When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives
Abstract
1. Introduction
2. The Activity of the LC in Sleep: Pioneering Studies
2.1. Animal Studies
2.2. Human Studies
3. The Activity of the LC in Sleep: Novel Insights
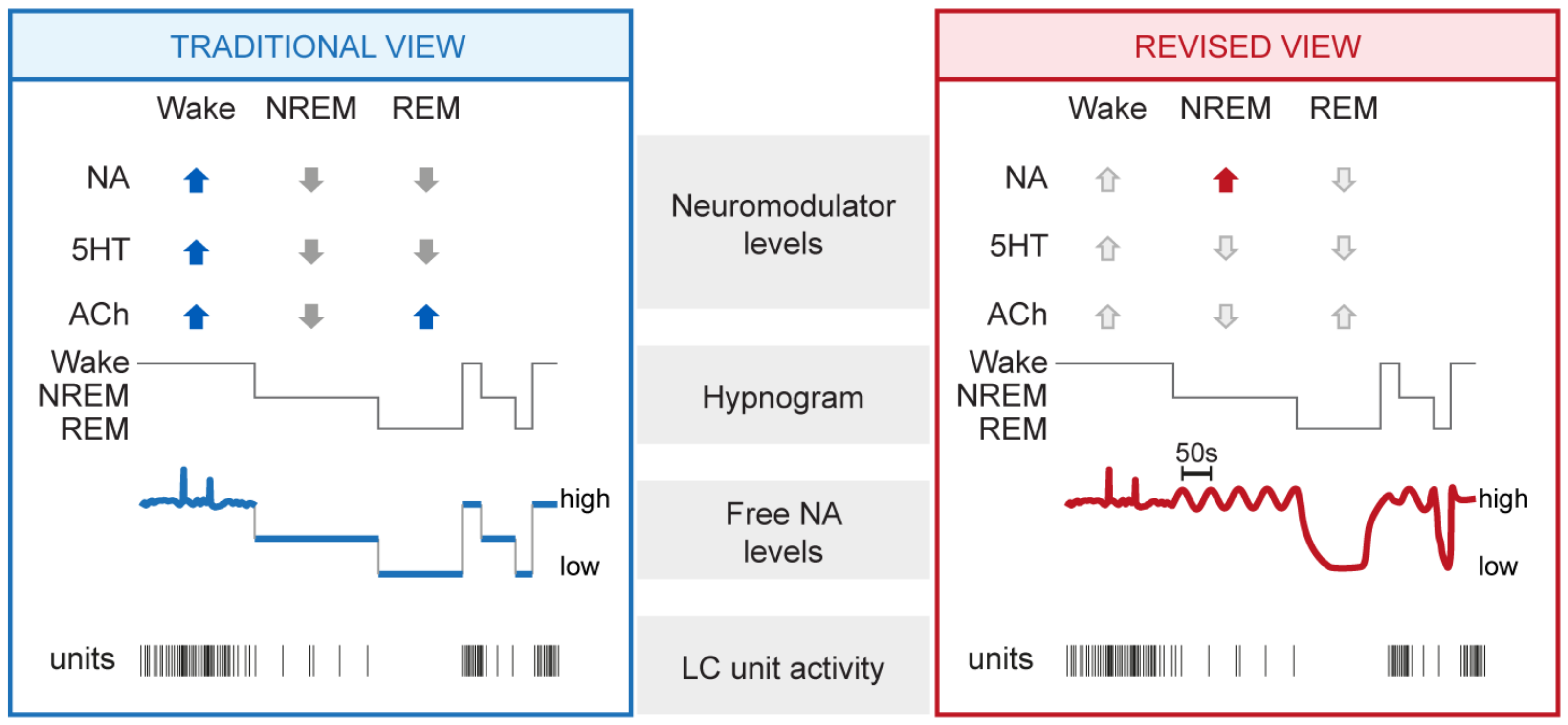
3.1. Mean NA Levels Differ across the Sleep–Wake Cycle
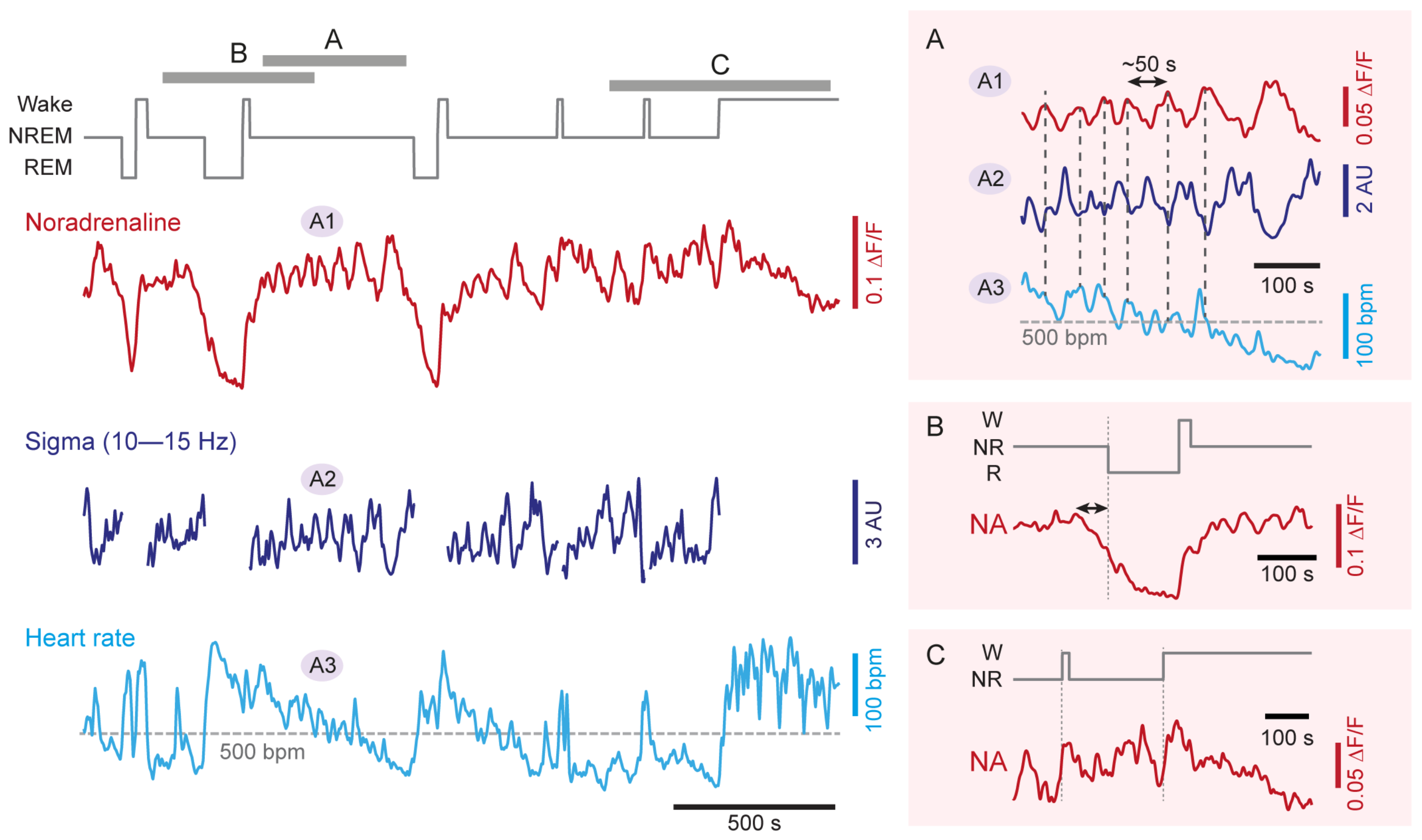
3.2. NA Levels and LC Activity Fluctuate during NREM Sleep
3.3. NA Levels Decay to Low Levels during REM Sleep
3.4. NA Levels Show Characteristic Dynamics at Behavioral State Transitions
3.5. Emerging Dynamics of Other Monoamines and Wake-Promoting Neurotransmitters
4. The Role of the LC in the Regulation of Sleep and Sleep Functions
4.1. LC as Part of Sensory Arousal Circuits during NREM Sleep
4.2. The LC as Part of the Regulatory Mechanisms of NREM Sleep
4.3. The LC in REM Sleep Control
4.4. The LC in Hippocampus-Dependent and Independent Memory Consolidation
4.5. The LC as Mediator of Vagal Afferent Information
4.6. The Role of the LC in the Regulation of Brain Vascular Activity
5. The LC and Sleep Function in Pathology
5.1. Aging and Neurodegenerative Disorders
5.2. Stress-Related Disorders
5.3. Sleep and Cardiovascular Regulation
6. Closing Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Locus Coeruleus |
| NA | Noradrenaline |
| NREM | Non-Rapid-Eye-Movement |
| REM | Rapid-Eye-Movement |
| DBH | Dopamine--Hydroxylase |
| MRI | Magnetic Resonance Imaging |
| 5HT | 5-hydroxytryptamine (or serotonin) |
| ACh | Acetylcholine |
| GRAB | G-Protein-Coupled-Receptor-Activation-Based |
| EEG | Electroencephalogram |
| VNS | Vagus Nerve Stimulation |
| AD | Alzheimer’s Disease |
References
- Manger, P.R.; Eschenko, O. The Mammalian Locus Coeruleus Complex—Consistencies and Variances in Nuclear Organization. Brain Sci. 2021, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, D. Evidence for existence of monoamine-containing neurons in central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 1964, 62, 1–55. [Google Scholar]
- Maeda, T.; Pin, C.; Salvert, D.; Ligier, M.; Jouvet, M. Les neurones contenant des catecholamines du tegmentum pontique et leurs voies de projection chez le chat. Brain Res. 1973, 57, 119–152. [Google Scholar] [CrossRef]
- Bogerts, B. A brainstem atlas of catecholaminergic neurons in man, using melanin as a natural marker. J. Comp. Neurol. 1981, 197, 63–80. [Google Scholar] [CrossRef]
- Robertson, S.D.; Plummer, N.W.; De Marchena, J.; Jensen, P. Developmental origins of central norepinephrine neuron diversity. Nat. Neurosci. 2013, 16, 1016–1023. [Google Scholar] [CrossRef]
- Aston-Jones, G. Locus Coeruleus, A5 and A7 Noradrenergic Cell Groups. In The Rat Nervous System, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 259–294. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J. Comp. Neurol. 2005, 493, 99–110. [Google Scholar] [CrossRef]
- Sara, S.J.; Bouret, S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Berridge, C.W.; Schmeichel, B.E.; España, R.A. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev. 2012, 16, 187–197. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal systems. Front. Biosci. 2003, 8, 438–451. [Google Scholar] [CrossRef]
- Touret, M.; Valatx, J.L.; Jouvet, M. The locus coeruleus: A quantitative and genetic study in mice. Brain Res. 1982, 250, 353–357. [Google Scholar] [CrossRef]
- Sturrock, R.; Rao, K. A quantitative histological study of neuronal loss from the locus coeruleus of ageing mice. Neuropath. Appl. Neurobiol. 1985, 11, 55–60. [Google Scholar] [CrossRef]
- Mouton, P.R.; Pakkenberg, B.; Gundersen, H.J.G.; Price, D.L. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanatom. 1994, 7, 185–190. [Google Scholar] [CrossRef]
- Descarries, L.; Mechawar, N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000, 125, 27–47. [Google Scholar]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef]
- Cohen, Z.; Molinatti, G.; Hamel, E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 1997, 17, 894–904. [Google Scholar] [CrossRef]
- McCormick, D.A.; Bal, T. Sleep and arousal: Thalamocortical mechanisms. Ann. Rev. Neurosci. 1997, 20, 185–215. [Google Scholar] [CrossRef]
- Wahis, J.; Holt, M.G. Astrocytes, Noradrenaline,α1-Adrenoreceptors, and Neuromodulation: Evidence and Unanswered Questions. Front. Cell. Neurosci. 2021, 15, 42. [Google Scholar] [CrossRef]
