Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging
Abstract
:1. Introduction
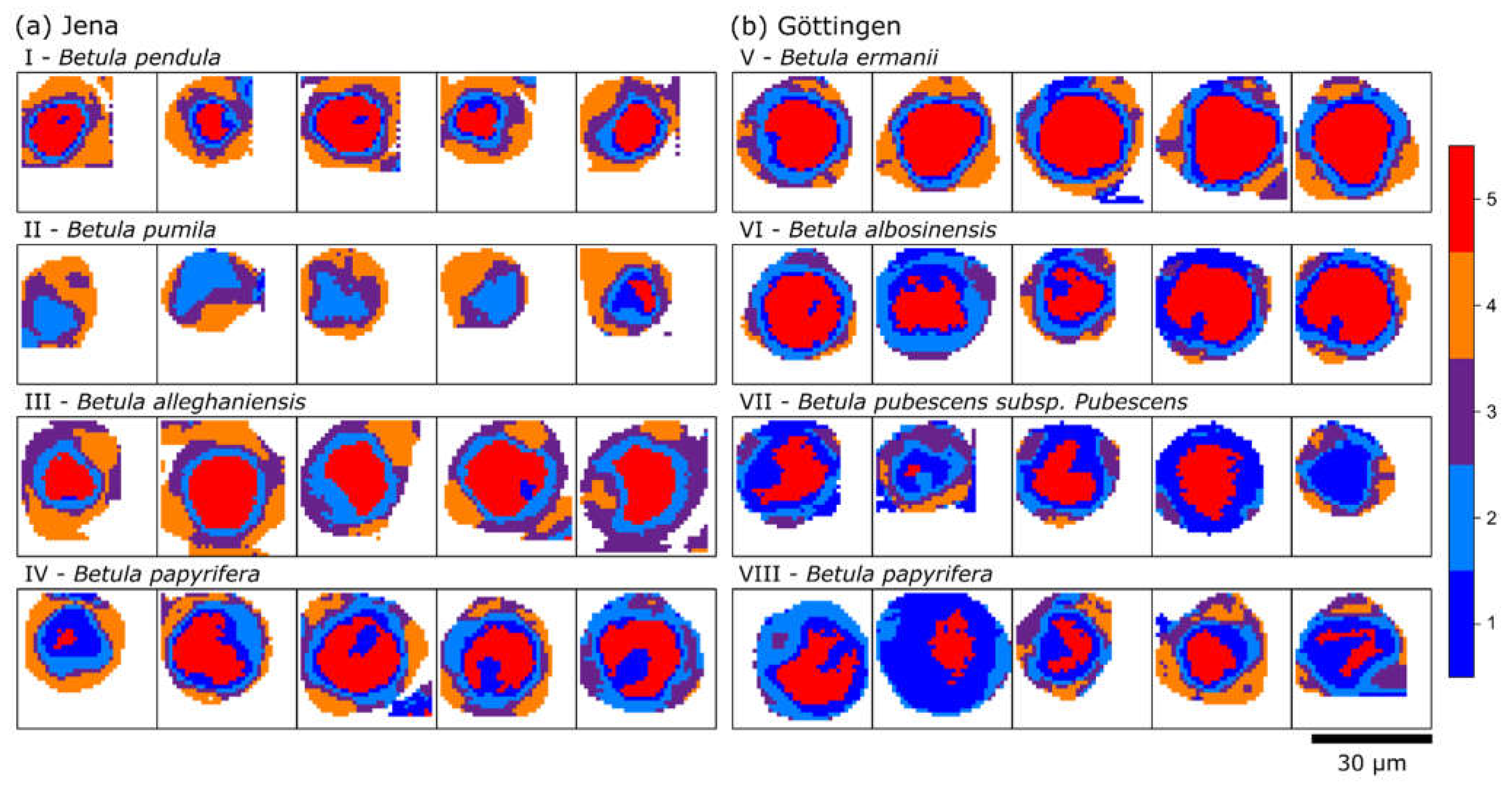
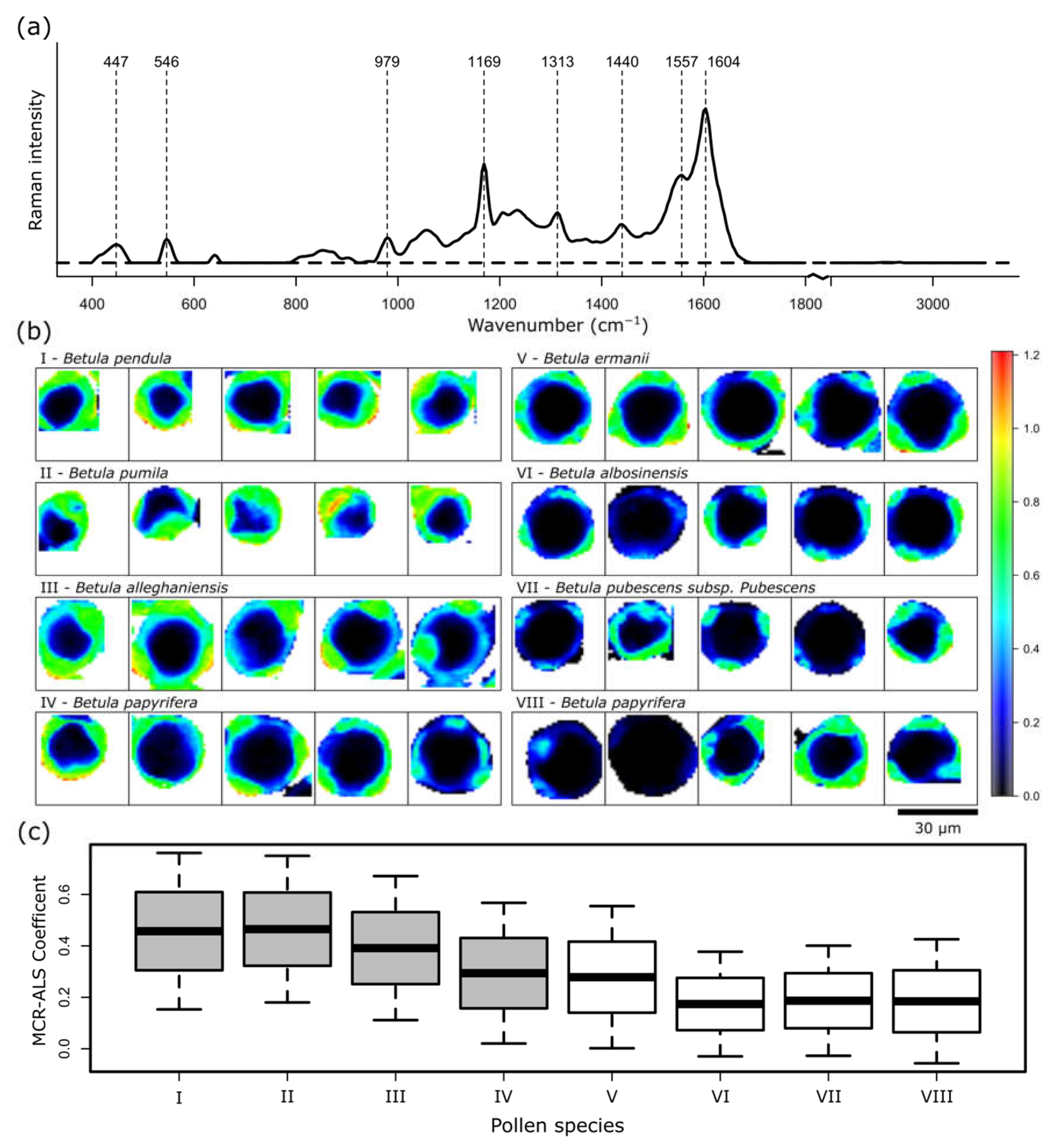
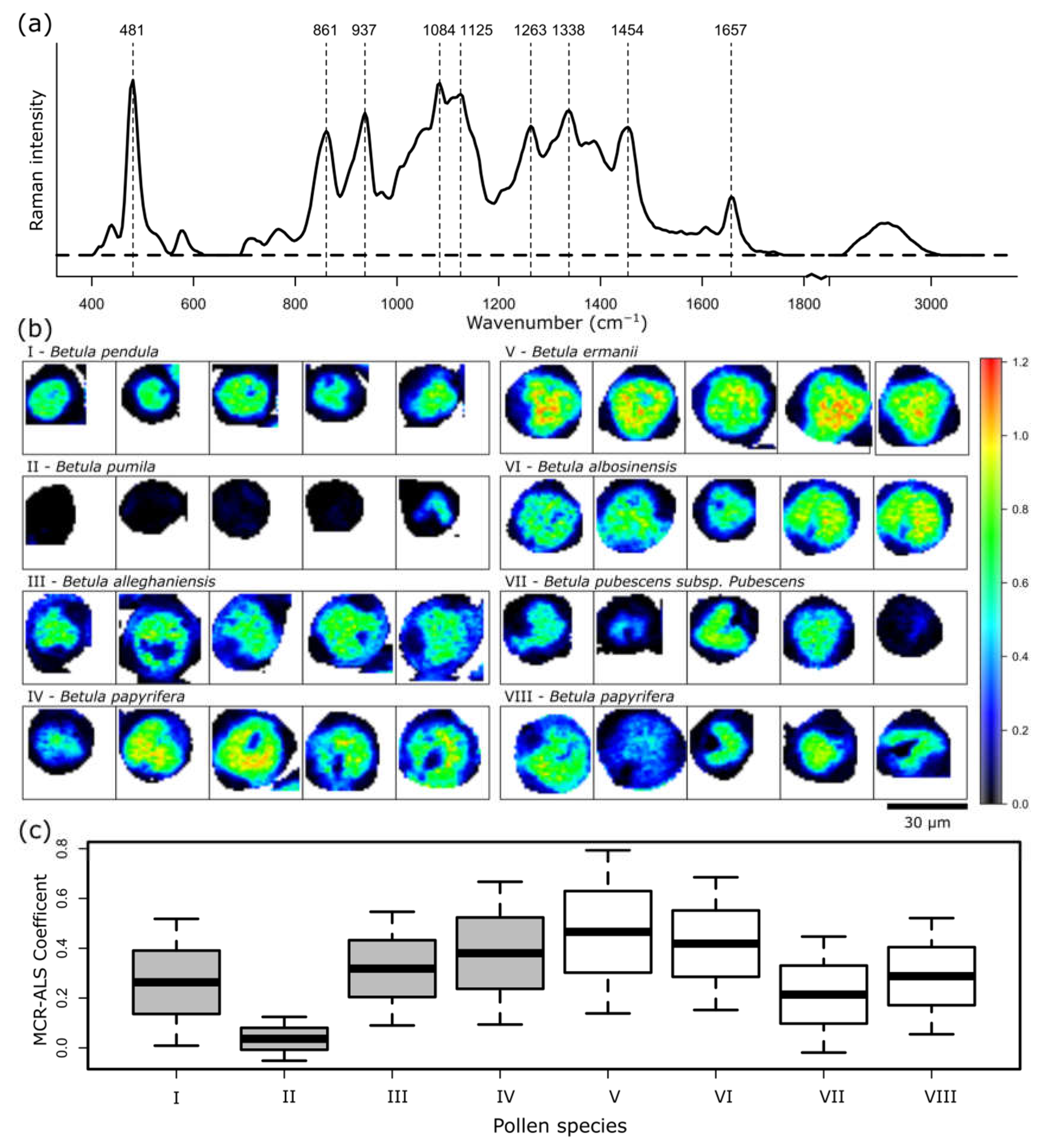
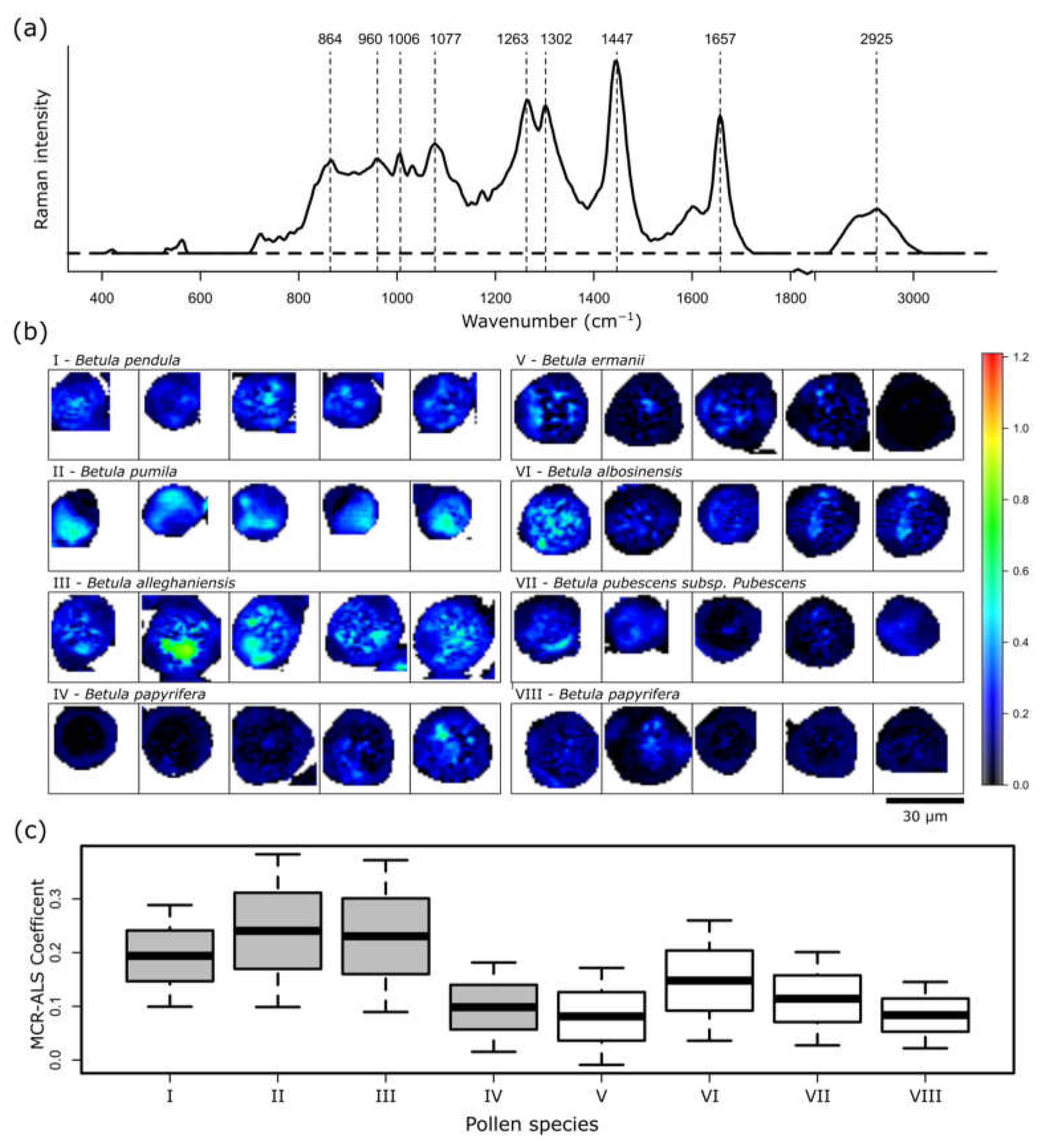
2. Results and Discussion
3. Material and Methods
3.1. Sample Collection
3.2. Sample Preparation for the Raman Analysis
3.3. Raman Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biedermann, T.; Winther, L.; Till, S.J.; Panzner, P.; Knulst, A.; Valovirta, E. Birch pollen allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, H.; Ring, J. Climate change, environment and allergy. Chem. Immunol. Allergy 2012, 96, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L.; WAO Special Committee on Climate Change Biodiversity. The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesper, D.A.; Kilic-Niebergall, E.; Pfefferle, P.I. Allergien und Umwelt. Allergo J. 2013, 22, 464–470. [Google Scholar] [CrossRef]
- Höflich, C. Climate Change and Pollen Associated Respiratory Allergies. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/377/publikationen/klimawandel_allergien_5-10.pdf (accessed on 17 December 2021).
