Eosinophils and Lung Cancer: From Bench to Bedside
Abstract
:1. Introduction
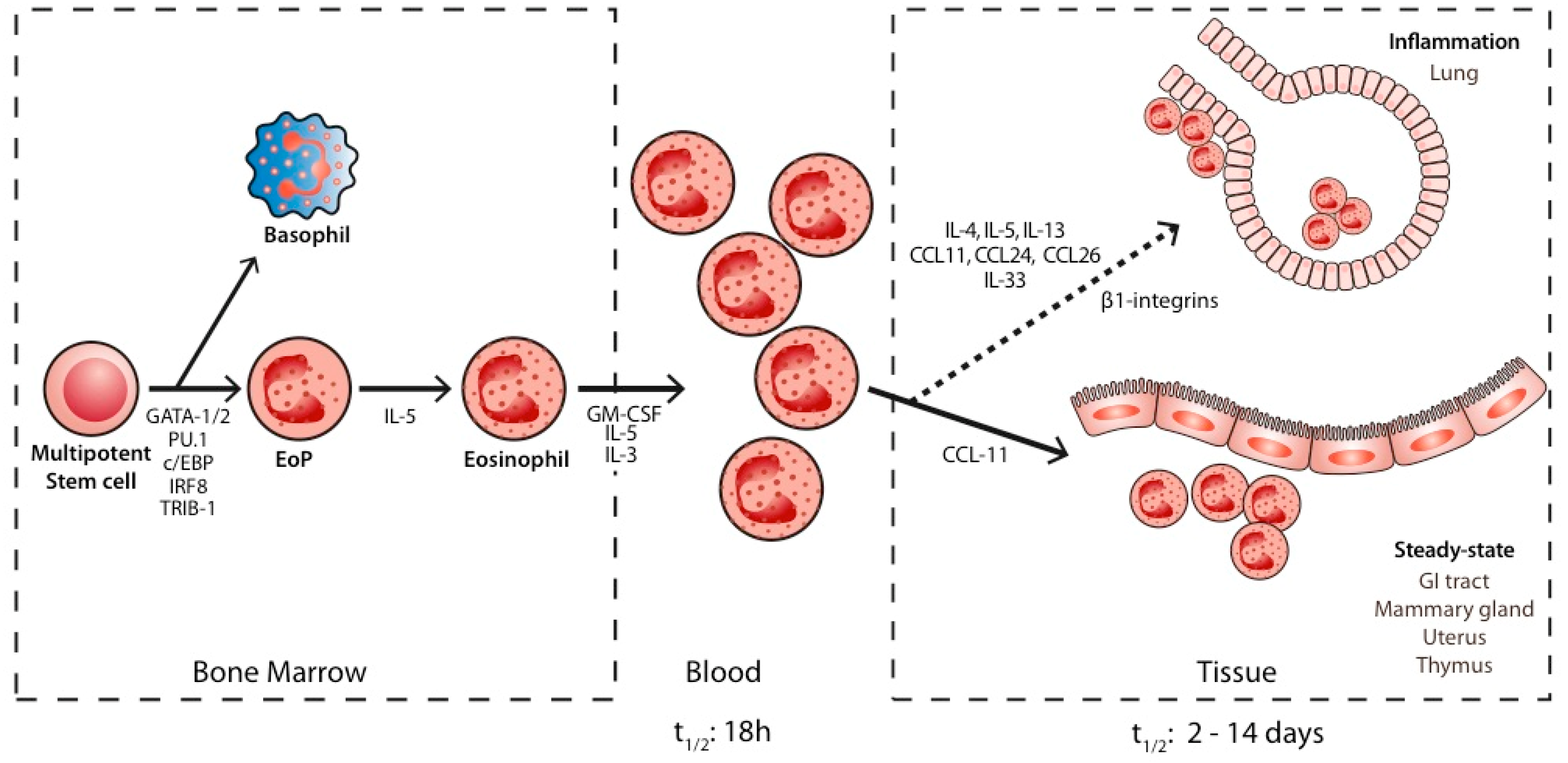
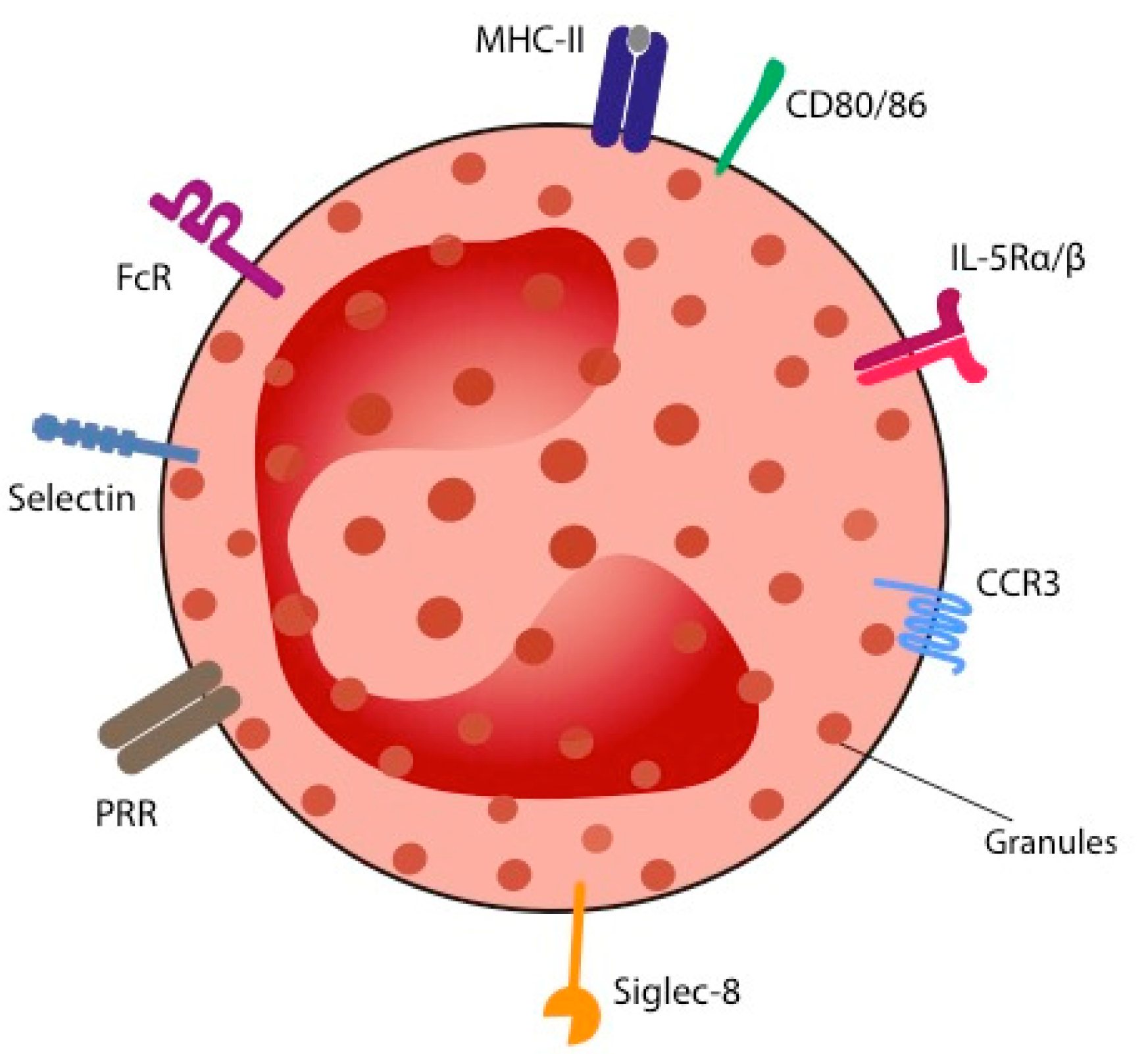
2. Biology of Eosinophils
3. Role of Eosinophils in Physiological Steady-State Conditions