- Waterhouse, B.D.; Navarra, R.L. The locus coeruleus-norepinephrine system and sensory signal processing: A historical review and current perspectives. Brain Res. 2019, 1709, 1–15. [Google Scholar] [CrossRef]
- Arrigoni, E.; Chen, M.C.; Fuller, P.M. The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep. J. Physiol. 2016, 594, 5391–5414. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Bayliss, D.A. Neural control of breathing and CO2 homeostasis. Neuron 2015, 87, 946–961. [Google Scholar] [CrossRef]
- Yu, X.; Franks, N.P.; Wisden, W. Sleep and sedative states induced by targeting the histamine and noradrenergic systems. Front. Neural Circuits 2018, 12, 4. [Google Scholar] [CrossRef]
- Valentino, R.J.; Van Bockstaele, E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008, 583, 194–203. [Google Scholar] [CrossRef]
- Vanderheyden, W.M.; Poe, G.R.; Liberzon, I. Trauma exposure and sleep: Using a rodent model to understand sleep function in PTSD. Exp. Brain Res. 2014, 232, 1575–1584. [Google Scholar] [CrossRef]
- Hirschberg, S.; Li, Y.; Randall, A.; Kremer, E.J.; Pickering, A.E. Functional dichotomy in spinal-vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 2017, 6, e29808. [Google Scholar] [CrossRef]
- España, R.A.; Schmeichel, B.E.; Berridge, C.W. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 2016, 1641, 207–216. [Google Scholar] [CrossRef]
- Kaur, S.; Saper, C.B. Neural circuitry underlying waking up to hypercapnia. Front. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef]
- Wood, S.K.; Valentino, R.J. The brain norepinephrine system, stress and cardiovascular vulnerability. Neurosci. Biobehav. Rev. 2017, 74, 393–400. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Drummond, G.T.; Sur, M. Locus coeruleus norepinephrine in learned behavior: Anatomical modularity and spatiotemporal integration in targets. Front. Neural Circuits 2021, 15, 638007. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Totah, N.K.; Logothetis, N.K.; Eschenko, O. Noradrenergic ensemble-based modulation of cognition over multiple timescales. Brain Res. 2019, 1709, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, V.; Floriou-Servou, A.; Markicevic, M.; Vermeiren, Y.; Sturman, O.; Privitera, M.; von Ziegler, L.; Ferrari, K.D.; Weber, B.; De Deyn, P.P.; et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 2019, 103, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.J.; Jensen, P.; McCall, J.G.; Pickering, A.E.; Schwarz, L.A.; Totah, N.K. Redefining noradrenergic neuromodulation of behavior: Impacts of a modular locus coeruleus architecture. J. Neurosci. 2019, 39, 8239–8249. [Google Scholar] [CrossRef] [PubMed]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Van Egroo, M.; Koshmanova, E.; Vandewalle, G.; Jacobs, H.I. Importance of the locus coeruleus-norepinephrine system in sleep-wake regulation: Implications for aging and Alzheimer’s disease. Sleep Med. Rev. 2022, 62, 101592. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Hobson, J.A. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002, 3, 591–605. [Google Scholar] [CrossRef]
- Foote, S.; Aston-Jones, G.; Bloom, F. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA 1980, 77, 3033–3037. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Bloom, F. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981, 1, 876–886. [Google Scholar] [CrossRef]
- Chu, N.S.; Bloom, F.E. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J. Neurobiol. 1974, 5, 527–544. [Google Scholar] [CrossRef]
- Hobson, J.A.; McCarley, R.W.; Wyzinski, P.W. Sleep cycle oscillation: Reciprocal discharge by two brainstem neuronal groups. Science 1975, 189, 55–58. [Google Scholar] [CrossRef]
- Rasmussen, K.; Morilak, D.A.; Jacobs, B.L. Single unit activity of locus coeruleus neurons in the freely moving cat: I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986, 371, 324–334. [Google Scholar] [CrossRef]
- Rajkowski, J.; Kubiak, P.; Aston-Jones, G. Locus coeruleus activity in monkey: Phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994, 35, 607–616. [Google Scholar] [CrossRef]
- Eschenko, O.; Sara, S.J. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: Brain stem–cortex interplay for memory consolidation? Cereb. Cort. 2008, 18, 2596–2603. [Google Scholar] [CrossRef]