- Wayne, P.; Foster, S.; Connolly, J.; Bazzaz, F.; Epstein, P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann. Allergy Asthma Immunol. 2002, 88, 279–282. [Google Scholar] [CrossRef]
- Ziska, L.H.; Epstein, P.R.; Schlesinger, W.H. Rising CO2, climate change, and public health: Exploring the links to plant biology. Environ. Health Perspect. 2009, 117, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Singer, B.D.; Ziska, L.H.; Frenz, D.A.; Gebhard, D.E.; Straka, J.G. Research note: Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct. Plant Biol. 2005, 32, 667–670. [Google Scholar] [CrossRef]
- Ziska, L.H.; Gebhard, D.E.; Frenz, D.A.; Faulkner, S.; Singer, B.D.; Straka, J.G. Cities as harbingers of climate change: Common ragweed, urbanization, and public health. J. Allergy Clin. Immunol. 2003, 111, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Song, U.; Mun, S.; Ho, C.H.; Lee, E.J. Responses of two invasive plants under various microclimate conditions in the Seoul metropolitan region. Environ. Manag. 2012, 49, 1238–1246. [Google Scholar] [CrossRef]
- Ziello, C.; Sparks, T.H.; Estrella, N.; Belmonte, J.; Bergmann, K.C.; Bucher, E.; Brighetti, M.A.; Damialis, A.; Detandt, M.; Galan, C.; et al. Changes to airborne pollen counts across Europe. PLoS ONE 2012, 7, e34076. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Jin, S.; Jiang, L.; Zou, Y. Raman spectroscopy of potential bio-hazards commonly found in bio-aerosols. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 243, 118753. [Google Scholar] [CrossRef] [PubMed]
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F.V. The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B. Characterization of pollen by vibrational spectroscopy. Appl. Spectrosc. 2010, 64, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Mularczyk-Oliwa, M.; Bombalska, A.; Kaliszewski, M.; Wlodarski, M.; Kopczynski, K.; Kwasny, M.; Szpakowska, M.; Trafny, E.A. Comparison of fluorescence spectroscopy and FTIR in differentiation of plant pollens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 246–254. [Google Scholar] [CrossRef]
- Lahlali, R.; Jiang, Y.; Kumar, S.; Karunakaran, C.; Liu, X.; Borondics, F.; Hallin, E.; Bueckert, R. ATR-FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front. Plant Sci. 2014, 5, 747. [Google Scholar] [CrossRef] [Green Version]
- Depciuch, J.; Kasprzyk, I.; Drzymała, E.; Parlinska-Wojtan, M. Identification of birch pollen species using FTIR spectroscopy. Aerobiologia 2018, 34, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Laucks, M.L.; Davis, E.J. Surface-enhanced Raman spectroscopy of bacteria and pollen. Appl. Spectrosc. 2005, 59, 1016–1023. [Google Scholar] [CrossRef]
- Seifert, S.; Merk, V.; Kneipp, J. Identification of aqueous pollen extracts using surface enhanced Raman scattering (SERS) and pattern recognition methods. J. Biophotonics 2016, 9, 181–189. [Google Scholar] [CrossRef]
- Johnstone, L.R.; Gomez, I.J.; Lin, H.; Fadiran, O.O.; Chen, V.W.; Meredith, J.C.; Perry, J.W. Adhesion Enhancements and Surface-Enhanced Raman Scattering Activity of Ag and Ag@SiO2 Nanoparticle Decorated Ragweed Pollen Microparticle Sensor. ACS Appl. Mater. Interfaces 2017, 9, 24804–24811. [Google Scholar] [CrossRef]