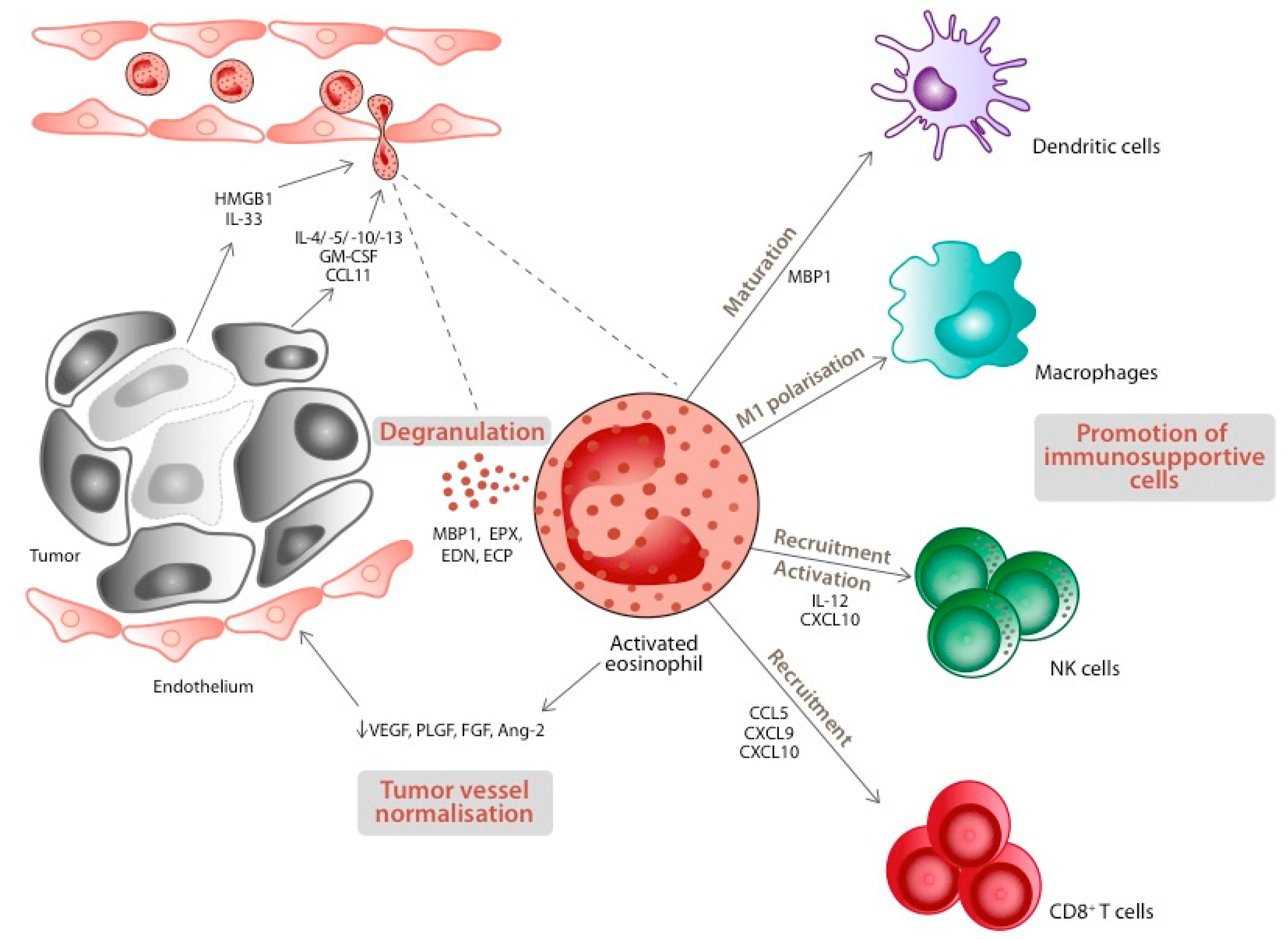
4. Eosinophils and Cancer: The Bench Side
5. Eosinophils and Lung Cancer: The Bedside
5.1. Blood Eosinophils (B-Eos)
5.2. Tissue Eosinophils (T-Eos)
6. Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kay, A.B. The early history of the eosinophil. Clin. Exp. Allergy 2015, 45, 575–582. [Google Scholar] [CrossRef] [PubMed]
- GINA Guidelines. Global Initiative for Asthma. Available online: https://ginasthma.org/gina-reports/ (accessed on 16 December 2021).
