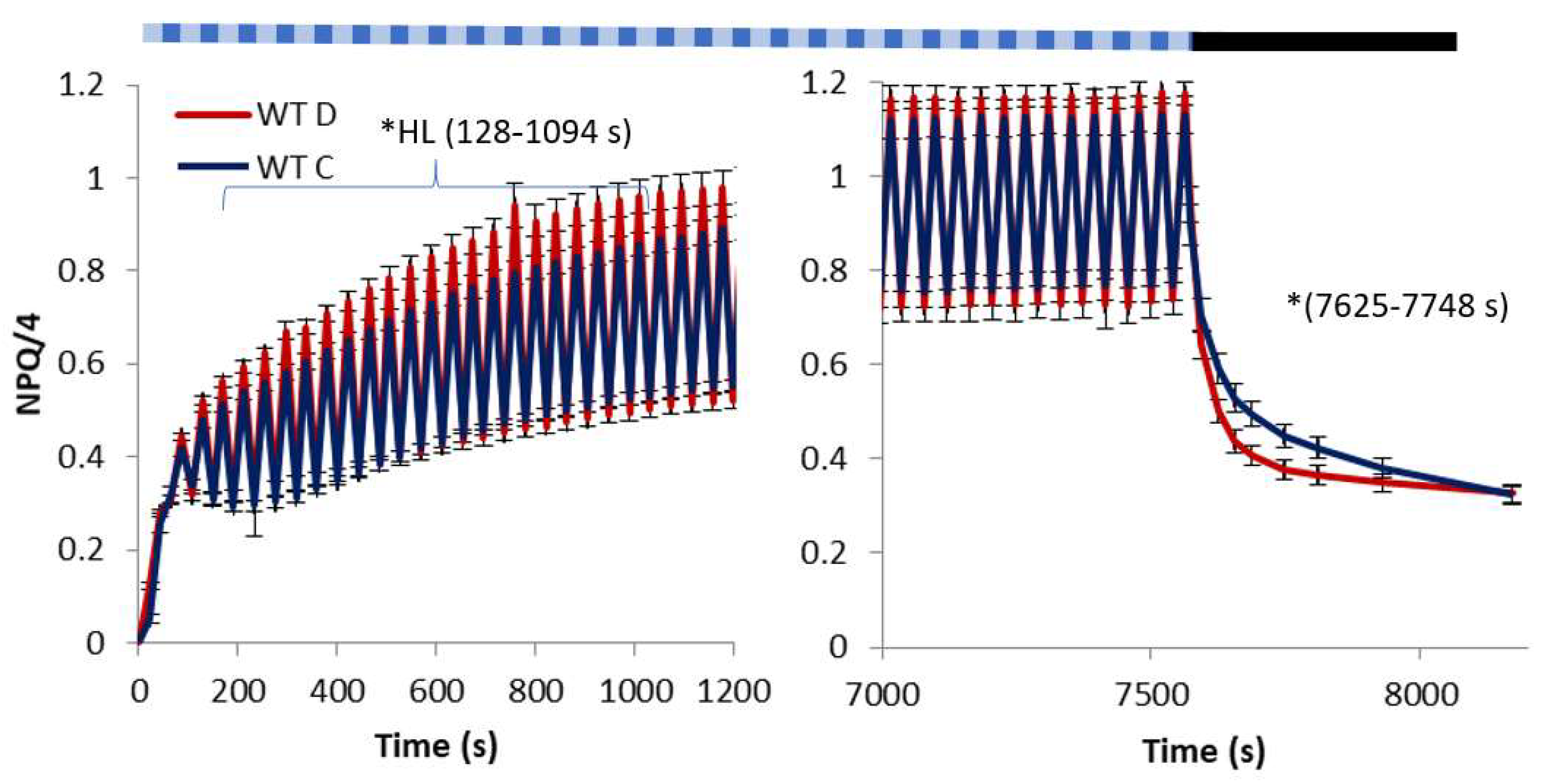
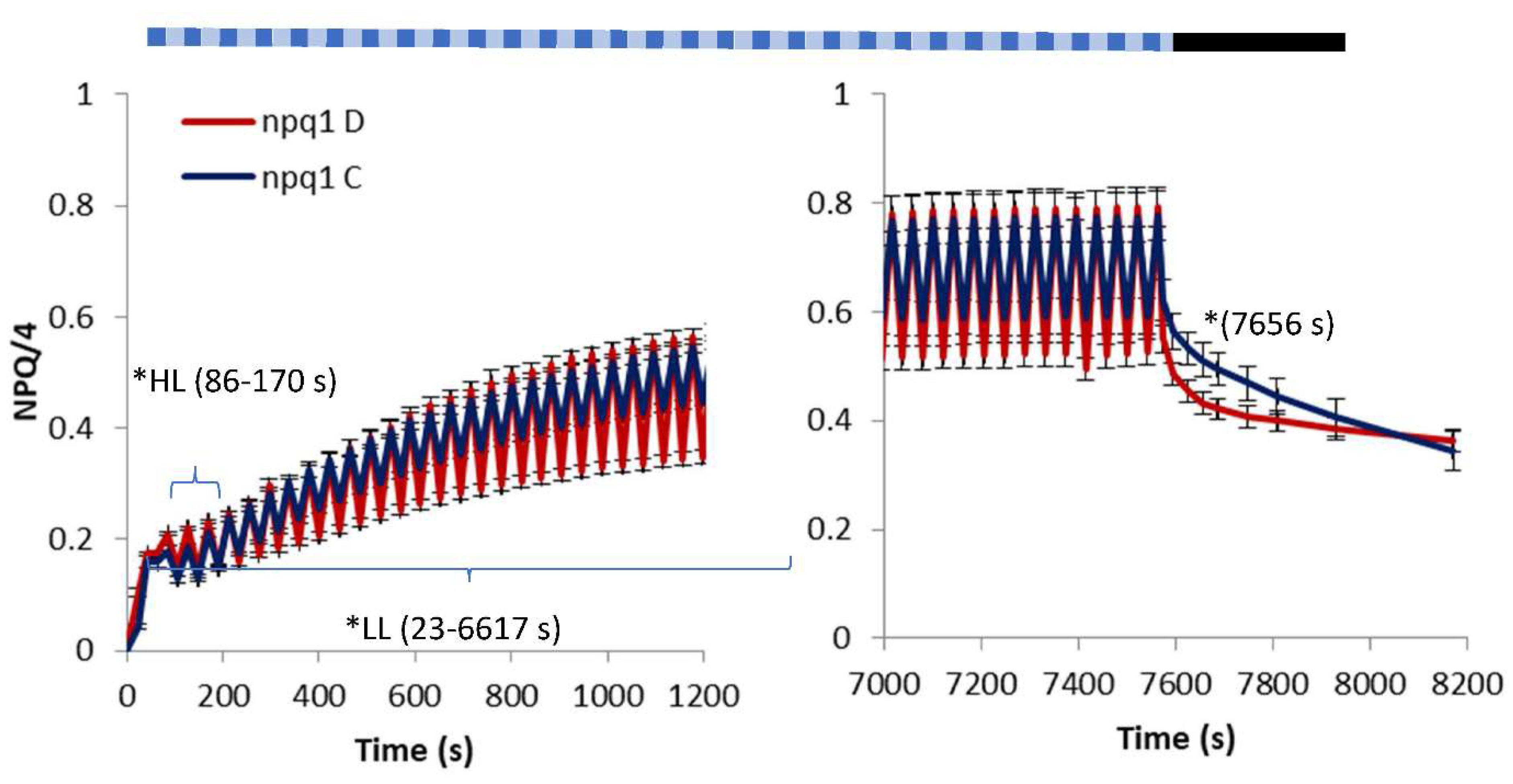
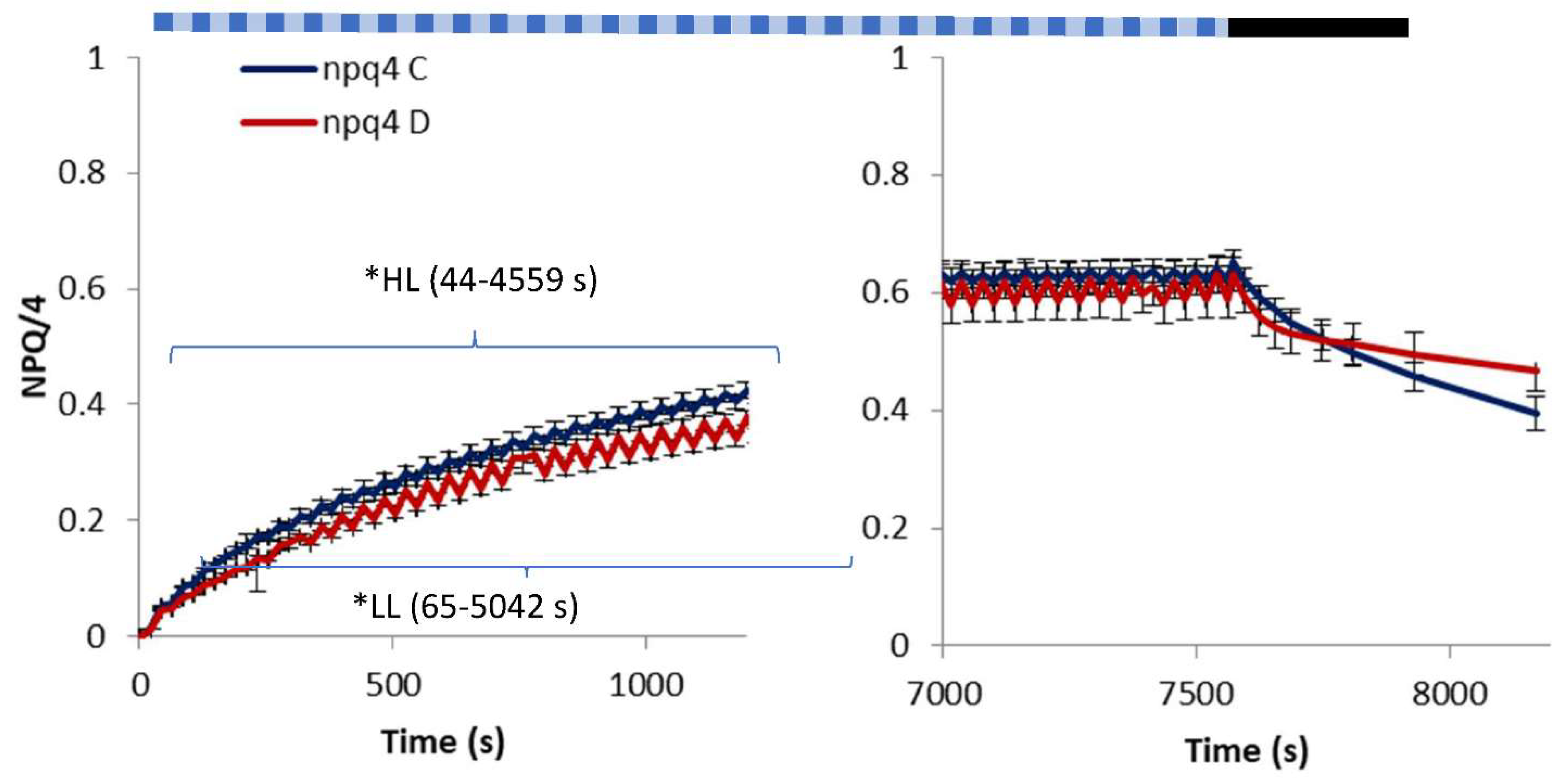
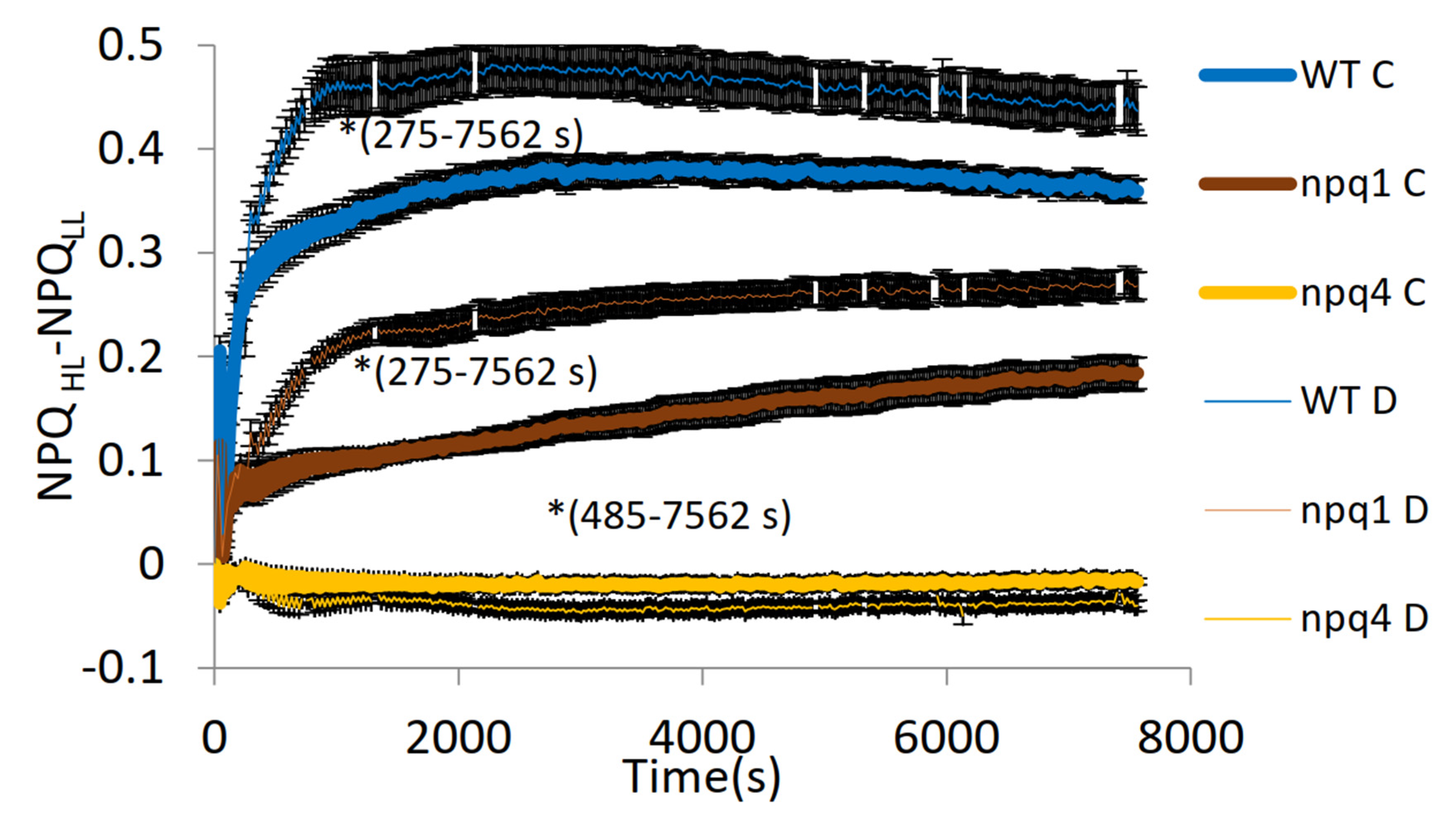
2. Results
3. Discussion
4. Materials and Methods
4.1. Arabidopsis Thaliana
- -
- pot capacity for plants grown at optimum soil water availability (C);
- -
- 60% of pot capacity for plants grown in water deficit conditions (D);
4.2. Determination of Chlorophyll Fluorescence
- light of constant intensity equal to 280 μmol m−2 s−1 for 7562 s followed by a 560-s period of relaxation in the dark;
- fluctuating light characterized by frequent (20 s long) changes from low (55 μmol m−2 s−1) to high intensity (530 μmol m−2 s−1) lasting in total for 7562 s followed by a 560-s period of relaxation in the dark.