- Eschenko, O.; Magri, C.; Panzeri, S.; Sara, S.J. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cort. 2012, 22, 426–435. [Google Scholar] [CrossRef]
- Swift, K.M.; Gross, B.A.; Frazer, M.A.; Bauer, D.S.; Clark, K.J.; Vazey, E.M.; Aston-Jones, G.; Li, Y.; Pickering, A.E.; Sara, S.J.; et al. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol. 2018, 28, 3599–3609. [Google Scholar] [CrossRef]
- Fernandez, L.M.; Vantomme, G.; Osorio-Forero, A.; Cardis, R.; Béard, E.; Lüthi, A. Thalamic reticular control of local sleep in mouse sensory cortex. eLife 2018, 7, e39111. [Google Scholar] [CrossRef]
- Menon, J.M.L.; Nolten, C.; Achterberg, E.J.M.; Joosten, R.N.J.M.A.; Dematteis, M.; Feenstra, M.G.P.; Drinkenburg, W.H.P.; Leenaars, C.H.C. Brain microdialysate monoamines in relation to circadian rhythms, sleep, and sleep deprivation—A systematic review, network meta-analysis, and new primary data. J. Circ. Rhythms 2019, 17, 1. [Google Scholar] [CrossRef]
- Devoto, P.; Flore, G.; Saba, P.; Fa, M.; Gessa, G.L. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 2005, 92, 368–374. [Google Scholar] [CrossRef]
- Florin-Lechner, S.M.; Druhan, J.P.; Aston-Jones, G.; Valentino, R.J. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996, 742, 89–97. [Google Scholar] [CrossRef]
- Berridge, C.; Abercrombie, E. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience 1999, 93, 1263–1270. [Google Scholar] [CrossRef]
- Dugast, C.; Cespuglio, R.; Suaud-Chagny, M. In vivo monitoring of evoked noradrenaline release in the rat anteroventral thalamic nucleus by continuous amperometry. J. Neurochem. 2002, 82, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Jouvet, M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. In Neurophysiology and Neurochemistry of Sleep and Wakefulness; Springer: Berlin/Heidelberg, Germany, 1972; pp. 166–307. [Google Scholar]
- Monti, J.M.; D’Angelo, L.; Jantos, H.; Barbeito, L.; Abo, V. Effect of DSP-4, a noradrenergic neurotoxin, on sleep and wakefulness and sensitivity to drugs acting on adrenergic receptors in the rat. Sleep 1988, 11, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Hellman, K.; Abel, T.; Thomas, S.A. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J. Neurophysiol. 2004, 92, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Gerashchenko, D.; Salin-Pascual, R.J.; Shiromani, P.J. Effects of hypocretin2-saporin and antidopamine-β-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur. J. Neurosci. 2004, 19, 2741–2752. [Google Scholar] [CrossRef]
- Manns, I.D.; Lee, M.G.; Modirrousta, M.; Hou, Y.P.; Jones, B.E. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur. J. Neurosci. 2003, 18, 723–727. [Google Scholar] [CrossRef]
- Langer, S.Z. α2-Adrenoceptors in the treatment of major neuropsychiatric disorders. Trends Pharm. Sci. 2015, 36, 196–202. [Google Scholar] [CrossRef]
- Washburn, M.; Moises, H.C. Electrophysiological correlates of presynaptic alpha 2-receptor-mediated inhibition of norepinephrine release at locus coeruleus synapses in dentate gyrus. J. Neurosci. 1989, 9, 2131–2140. [Google Scholar] [CrossRef]
- Svensson, T.; Bunney, B.; Aghajanian, G. Inhibition of both noradrenergic and serotonergic neurons in brain by the α-adrenergic agonist clonidine. Brain Res. 1975, 92, 291–306. [Google Scholar] [CrossRef]
- Tononi, G.; Pompeiano, M.; Cirelli, C. Suppression of desynchronized sleep through microinjection of the α2-adrenergic agonist clonidine in the dorsal pontine tegmentum of the cat. Pflügers Arch. 1991, 418, 512–518. [Google Scholar] [CrossRef]
- Crochet, S.; Sakai, K. Alpha-2 adrenoceptor mediated paradoxical (REM) sleep inhibition in the cat. Neuroreport 1999, 10, 2199–2204. [Google Scholar] [CrossRef]
- Hayat, H.; Regev, N.; Matosevich, N.; Sales, A.; Paredes-Rodriguez, E.; Krom, A.J.; Bergman, L.; Li, Y.; Lavigne, M.; Kremer, E.J.; et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020, 6, eaaz4232. [Google Scholar] [CrossRef]
- Dang-Vu, T.T.; Schabus, M.; Desseilles, M.; Albouy, G.; Boly, M.; Darsaud, A.; Gais, S.; Rauchs, G.; Sterpenich, V.; Vandewalle, G.; et al. Spontaneous neural activity during human slow wave sleep. Proc. Natl. Acad. Sci. USA 2008, 105, 15160–15165. [Google Scholar] [CrossRef]
- Kelberman, M.; Keilholz, S.; Weinshenker, D. What’s that (blue) spot on my MRI? Multimodal neuroimaging of the locus coeruleus in neurodegenerative disease. Front. Neurosci. 2020. [Google Scholar] [CrossRef]