- GOLD Guidelines. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 16 December 2021).
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.M.; Routier, E.; Robert, C. Experience in daily practice with ipilimumab for thetreatment of patients with metastatic melanoma: Anearly increase in lymphocyte and eosinophil countsis associated with improved survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29170120 (accessed on 28 April 2022). [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Iwasaki, H.; Kohno, K.; Yoshimoto, G.; Kikushige, Y.; Okeda, A.; Uike, N.; Niiro, H.; Takenaka, K.; Nagafuji, K.; et al. Identification of the human eosinophil lineage-committed progenitor: Revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 2009, 206, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Mizuno, S.I.; Mayfield, R.; Shigematsu, H.; Arinobu, Y.; Seed, B.; Gurish, M.F.; Takatsu, K.; Akashi, K. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 2005, 201, 1891–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drissen, R.; Buza-Vidas, N.; Woll, P.; Thongjuea, S.; Gambardella, A.; Giustacchini, A.; Mancini, E.; Zriwil, A.; Lutteropp, M.; Grover, A.; et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat. Immunol. 2016, 17, 666–676. [Google Scholar] [CrossRef] [Green Version]
- Mack, E.A.; Pear, W.S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr. Opin. Hematol. 2020, 27, 27–33. [Google Scholar] [CrossRef]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Wagner, N.; Crossman, M.W.; Foster, P.S.; Rothenberg, M.E. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J. Biol. Chem. 2002, 277, 4406–4412. [Google Scholar] [CrossRef] [Green Version]
- Foster, P.S.; Hogan, S.P.; Ramsay, A.J.; Matthaei, K.I.; Young, I.G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996, 183, 195–201. [Google Scholar] [CrossRef]
- Walsh, G.M. Anti-IL-5 monoclonal antibodies for the treatment of asthma: An update. Expert Opin. Biol. Ther. 2020, 20, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, P.C.; Schollaert, K.L.; Bouffi, C.; Rothenberg, M.E. IL-5 Triggers a Cooperative Cytokine Network That Promotes Eosinophil Precursor Maturation. J. Immunol. 2014, 193, 4043–4052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbach, K.; Schick, P.; Trepel, F.; Raffler, H.; Döhrmann, J.; Heilgeist, G.; Heltzel, W.; Li, K.; Past, W.; van der Woerd-de Lange, J.; et al. Estimation of Kinetic Parameters of Neutrophilic, Eosinophilic, and Basophilic Granulocytes in Human Blood. Blut 1979, 39, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouon-Evans, V.; Pollard, J.W. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology 2001, 142, 4515–4521. [Google Scholar] [CrossRef]
- Gouon-Evans, V.; Lin, E.Y.; Pollard, J.W. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002, 4, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Moser, R.; Fehr, J.; Bruinzeel, P.L.B. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J. Imunol. 1992, 149, 1432–1438. [Google Scholar]
- Horie, S.; Okubo, Y.; Hossain, M.; Sato, E.; Nomura, H.; Koyama, S.; Suzuki, J.I.; Isobe, M.; Sekiguchi, M. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern. Med. 1997, 36, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Sher, A.; Coffman, R.L.; Hieny, S.; Cheever, A.W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J. Immunol. 1990, 145, 3911–3916. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2123226 (accessed on 28 April 2022).