- Schulte, F.; Mader, J.; Kroh, L.W.; Panne, U.; Kneipp, J. Characterization of pollen carotenoids with in situ and high-performance thin-layer chromatography supported resonant Raman spectroscopy. Anal. Chem. 2009, 81, 8426–8433. [Google Scholar] [CrossRef]
- Korinth, F.; Mondol, A.S.; Stiebing, C.; Schie, I.W.; Krafft, C.; Popp, J. New methodology to process shifted excitation Raman difference spectroscopy data: A case study of pollen classification. Sci. Rep. 2020, 10, 11215. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.; Ribeiro, H.; Fernandez-Gonzalez, M.; Aira, M.J.; Abreu, I. Pollen Raman spectra database: Application to the identification of airborne pollen. Talanta 2014, 119, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.; Lingott, J.; Panne, U.; Kneipp, J. Chemical characterization and classification of pollen. Anal. Chem. 2008, 80, 9551–9556. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P.; Niessner, R.; Panne, U. Characterization and discrimination of pollen by Raman microscopy. Anal. Bioanal. Chem. 2005, 381, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bleha, R.; Shevtsova, T.V.; Zivcakova, M.; Korbarova, A.; Jezkova, M.; Salon, I.; Brindza, J.; Synytsya, A. Spectroscopic Discrimination of Bee Pollen by Composition, Color, and Botanical Origin. Foods 2021, 10, 1682. [Google Scholar] [CrossRef]
- Diehn, S.; Zimmermann, B.; Tafintseva, V.; Seifert, S.; Bagcioglu, M.; Ohlson, M.; Weidner, S.; Fjellheim, S.; Kohler, A.; Kneipp, J. Combining Chemical Information from Grass Pollen in Multimodal Characterization. Front. Plant Sci. 2019, 10, 1788. [Google Scholar] [CrossRef] [Green Version]
- Joester, M.; Seifert, S.; Emmerling, F.; Kneipp, J. Physiological influence of silica on germinating pollen as shown by Raman spectroscopy. J. Biophotonics 2017, 10, 542–552. [Google Scholar] [CrossRef]
- Zimmermann, B.; Kohler, A. Infrared spectroscopy of pollen identifies plant species and genus as well as environmental conditions. PLoS ONE 2014, 9, e95417. [Google Scholar] [CrossRef]
- Weglinska, M.; Szostak, R.; Kita, A.; Nems, A.; Mazurek, S. Determination of nutritional parameters of bee pollen by Raman and infrared spectroscopy. Talanta 2020, 212, 120790. [Google Scholar] [CrossRef]
- Pereira, S.G.; Guedes, A.; Abreu, I.; Ribeiro, H. Testing the Raman parameters of pollen spectra in automatic identification. Aerobiologia 2020, 37, 15–28. [Google Scholar] [CrossRef]
- Zimmermann, B.; Bagcioglu, M.; Tafinstseva, V.; Kohler, A.; Ohlson, M.; Fjellheim, S. A high-throughput FTIR spectroscopy approach to assess adaptive variation in the chemical composition of pollen. Ecol. Evol. 2017, 7, 10839–10849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondol, A.S.; Patel, M.D.; Ruger, J.; Stiebing, C.; Kleiber, A.; Henkel, T.; Popp, J.; Schie, I.W. Application of High-Throughput Screening Raman Spectroscopy (HTS-RS) for Label-Free Identification and Molecular Characterization of Pollen. Sensors 2019, 19, 4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlmann, S.; Shang, X.; Giannakaki, E.; Filioglou, M.; Saarto, A.; Romakkaniemi, S.; Komppula, M. Detection and characterization of birch pollen in the atmosphere using a multiwavelength Raman polarization lidar and Hirst-type pollen sampler in Finland. Atmos. Chem. Phys. 2019, 19, 14559–14569. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.; Giannakaki, E.; Bohlmann, S.; Filioglou, M.; Saarto, A.; Ruuskanen, A.; Leskinen, A.; Romakkaniemi, S.; Komppula, M. Optical characterization of pure pollen types using a multi-wavelength Raman polarization lidar. Atmos. Chem. Phys. 2020, 20, 15323–15339. [Google Scholar] [CrossRef]