- Doppler, C.E.; Smit, J.A.; Hommelsen, M.; Seger, A.; Horsager, J.; Kinnerup, M.B.; Hansen, A.K.; Fedorova, T.D.; Knudsen, K.; Otto, M.; et al. Microsleep disturbances are associated with noradrenergic dysfunction in Parkinson’s disease. Sleep 2021, 44, zsab040. [Google Scholar] [CrossRef]
- Spiegel, R.; DeVos, J. Central effects of guanfacine and clonidine during wakefulness and sleep in healthy subjects. Brit. J. Clin. Pharmacol. 1980, 10, 165S–168S. [Google Scholar] [CrossRef]
- Nicholson, A.; Pascoe, P.A. Presynaptic alpha2-adrenoceptor function and sleep in man: Studies with clonidine and idazoxan. Neuropharmacology 1991, 30, 367–372. [Google Scholar] [CrossRef]
- Gais, S.; Rasch, B.; Dahmen, J.C.; Sara, S.; Born, J. The memory function of noradrenergic activity in non-REM sleep. J. Cogn. Neurosci. 2011, 23, 2582–2592. [Google Scholar] [CrossRef]
- Nicholson, A.; Pascoe, P.A. Rapid Eye Movement Sleep and Sleep Continuity Depression and Antidepressants. Drugs 1989, 38, 4–13. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 2019, 102, 745–761. [Google Scholar] [CrossRef]
- Osorio-Forero, A.; Cardis, R.; Vantomme, G.; Guillaume-Gentil, A.; Katsioudi, G.; Devenoges, C.; Fernandez, L.M.; Lüthi, A. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 2021, 31, 5009–5023. [Google Scholar] [CrossRef]
- Kjaerby, C.; Andersen, M.; Hauglund, N.L.; Ding, F.; Wang, W.; Xu, Q.; Deng, S.; Kang, N.; Peng, S.; Sun, Q.; et al. Dynamic fluctuations of the locus coeruleus-norepinephrine system underlie sleep state transitions. BioRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Totah, N.K.; Neves, R.M.; Panzeri, S.; Logothetis, N.K.; Eschenko, O. The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 2018, 99, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Cardis, R.; Lecci, S.; Fernandez, L.M.; Osorio-Forero, A.; Chung, P.C.S.; Fulda, S.; Decosterd, I.; Lüthi, A. Cortico-autonomic local arousals and heightened somatosensory arousability during NREMS of mice in neuropathic pain. eLife 2021, 10, e65835. [Google Scholar] [CrossRef] [PubMed]
- Lecci, S.; Fernandez, L.M.; Weber, F.D.; Cardis, R.; Chatton, J.Y.; Born, J.; Lüthi, A. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 2017, 3, e1602026. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.O. Cognitive and physiologic impacts of the infraslow oscillation. Front. Syst. Neurosci. 2018, 44. [Google Scholar] [CrossRef]
- Fernandez, L.M.; Lüthi, A. Sleep spindles: Mechanisms and functions. Physiol. Rev. 2020, 100, 805–868. [Google Scholar] [CrossRef]
- Yüzgeç, Ö.; Prsa, M.; Zimmermann, R.; Huber, D. Pupil size coupling to cortical states protects the stability of deep sleep via parasympathetic modulation. Curr. Biol. 2018, 28, 392–400. [Google Scholar] [CrossRef]
- Glin, L.; Arnaud, C.; Berracochea, D.; Galey, D.; Jaffard, R.; Gottesmann, C. The intermediate stage of sleep in mice. Physiol. Behav. 1991, 50, 951–953. [Google Scholar] [CrossRef]
- Gottesmann, C.; Gandolfo, G.; Zernicki, B. Intermediate stage of sleep in the cat. J. Physiol. 1984, 79, 365–372. [Google Scholar]
- Aeschbach, D.; Borbély, A.A. All-night dynamics of the human sleep EEG. J. Sleep Res. 1993, 2, 70–81. [Google Scholar] [CrossRef]
- Purcell, S.; Manoach, D.; Demanuele, C.; Cade, B.; Mariani, S.; Cox, R.; Panagiotaropoulou, G.; Saxena, R.; Pan, J.; Smoller, J.; et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Comm. 2017, 8, 15930. [Google Scholar] [CrossRef]
- El Mansari, M.; Sakai, K.; Jouvet, M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp. Brain Res. 1989, 76, 519–529. [Google Scholar] [CrossRef]
- Steriade, M.; Datta, S.; Pare, D.; Oakson, G.; Dossi, R.C. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990, 10, 2541–2559. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.w.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The serotonergic raphe promote sleep in zebrafish and mice. Neuron 2019, 103, 686–701. [Google Scholar] [CrossRef]
- Wan, J.; Peng, W.; Li, X.; Qian, T.; Song, K.; Zeng, J.; Deng, F.; Hao, S.; Feng, J.; Zhang, P.; et al. A genetically encoded sensor for measuring serotonin dynamics. Nat. Neurosci. 2021, 24, 746–752. [Google Scholar] [CrossRef]