- Milovanovic, M.; Volarevic, V.; Radosavljevic, G.; Jovanovic, I.; Pejnovic, N.; Arsenijevic, N.; Lukic, M.L. IL-33/ST2 axis in inflammation and immunopathology. Immunol. Res. 2012, 52, 89–99. [Google Scholar] [CrossRef]
- Bochner, B.S.; Schleimer, R.P. The role of adhesion molecules in human eosinophil and basophil recruitment. J. Allergy Clin. Immunol. 1994, 94, 427–438. [Google Scholar] [CrossRef]
- Zimmermann, N.; Hershey, G.K.; Foster, P.S.; Rothenberg, M.E. Chemokines in asthma: Cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003, 111, 227–243. [Google Scholar] [PubMed]
- Kita, H.; Adolphson, C.R.; Gleich, G.J. Biology of Eosinophils. In Allergy: Principles and Practice, 4th ed.; Mosby: St Louis, MO, USA, 1998. [Google Scholar]
- Sur, S.; Adolphson, C.R.; Gleich, G.J. Eosinophils: Biochemical and cellular aspects. In Allergy: Principles and Practice, 4th ed.; Mosby: St Louis, MO, USA, 1998. [Google Scholar]
- Gleich, G.J.; Adolphson, C.R.; Leiferman, K.M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993, 44, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.P.; Rosenberg, H.F.; Moqbel, R.; Phipps, S.; Foster, P.S.; Lacy, P.; Kay, A.B.; Rothenberg, M.E. Eosinophils: Biological properties and role in health and disease. Clin. Exp. Allergy 2008, 38, 709–750. [Google Scholar] [CrossRef] [PubMed]
- Kvarnhammar, A.M.; Cardell, L.O. Pattern-recognition receptors in human eosinophils. Immunology 2012, 136, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [Green Version]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Rosenberg, H.F. Eosinophils in Health and Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 111–119. [Google Scholar]
- Croxford, A.L.; Buch, T. Cytokine reporter mice in immunological research: Perspectives and lessons learned. Immunology 2011, 132, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Lathbury, L.J.; Salamonsen, L.A. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol. Reprod. 2000, 62, 404–411. [Google Scholar] [CrossRef]
- Timmons, B.C.; Fairhurst, A.-M.; Mahendroo, M.S. Temporal Changes in Myeloid Cells in the Cervix during Pregnancy and Parturition. J. Immunol. 2009, 182, 2700–2707. [Google Scholar] [CrossRef] [Green Version]
- Chu, V.T.; Beller, A.; Rausch, S.; Strandmark, J.; Zänker, M.; Arbach, O.; Kruglov, A.; Berek, C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 2014, 40, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, Y.P.S.; Henderson, N.C.; Heredia, J.E.; Eagle, A.R.; Odegaard, J.I.; Lehwald, N.; Nguyen, K.D.; Sheppard, D.; Mukundan, L.; Locksley, R.M.; et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9914–9919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils Sustain Adipose Alternatively Activated macrophages Associated with Glucose Homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-B.; Weller, P.F. Pivotal Advance: Eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J. Leukoc. Biol. 2008, 83, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Jordan, M.B.; Mills, D.M.; Kappler, J.; Marrack, P.; Cambier, J.C. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 2004, 304, 1808–1810. [Google Scholar] [CrossRef]
- Chu, V.T.; Fröhlich, A.; Steinhauser, G.; Scheel, T.; Roch, T.; Fillatreau, S.; Lee, J.J.; Löhning, M.; Berek, C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011, 12, 151–159. [Google Scholar] [CrossRef]