- Kendel, A.; Zimmermann, B. Chemical Analysis of Pollen by FT-Raman and FTIR Spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Bagcioglu, M.; Zimmermann, B.; Kohler, A. A Multiscale Vibrational Spectroscopic Approach for Identification and Biochemical Characterization of Pollen. PLoS ONE 2015, 10, e0137899. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, B.; Bagcioglu, M.; Sandt, C.; Kohler, A. Vibrational microspectroscopy enables chemical characterization of single pollen grains as well as comparative analysis of plant species based on pollen ultrastructure. Planta 2015, 242, 1237–1250. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Methods 2014, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635. [Google Scholar] [CrossRef] [Green Version]
- Mullen, K.M. ALS: Multivariate Curve Resolution Alternating Least Squares (MCR-ALS), R Package Version 0.0.6. 2015. Available online: https://CRAN.R-project.org/package=ALS (accessed on 30 March 2022).
- De Juan, A.; Jaumot, J.; Tauler, R. Multivariate Curve Resolution (MCR). Solving the mixture analysis problem. Anal. Methods 2014, 6, 4964–4976. [Google Scholar] [CrossRef]
- Ischebeck, T. Lipids in pollen—They are different. Biochim. Biophys. Acta 2016, 1861, 1315–1328. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Beleites, C.; Sergo, V. HyperSpec: A Package to Handle Hyperspectral Data Sets in R, R Package Version 0.99-20200527. 2020. Available online: https://github.com/cbeleites/hyperSpec (accessed on 30 March 2022).
- Ryabchykov, O.; Bocklitz, T.; Ramoji, A.; Neugebauer, U.; Foerster, M.; Kroegel, C.; Bauer, M.; Kiehntopf, M.; Popp, J. Automatization of spike correction in Raman spectra of biological samples. Chemom. Intell. Lab. Syst. 2016, 155, 1–6. [Google Scholar] [CrossRef]
- Borchers, H.W. Pracma: Practical Numerical Math Functions; R Package Version 2.2.9. 2019. Available online: https://CRAN.R-project.org/package=pracma(accessed on 30 March 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiebing, C.; Post, N.; Schindler, C.; Göhrig, B.; Lux, H.; Popp, J.; Heutelbeck, A.; Schie, I.W. Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging. Int. J. Mol. Sci. 2022, 23, 5112. https://doi.org/10.3390/ijms23095112
Stiebing C, Post N, Schindler C, Göhrig B, Lux H, Popp J, Heutelbeck A, Schie IW. Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging. International Journal of Molecular Sciences. 2022; 23(9):5112. https://doi.org/10.3390/ijms23095112
Chicago/Turabian StyleStiebing, Clara, Nele Post, Claudia Schindler, Bianca Göhrig, Harald Lux, Jürgen Popp, Astrid Heutelbeck, and Iwan W. Schie. 2022. "Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging" International Journal of Molecular Sciences 23, no. 9: 5112. https://doi.org/10.3390/ijms23095112
APA StyleStiebing, C., Post, N., Schindler, C., Göhrig, B., Lux, H., Popp, J., Heutelbeck, A., & Schie, I. W. (2022). Revealing the Chemical Composition of Birch Pollen Grains by Raman Spectroscopic Imaging. International Journal of Molecular Sciences, 23(9), 5112. https://doi.org/10.3390/ijms23095112