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T.; et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 2018, 174, 481–496. [Google Scholar] [CrossRef]
- Patriarchi, T.; Mohebi, A.; Sun, J.; Marley, A.; Liang, R.; Dong, C.; Puhger, K.; Mizuno, G.O.; Davis, C.M.; Wiltgen, B.; et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Meth. 2020, 17, 1147–1155. [Google Scholar] [CrossRef]
- Jing, M.; Li, Y.; Zeng, J.; Huang, P.; Skirzewski, M.; Kljakic, O.; Peng, W.; Qian, T.; Tan, K.; Zou, J.; et al. An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat. Meth. 2020, 17, 1139–1146. [Google Scholar] [CrossRef]
- Duffet, L.; Kosar, S.; Panniello, M.; Viberti, B.; Bracey, E.; Zych, A.D.; Radoux-Mergault, A.; Zhou, X.; Dernic, J.; Ravotto, L.; et al. A genetically encoded sensor for in vivo imaging of orexin neuropeptides. Nat. Meth. 2022, 19, 231–241. [Google Scholar] [CrossRef]
- Hasegawa, E.; Miyasaka, A.; Sakurai, K.; Cherasse, Y.; Li, Y.; Sakurai, T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science 2022, 375, 994–1000. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Bloom, F. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1981, 1, 887–900. [Google Scholar] [CrossRef]
- Takahashi, K.; Kayama, Y.; Lin, J.; Sakai, K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 2010, 169, 1115–1126. [Google Scholar] [CrossRef]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hopf, F.W.; Li, S.B.; de Lecea, L. In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness. Nat. Comm. 2018, 9, 5211. [Google Scholar] [CrossRef] [PubMed]
- Devilbiss, D.M.; Waterhouse, B.D. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J. Neurosci. 2004, 24, 10773–10785. [Google Scholar] [CrossRef] [PubMed]
- Manella, L.C.; Petersen, N.; Linster, C. Stimulation of the locus ceruleus modulates signal-to-noise ratio in the olfactory bulb. J. Neurosci. 2017, 37, 11605–11615. [Google Scholar] [CrossRef] [PubMed]
- Rodenkirch, C.; Liu, Y.; Schriver, B.J.; Wang, Q. Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci. 2019, 22, 120–133. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Chen, S.; Zhu, Y.; Oshinsky, M.L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 2001, 4, 732–738. [Google Scholar] [CrossRef]
- Park, S.H.; Weber, F. Neural and homeostatic regulation of REM sleep. Front. Psychol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Luppi, P.H.; Gervasoni, D.; Verret, L.; Goutagny, R.; Peyron, C.; Salvert, D.; Leger, L.; Fort, P. Paradoxical (REM) sleep genesis: The switch from an aminergic–cholinergic to a GABAergic–glutamatergic hypothesis. J. Physiol. Paris 2006, 100, 271–283. [Google Scholar] [CrossRef]
- Singh, S.; Mallick, B.N. Mild electrical stimulation of pontine tegmentum around locus coeruleus reduces rapid eye movement sleep in rats. Neurosci. Res. 1996, 24, 227–235. [Google Scholar] [CrossRef]
- Kaur, S.; Saxena, R.; Mallick, B.N. GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci. Lett. 1997, 223, 105–108. [Google Scholar] [CrossRef]
- Crochet, S.; Sakai, K. Effects of microdialysis application of monoamines on the EEG and behavioural states in the cat mesopontine tegmentum. Eur. J. Neurosci. 1999, 11, 3738–3752. [Google Scholar] [CrossRef]
- Cirelli, C.; Tononi, G.; Pompeiano, M.; Pompeiano, O.; Gennari, A. Modulation of desynchronized sleep through microinjection of α 1-adrenergic agonists and antagonists in the dorsal pontine tegmentum of the cat. Pflügers Arch. 1992, 422, 273–279. [Google Scholar] [CrossRef]
- Clément, O.; Valencia Garcia, S.; Libourel, P.A.; Arthaud, S.; Fort, P.; Luppi, P.H. The inhibition of the dorsal paragigantocellular reticular nucleus induces waking and the activation of all adrenergic and noradrenergic neurons: A combined pharmacological and functional neuroanatomical study. PLoS ONE 2014, 9, e96851. [Google Scholar] [CrossRef]
- Nitz, D.; Siegel, J. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience 1997, 78, 795–801. [Google Scholar] [CrossRef]
- Mallick, B.; Kaur, S.; Saxena, R. Interactions between cholinergic and GABAergic neurotransmitters in and around the locus coeruleus for the induction and maintenance of rapid eye movement sleep in rats. Neuroscience 2001, 104, 467–485. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Sur, M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 2019, 22, 218–228. [Google Scholar] [CrossRef]