- Berek, C. Eosinophils: Important players in humoral immunity. Clin. Exp. Immunol. 2016, 183, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Odemuyiwa, S.O.; Ghahary, A.; Li, Y.; Puttagunta, L.; Lee, J.E.; Musat-Marcu, S.; Ghahary, A.; Moqbel, R. Cutting Edge: Human Eosinophils Regulate T Cell Subset Selection through Indoleamine 2,3-Dioxygenase. J. Immunol. 2004, 173, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Wang, J.; Lee, P.; Sharma, S.; Mao, J.T.; Meissner, H.; Dubinett, S.M.; Dubinett, S.M.; Uyemura, K.; Modlin, R.; et al. Human Non-Small Cell Lung Cancer Cells Express a Type 2 Cytokine Pattern. Cancer Res. 1995, 55, 3847–3853. [Google Scholar] [PubMed]
- Curran, C.S.; Evans, M.D.; Bertics, P.J. GM-CSF Production by Glioblastoma Cells Has a Functional Role in Eosinophil Survival, Activation, and Growth Factor Production for Enhanced Tumor Cell Proliferation. J. Immunol. 2011, 187, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Simson, L.; Ellyard, J.I.; Dent, L.A.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of Carcinogenesis by IL-5 and CCL11: A Potential Role for Eosinophils in Tumor Immune Surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.A. Pivotal Advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006, 79, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, R.; Herzog, G.I.; DeMarco, R.A.; Beer-Stolz, D.; Lee, J.J.; Rubartelli, A.; Schrezenmeier, H.; Lotze, M.T. Eosinophils Oxidize Damage-Associated Molecular Pattern Molecules Derived from Stressed Cells. J. Immunol. 2009, 183, 5023–5031. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Tepper, R.I.; Coffman, R.L.; Leder, P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 1992, 257, 548–551. [Google Scholar] [CrossRef]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human Eosinophils Exert TNF-α and Granzyme A-Mediated Tumoricidal Activity toward Colon Carcinoma Cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25915731 (accessed on 28 April 2022). [CrossRef] [PubMed]
- O’Flaherty, S.M.; Sutummaporn, K.; Häggtoft, W.L.; Worrall, A.P.; Rizzo, M.; Braniste, V.; Höglund, P.; Kadri, N.; Chambers, B.J. TLR-Stimulated Eosinophils Mediate Recruitment and Activation of NK Cells In Vivo. Scand. J. Immunol. 2017, 85, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotfi, R.; Lotze, M.T. Eosinophils induce DC maturation, regulating immunity. J. Leukoc. Biol. 2008, 83, 456–460. [Google Scholar] [CrossRef]
- Wong, D.T.W.; Bowen, S.M.; Elovic, A.; Gallagher, G.T.; Weller, P.F. Eosinophil ablation and tumor development. Oral Oncol. 1999, 35, 496–501. [Google Scholar] [CrossRef]
- da Silva, J.M.; dos Santos, T.P.M.; Sobral, L.M.; Queiroz-Junior, C.M.; Rachid, M.A.; Proudfoot, A.E.I.; Garlet, G.P.; Batista, A.C.; Teixeira, M.M.; Leopoldino, A.M.; et al. Relevance of CCL3/CCR5 axis in oral carcinogenesis. Oncotarget 2017, 8, 51024–51036. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Liu, L.B.; Shang, W.Q.; Chang, K.K.; Meng, Y.H.; Mei, J.; Yu, J.J.; Li, D.J.; Li, M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S.; Fingleton, B.; et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015, 75, 1624–1634. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25691457 (accessed on 28 April 2022). [CrossRef] [Green Version]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005, 7, 390–396. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15967116 (accessed on 28 April 2022). [CrossRef] [Green Version]