4.3. Measurements of Leaf RWC
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| At | Arabidopsis thaliana |
| FL | fluctuating light |
| HL | high light |
| LL | low light |
| NPQ | non-photochemical quenching |
| npq1 | Arabidopsis thaliana mutant, see description in Materials and methods |
| npq4 | Arabidopsis thaliana mutant, see description in Materials and methods |
| PsbS | photoprotective protein from the light harvesting complex family |
| WT | wild type |
| Zx | zeaxanthin |
| Ax | antheraxanthin |
| Vx | violaxanthin |
| VDE | violaxanthin de-epoxidase |
References
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Brachmann, G.; et al. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaks, J.; Amarnath, K.; Kramer, D.M.; Niyogi, K.K.; Fleming, G.R. Mathematical model of nonphotochemical quenching. Proc. Natl. Acad. Sci. USA 2012, 109, 15757–15762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, A.; Kaiser, E.; Yin, X.; Harbinson, J.; Molenaar, J.; Driever, S.M.; Struik, P.C. Dynamic modelling of limitations on improving leaf CO2 assimilation under fluctuating irradiance. Plant Cell Environ. 2018, 41, 589–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.D.B.; Ort, D.R.; Niyogi, K.K. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle-a mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Schwarz, N.; Armbruster, U.; Iven, T.; Brückle, L.; Melzer, M.; Feussner, I.; Jahns, P. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 2015, 56, 346–357. [Google Scholar] [CrossRef] [Green Version]
- North, H.M.; Frey, A.; Boutin, J.-P.; Sotta, B.; Marion-Poll, A. Analysis of xanthophyll cycle gene expression during the adaptation of Arabidopsis to excess light and drought stress: Changes in RNA steady-state levels do not contribute to short-term responses. Plant Sci. 2005, 169, 115–124. [Google Scholar] [CrossRef]
- Latowski, D.; Kruk, J.; Burda, K.; Skrzynecka-Jaskier, M.; Kostecka-Gugała, A.; Strzałka, K. Kinetics of violaxanthin de-epoxidation by violaxanthin de-epoxidase, a xanthophyll cycle enzyme, is regulated by membrane fluidity in model lipid bilayers. Eur. J. Biochem. 2002, 269, 4656–4665. [Google Scholar] [CrossRef] [PubMed]
- Kress, E.; Jahns, P. The Dynamics of energy dissipation and xanthophyll conversion in Arabidopsis indicate an indirect photoprotective role of zeaxanthin in slowly inducible and relaxing components of non-photochemical quenching of excitation energy. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Matuszyńska, A.; Heidari, S.; Jahns, P.; Ebenhöh, O. A mathematical model of non-photochemical quenching to study short-term light memory in plants. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welc, R.; Luchowski, R.; Kluczyk, D.; Zubik-Duda, M.; Grudzinski, W.; Maksim, M.; Reszczynska, E.; Sowinski, K.; Mazur, R.; Nosalewicz, A.; et al. Mechanisms shaping the synergism of zeaxanthin and PsbS in photoprotective energy dissipation in the photosynthetic apparatus of plants. Plant J. 2021, 107, 418–433. [Google Scholar] [CrossRef]
- Acebron, K.; Matsubara, S.; Jedmowski, C.; Emin, D.; Muller, O.; Rascher, U. Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. New Phytol. 2021, 229, 2104–2119. [Google Scholar] [CrossRef]
- Li, Z.; Ahn, T.K.; Avenson, T.J.; Ballottari, M.; Cruz, J.A.; Kramer, D.M.; Bassi, R.; Fleming, G.R.; Keasling, J.D.; Niyogi, K.K. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 2009, 21, 1798–1812. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.C. Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and water stress. Plant Physiol. 1988, 87, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C.; Leitão, A.E.; Fortunato, A.S.; Pais, I.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; et al. Stress cross-response of the antioxidative system promoted by superimposed drought and cold conditions in Coffea spp. PLoS ONE 2018, 13, e0198694. [Google Scholar] [CrossRef]