- Weber, F.; Do, J.P.H.; Chung, S.; Beier, K.T.; Bikov, M.; Doost, M.S.; Dan, Y. Regulation of REM and non-REM sleep by periaqueductal GABAergic neurons. Nat. Comm. 2018, 9, 354. [Google Scholar] [CrossRef]
- Kaur, S.; Saxena, R.; Mallick, B.N. GABAergic neurons in prepositus hypoglossi regulate REM sleep by its action on locus coeruleus in freely moving rats. Synapse 2001, 42, 141–150. [Google Scholar] [CrossRef]
- Stucynski, J.A.; Schott, A.L.; Baik, J.; Chung, S.; Weber, F. Regulation of REM sleep by inhibitory neurons in the dorsomedial medulla. Curr. Biol. 2022, 32, 37–50. [Google Scholar] [CrossRef]
- Ennis, M.; Aston-Jones, G. Evidence for self-and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res. 1986, 374, 299–305. [Google Scholar] [CrossRef]
- Machida, M.; Sutton, A.M.; Williams, B.L.; Wellman, L.L.; Sanford, L.D. Differential behavioral, stress, and sleep responses in mice with different delays of fear extinction. Sleep 2019, 42, zsz147. [Google Scholar] [CrossRef]
- Boyce, R.; Glasgow, S.D.; Williams, S.; Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 2016, 352, 812–816. [Google Scholar] [CrossRef]
- Nollet, M.; Hicks, H.; McCarthy, A.P.; Wu, H.; Möller-Levet, C.S.; Laing, E.E.; Malki, K.; Lawless, N.; Wafford, K.A.; Dijk, D.J.; et al. REM sleep’s unique associations with corticosterone regulation, apoptotic pathways, and behavior in chronic stress in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 2733–2742. [Google Scholar] [CrossRef]
- Machida, M.; Wellman, L.L.; Fitzpatrick, B.; Mairen, E.; Hallum, B.; Sutton, B.; Amy, M.; Lonart, G.; Sanford, L.D. Effects of optogenetic inhibition of BLA on sleep brief optogenetic inhibition of the basolateral amygdala in mice alters effects of stressful experiences on rapid eye movement sleep. Sleep 2017, 40, zsx020. [Google Scholar] [CrossRef]
- Lo, Y.; Yi, P.L.; Hsiao, Y.T.; Chang, F.C. Hypocretin in locus coeruleus and dorsal raphe nucleus mediates inescapable footshock stimulation-induced REM sleep alteration. Sleep, 2022; in press. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Joshi, N.; Chandler, D. Persistent stress-induced neuroplastic changes in the locus coeruleus/norepinephrine system. Neural Plast. 2018, 2018, 1892570. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.M.; Geiller, T.; Losonczy, A. A role for the locus coeruleus in hippocampal CA1 place cell reorganization during spatial reward learning. Neuron 2020, 105, 1018–1026. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Okuyama, T.; Sun, C.; Smith, L.M.; Abe, K.; Tonegawa, S. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. USA 2018, 115, E310–E316. [Google Scholar] [CrossRef]
- Debiec, J.; LeDoux, J.E. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 521–524. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.J.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
- Glennon, E.; Carcea, I.; Martins, A.R.O.; Multani, J.; Shehu, I.; Svirsky, M.A.; Froemke, R.C. Locus coeruleus activation accelerates perceptual learning. Brain Res. 2019, 1709, 39–49. [Google Scholar] [CrossRef]
- Martins, A.R.O.; Froemke, R.C. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 2015, 18, 1483–1492. [Google Scholar] [CrossRef]
- Palacios-Filardo, J.; Mellor, J.R. Neuromodulation of hippocampal long-term synaptic plasticity. Curr. Opin. Neurobiol. 2019, 54, 37–43. [Google Scholar] [CrossRef]
- Bacon, T.J.; Pickering, A.E.; Mellor, J.R. Noradrenaline release from locus coeruleus terminals in the hippocampus enhances excitation-spike coupling in ca1 pyramidal neurons via β-adrenoceptors. Cereb. Cortex 2020, 30, 6135–6151. [Google Scholar] [CrossRef]
- Sara, S.J.; Roullet, P.; Przybyslawski, J. Consolidation of memory for odor–reward association: β-adrenergic receptor involvement in the late phase. Learn. Mem. 1999, 6, 88–96. [Google Scholar] [CrossRef]
- Tronel, S.; Feenstra, M.G.; Sara, S.J. Noradrenergic action in prefrontal cortex in the late stage of memory consolidation. Learn. Mem. 2004, 11, 453–458. [Google Scholar] [CrossRef]
- Novitskaya, Y.; Sara, S.J.; Logothetis, N.K.; Eschenko, O. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem. 2016, 23, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Locus coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 2015, 35, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, E.J.; Peoples, J.; Telegan, P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: Evidence for a monosynaptic pathway. J. Comp. Neurol. 1999, 412, 410–428. [Google Scholar] [CrossRef]