- Kratochvill, F.; Neale, G.; Haverkamp, J.M.; de Velde, L.A.; Smith, A.M.; Kawauchi, D.; McEvoy, J.; Roussel, M.F.; Dyer, M.A.; Qualls, J.E.; et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015, 12, 1902–1914. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26365184 (accessed on 28 April 2022). [CrossRef] [Green Version]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Pas de Deux: Control of Anti-tumor Immunity by Cancer-Associated Inflammation. Immunity 2019, 51, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic plasticity and targeting of Siglec-FhighCD11clow eosinophils to the airway in a murine model of asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 267–271. [Google Scholar] [CrossRef]
- Percopo, C.M.; Brenner, T.A.; Ma, M.; Kraemer, L.S.; Hakeem, R.M.A.; Lee, J.J.; Rosenberg, H.F. SiglecF + Gr1 hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J. Leukoc. Biol. 2017, 101, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.; Lotze, M.T.; Muul, L.; Leitman, S.; Chang, A.; Ettinghausen, S.; Matory, Y.; Skibber, J.; Shiloni, E.; JT, V. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- van Haelst Pisani, C.; Kovach, J.; Kita, H.; Leiferman, K.; Gleich, G.; Silver, J.; Dennin, R.; Abrams, J. Administration of interleukin-2 (IL-2) results in increased plasma concentrations of IL-5 and eosinophilia in patients with cancer. Blood 1991, 78, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26289067 (accessed on 28 April 2022). [CrossRef] [PubMed] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27185375 (accessed on 28 April 2022). [CrossRef] [PubMed] [Green Version]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28128709 (accessed on 28 April 2022). [CrossRef]
- Bernard-Tessier, A.; Jeanville, P.; Champiat, S.; Lazarovici, J.; Voisin, A.L.; Mateus, C.; Lambotte, O.; Annereau, M.; Michot, J.M. Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur. J. Cancer 2017, 81, 135–137. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28624693 (accessed on 28 April 2022). [CrossRef]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Fengying, F.; Ren, S.; et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar] [CrossRef]
- Sibille, A.; Henket, M.; Corhay, J.L.; Alfieri, R.; Louis, R.; Duysinx, B. White Blood Cells in Patients Treated with Programmed Cell Death-1 Inhibitors for Non-small Cell Lung Cancer. Lung 2021, 199, 549–557. [Google Scholar] [CrossRef]
- Okauchi, S.; Shiozawa, T.; Miyazaki, K.; Nishino, K.; Sasatani, Y.; Ohara, G.; Kagohashi, K.; Sato, S.; Kodama, T.; Satoh, H.; et al. Association between peripheral eosinophils and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Polish Arch. Intern. Med. 2021, 131, 152–160. [Google Scholar] [CrossRef]
- Voorwerk, L.; Garner, H.; Blomberg, O.S.; Spagnuolo, L.; Chalabi, M.; van Dyk, E.; Isaeva, O.I.; Bakker, N.; Klaver, C.; Duijst, M.; et al. LBA10 Critical role of eosinophils during response to immune checkpoint blockade in breast cancer and other cancer types. Ann. Oncol. 2020, 31, S1142. [Google Scholar] [CrossRef]
- Postow, M.A.; Hellmann, M.D. Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 1165. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29562154 (accessed on 28 April 2022). [CrossRef]
- Scanvion, Q.; Béné, J.; Gautier, S.; Grandvuillemin, A.; Le Beller, C.; Chenaf, C.; Etienne, N.; Brousseau, S.; Cortot, A.B.; Mortier, L.; et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology 2020, 9, 1722022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Suda, T.; Shiozaki, H.; Miura, Y.; Hitoshi, Y.; Takatsu, K.; Kasahara, T. Role of IL-5 in IL-2-induced eosinophilia. In vivo and in vitro expression of IL-5 mRNA by IL-2. J. Immunol. 2021, 45, 873–877. [Google Scholar]