- Baccari, S.; Elloumi, O.; Chaari-Rkhis, A.; Fenollosa, E.; Morales, M.; Drira, N.; Ben, A.F.; Fki, L.; Munné-Bosch, S. Linking leaf water potential, photosynthesis and chlorophyll loss with mechanisms of photo- and antioxidant protection in juvenile olive trees Subjected to severe drought. Front. Plant Sci. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Baraldi, R.; Canaccini, F.; Cortes, S.; Magnani, F.; Rapparini, F.; Zamboni, A.; Raddi, S. Role of xanthophyll cycle-mediated photoprotection in Arbutus unedo plants exposed to water stress during the Mediterranean summer. Photosynthetica 2008, 46, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Peguero-Pina, J.J.; Morales, F.; Flexas, J.; Gil-Pelegrín, E.; Moya, I. Photochemistry, remotely sensed physiological reflectance index and de-epoxidation state of the xanthophyll cycle in Quercus coccifera under intense drought. Oecologia 2008, 156, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-E.; Liu, W.-J.; Su, Y.-Q.; Cui, J.-M.; Zhang, Z.-W.; Yuan, M.; Zhang, H.-Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Johnson, M.P.; Ruban, A.V. Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana. Planta 2011, 233, 1253–1264. [Google Scholar] [CrossRef]
- Steen, C.J.; Morris, J.M.; Short, A.H.; Niyogi, K.K.; Fleming, G.R. Complex roles of PsbS and xanthophylls in the regulation of nonphotochemical quenching in Arabidopsis thaliana under fluctuating light. J. Phys. Chem. 2020, 124, 10311–10325. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.P.; Ruban, A.V. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced pH. J. Biol. Chem. 2011, 286, 19973–19981. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-photochemical quenching plays a key role in light acclimation of rice plants differingin leaf color. Front. Plant Sci. 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Samson, G.; Bonin, L.; Maire, V. Dynamics of regulated YNPQ and non-regulated YNO energy dissipation in sunflower leaves exposed to sinusoidal lights. Photosynth. Res. 2019, 141, 315–330. [Google Scholar] [CrossRef]
- Hubbart, S.; Smillie, I.R.A.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-P.; Müller-Moulé, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Segarra-Medina, C.; González-Guzmán, M.; Puertolas, J.; Gómez-Cadenas, A. A customizable method to characterize Arabidopsis thaliana transpiration under drought conditions. Plant Methods 2019, 15, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puértolas, J.; Larsen, E.K.; Davies, W.J.; Dodd, I.C. Applying ‘drought’ to potted plants by maintaining suboptimal soil moisture improves plant water relations. J. Exp. Bot. 2017, 68, 2413–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Y(II) | Y(NPQ) | Y(NO) | |||||
|---|---|---|---|---|---|---|---|
| LL | HL | LL | HL | LL | HL | ||
| WT | C | 0.417 * | 0.165 ** | 0.439 ns | 0.671 ** | 0.144 * | 0.165 ** |
| D | 0.356 * | 0.074 ** | 0.481 ns | 0.729 ** | 0.164 * | 0.197 ** | |
| npq1 | C | 0.412 ** | 0.158 | 0.412 ns | 0.633 * | 0.176 ** | 0.210 ** |
| D | 0.355 ** | 0.084 | 0.436 ns | 0.690 * | 0.210 ** | 0.226 ** | |
| npq4 | C | 0.399 ** | 0.175 | 0.432 * | 0.588 ns | 0.170 ** | 0.237 ** |
| D | 0.303 ** | 0.103 | 0.499 * | 0.625 ns | 0.199 ** | 0.273 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosalewicz, A.; Okoń, K.; Skorupka, M. Non-Photochemical Quenching under Drought and Fluctuating Light. Int. J. Mol. Sci. 2022, 23, 5182. https://doi.org/10.3390/ijms23095182
Nosalewicz A, Okoń K, Skorupka M. Non-Photochemical Quenching under Drought and Fluctuating Light. International Journal of Molecular Sciences. 2022; 23(9):5182. https://doi.org/10.3390/ijms23095182
Chicago/Turabian StyleNosalewicz, Artur, Karolina Okoń, and Maria Skorupka. 2022. "Non-Photochemical Quenching under Drought and Fluctuating Light" International Journal of Molecular Sciences 23, no. 9: 5182. https://doi.org/10.3390/ijms23095182
APA StyleNosalewicz, A., Okoń, K., & Skorupka, M. (2022). Non-Photochemical Quenching under Drought and Fluctuating Light. International Journal of Molecular Sciences, 23(9), 5182. https://doi.org/10.3390/ijms23095182