- Lopes, L.T.; Patrone, L.G.A.; Li, K.Y.; Imber, A.N.; Graham, C.D.; Gargaglioni, L.H.; Putnam, R.W. Anatomical and functional connections between the locus coeruleus and the nucleus tractus solitarius in neonatal rats. Neuroscience 2016, 324, 446–468. [Google Scholar] [CrossRef]
- Aston-Jones, G. The locus coeruleus, A5 and A7 noradrenergic cell groups. In The Rat Nervous System, 2nd ed.; Academic Press: Cambridge, MA, USA, 1994; Chapter 11; pp. 259–294. [Google Scholar]
- Ruggiero, D.A.; Pickel, V.M.; Milner, T.A.; Anwar, M.; Otake, K.; Mtui, E.P.; Park, D. Viscerosensory Processing in Nucleus Tractus Solitarii: Structural and Neurochemical Substrates. In Nucleus of the Solitary Tract; CRC Press: Boca Raton, FL, USA, 1994; Chapter 1; pp. 3–34. [Google Scholar]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A neural circuit for gut-induced reward. Cell 2018, 175, 665–678. [Google Scholar] [CrossRef]
- Childs, J.E.; Alvarez-Dieppa, A.C.; McIntyre, C.K.; Kroener, S. Vagus nerve stimulation as a tool to induce plasticity in pathways relevant for extinction learning. J. Vis. Exp. 2015, 102, e53032. [Google Scholar] [CrossRef]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299. [Google Scholar]
- Broncel, A.; Bocian, R.; Kłos-Wojtczak, P.; Kulbat-Warycha, K.; Konopacki, J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res. Bull. 2020, 155, 37–47. [Google Scholar] [CrossRef]
- Englot, D.J.; Rolston, J.D.; Wright, C.W.; Hassnain, K.H.; Chang, E.F. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery 2016, 79, 345–353. [Google Scholar] [CrossRef]
- Schachter, S.C.; Saper, C.B. Vagus nerve stimulation. Epilepsia 1998, 39, 677–686. [Google Scholar] [CrossRef]
- Berger, A.; Vespa, S.; Dricot, L.; Dumoulin, M.; Iachim, E.; Doguet, P.; Vandewalle, G.; El Tahry, R. How is the norepinephrine system involved in the antiepileptic effects of Vagus Nerve Stimulation (VNS)? Front. Neurosci. 2021. [Google Scholar] [CrossRef]
- Rush, A.J.; George, M.S.; Sackeim, H.A.; Marangell, L.B.; Husain, M.M.; Giller, C.; Nahas, Z.; Haines, S.; Simpson, R.K., Jr.; Goodman, R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol. Psych. 2000, 47, 276–286. [Google Scholar] [CrossRef]
- Austelle, C.W.; O’Leary, G.H.; Thompson, S.; Gruber, E.; Kahn, A.; Manett, A.J.; Short, B.; Badran, B.W. A comprehensive review of vagus nerve stimulation for depression. Neuromodulation Technol. Neural Interface 2022, 25, 309–315. [Google Scholar] [CrossRef]
- Faris, P.L.; Hofbauer, R.D.; Daughters, R.; VandenLangenberg, E.; Iversen, L.; Goodale, R.L.; Maxwell, R.; Eckert, E.D.; Hartman, B.K. Destabilization of the positive vago-vagal reflex in bulimia nervosa. Physiol. Behav. 2008, 94, 136–153. [Google Scholar] [CrossRef][Green Version]
- Sjogren, M.J.; Hellstrom, P.T.; Jonsson, M.A.; Runnerstam, M.; Hans, C.; Ben-Menachem, E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: A pilot study. J. Clin. Psychiatry 2002, 63, 3113. [Google Scholar] [CrossRef]
- Gieroba, Z.; Blessing, W. Fos-containing neurons in medulla and pons after unilateral stimulation of the afferent abdominal vagus in conscious rabbits. Neuroscience 1994, 59, 851–858. [Google Scholar] [CrossRef]
- Naritoku, D.K.; Terry, W.J.; Helfert, R.H. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995, 22, 53–62. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B.; Smith, D.C.; Browning, R.A. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 1998, 39, 709–714. [Google Scholar] [CrossRef]
- Takigawa, M.; Mogenson, G. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977, 135, 217–230. [Google Scholar] [CrossRef]
- Groves, D.A.; Bowman, E.M.; Brown, V.J. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci. Lett. 2005, 379, 174–179. [Google Scholar] [CrossRef]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker II, R.L.; Kilgard, M.P.; Hays, S.A. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef]
- Dorr, A.E.; Debonnel, G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 2006, 318, 890–898. [Google Scholar] [CrossRef]