- Van Gool, F.; Molofsky, A.B.; Morar, M.M.; Rosenzwajg, M.; Liang, H.E.; Klatzmann, D.; Locksley, R.M.; Bluestone, J.A. Interleukin-5—Producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood 2014, 124, 3572–3576. [Google Scholar] [CrossRef] [Green Version]
- Werner, J.P. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003, 139, 683–693. [Google Scholar]
- Ye, L.; Wang, H.; Li, H.; Liu, H.; Lv, T.; Song, Y.; Zhang, F. Eosinophil peroxidase over-expression predicts the clinical outcome of patients with primary lung adenocarcinoma. J. Cancer 2019, 10, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Tataroǧlu, C.; Kargi, A.; Özkal, S.; Eşrefoǧlu, N.; Akkoçlu, A. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer 2004, 43, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Van Hulst, G.; Batugedara, H.M.; Jorssen, J.; Louis, R.; Bureau, F.; Desmet, C.J. Eosinophil diversity in asthma. Biochem. Pharmacol. 2020, 179, 113963. [Google Scholar] [CrossRef]
- Dolitzky, A.; Shapira, G.; Grisaru-Tal, S.; Hazut, I.; Avlas, S.; Gordon, Y.; Itan, M.; Shomron, N.; Munitz, A. Transcriptional Profiling of Mouse Eosinophils Identifies Distinct Gene Signatures Following Cellular Activation. Front. Immunol. 2021, 12, 5288. [Google Scholar] [CrossRef]
- Van Rossem, I.; Hanon, S.; Verbanck, S.; Vanderhelst, E. Blood Eosinophil Counts in COPD: Adding Within-day Variability to the Equation. Am. J. Respir. Crit. Care Med. 2021, 205, 727–729. [Google Scholar] [CrossRef]
- Abrahamsen, J.F.; Smaaland, R.; Sandberg, S.; Aakvaag, A.; Lote, K. Circadian variation in serum cortisol and circulating neutrophils are markers for circadian variation of bone marrow proliferation in cancer patients. Eur. J. Haematol. 1993, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
| Study | N | Stage of Disease | ICI | Eosinophils | Outcome | Effects | p Value |
|---|---|---|---|---|---|---|---|
| Tanizaki 2017 [5] | 134 | IIIB-IV | nivolumab | AEC t0; categorical; simple & composite biomarker | OS PFS | HR = 0.24 [95% CI 0.09−0.62] HR = 0.53 [95% CI 0.31−0.91] if AECt0 ≥ 0.15 cells/mL | 0.003 0.02 |
| Chu X 2020 [81] | 300 | IIIB-IV | PD-1i +/− CT +/− AAG | AEC t0; categorical; simple | ORR PFS | 40.9 % vs 28.8 % med. = 8.93 vs 5.87 mo HR = 0.744 [95% CI 0.56−0.99] if AECt0 ≥ 0.15 cells/mL | 0.029 0.038 |
| Sibille 2021 [82] | 191 | IIIA-IV | pembrolizumab nivolumab atezolizumab durvalumab | AEC & REC t1; continuous | ORR | OR = 0.53 [95% CI 0.32−0.88] if RECt1 > 5.3% | 0.014 |
| Okauchi 2021 [83] | 190 | IIIA-IV | nivolumab pembrolizumab atezolizumab +/− CT | AEC & REC t0 & q2–3 wk; RECmax. *; categorical | TTF | OR = 0.39 [95% CI 0.26−0.60] if RECmax. > 5% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibille, A.; Corhay, J.-L.; Louis, R.; Ninane, V.; Jerusalem, G.; Duysinx, B. Eosinophils and Lung Cancer: From Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 5066. https://doi.org/10.3390/ijms23095066
Sibille A, Corhay J-L, Louis R, Ninane V, Jerusalem G, Duysinx B. Eosinophils and Lung Cancer: From Bench to Bedside. International Journal of Molecular Sciences. 2022; 23(9):5066. https://doi.org/10.3390/ijms23095066
Chicago/Turabian StyleSibille, Anne, Jean-Louis Corhay, Renaud Louis, Vincent Ninane, Guy Jerusalem, and Bernard Duysinx. 2022. "Eosinophils and Lung Cancer: From Bench to Bedside" International Journal of Molecular Sciences 23, no. 9: 5066. https://doi.org/10.3390/ijms23095066
APA StyleSibille, A., Corhay, J.-L., Louis, R., Ninane, V., Jerusalem, G., & Duysinx, B. (2022). Eosinophils and Lung Cancer: From Bench to Bedside. International Journal of Molecular Sciences, 23(9), 5066. https://doi.org/10.3390/ijms23095066