- Collins, L.; Boddington, L.; Steffan, P.J.; McCormick, D. Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr. Biol. 2021, 31, 2088–2098. [Google Scholar] [CrossRef]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef]
- Manta, S.; Dong, J.; Debonnel, G.; Blier, P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psych. Neurosci. 2009, 34, 272. [Google Scholar]
- Manta, S.; El Mansari, M.; Debonnel, G.; Blier, P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int. J. Neuropsychopharm. 2013, 16, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Bianca, R.; Komisaruk, B.R. Pupil dilatation in response to vagal afferent electrical stimulation is mediated by inhibition of parasympathetic outflow in the rat. Brain Res. 2007, 1177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mridha, Z.; de Gee, J.W.; Shi, Y.; Alkashgari, R.; Williams, J.; Suminski, A.; Ward, M.P.; Zhang, W.; McGinley, M.J. Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat. Comm. 2021, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, V.D.; Lespérance, P.; Nguyen, D.K.; Fournier-Gosselin, M.P.; Richer, F. Effects of vagus nerve stimulation on pupillary function. Int. J. Psychophys. 2015, 98, 455–459. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous vagus nerve stimulation in humans induces pupil dilation and attenuates alpha oscillations. J. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef]
- Fernández-Guardiola, A.; Martinez, A.; Valdés-Cruz, A.; Magdaleno-Madrigal, V.; Martinez, D.; Fernández-Mas, R. Vagus nerve prolonged stimulation in cats: Effects on epileptogenesis (amygdala electrical kindling): Behavioral and electrographic changes. Epilepsia 1999, 40, 822–829. [Google Scholar] [CrossRef]
- Valdés-Cruz, A.; Magdaleno-Madrigal, V.M.; Martınez-Vargas, D.; Fernández-Mas, R.; Almazán-Alvarado, S.; Martınez, A.; Fernández-Guardiola, A. Chronic stimulation of the cat vagus nerve: Effect on sleep and behavior. Prog. Neuro-Psychopharm. Biol. Psych. 2002, 26, 113–118. [Google Scholar] [CrossRef]
- Valdés-Cruz, A.; Magdaleno-Madrigal, V.M.; Martínez-Vargas, D.; Fernández-Mas, R.; Almazán-Alvarado, S. Long-term changes in sleep and electroencephalographic activity by chronic vagus nerve stimulation in cats. Prog. Neuro-Psychopharm. Biol. Psych. 2008, 32, 828–834. [Google Scholar] [CrossRef]
- Malow, B.A.; Edwards, J.; Marzec, M.; Sagher, O.; Ross, D.; Fromes, G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology 2001, 57, 879–884. [Google Scholar] [CrossRef]
- Galli, R.; Bonanni, E.; Pizzanelli, C.; Maestri, M.; Lutzemberger, L.; Giorgi, F.S.; Iudice, A.; Murri, L. Daytime vigilance and quality of life in epileptic patients treated with vagus nerve stimulation. Epil. Behav. 2003, 4, 185–191. [Google Scholar] [CrossRef]
- Armitage, R.; Husain, M.; Hoffmann, R.; Rush, A.J. The effects of vagus nerve stimulation on sleep EEG in depression: A preliminary report. J. Psychosom. Res. 2003, 54, 475–482. [Google Scholar] [CrossRef]
- Rizzo, P.; Beelke, M.; De Carli, F.; Canovaro, P.; Nobili, L.; Robert, A.; Tanganelli, P.; Regesta, G.; Ferrillo, F. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep 2003, 26, 607–611. [Google Scholar] [CrossRef]
- Rizzo, P.; Beelke, M.; De Carli, F.; Canovaro, P.; Nobili, L.; Robert, A.; Fornaro, P.; Tanganelli, P.; Regesta, G.; Ferrillo, F. Modifications of sleep EEG induced by chronic vagus nerve stimulation in patients affected by refractory epilepsy. Clin. Neurophysiol. 2004, 115, 658–664. [Google Scholar] [CrossRef]
- Hallböök, T.; Lundgren, J.; Stjernqvist, K.; Blennow, G.; Strömblad, L.G.; Rosén, I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; Its impact on cognition, quality of life, behaviour and mood. Seizure 2005, 14, 504–513. [Google Scholar] [CrossRef]
- Ravan, M.; Begnaud, J. Investigating the effect of short term responsive VNS therapy on sleep quality using automatic sleep staging. IEEE Trans. Biomed. Eng. 2019, 66, 3301–3309. [Google Scholar] [CrossRef]
- Al-Biltagi, M.A. Childhood epilepsy and sleep. World J. Clin. Pediatr. 2014, 3, 45. [Google Scholar] [CrossRef]
- Cherrad, N.; Cardis, R.; Osorio-Forero, A.; Arnold, M.; Fernandez, L.M.J.; Lüthi, A. Chemogenetic activation of VGLUT2 neurons in the left nodose ganglion of the vagal nerve suppresses rapid-eye-movement sleep in mouse [Abstract]. J. Sleep Res. 2020, 29, e13181. [Google Scholar]
- Giorgi, F.S.; Galgani, A.; Puglisi-Allegra, S.; Limanaqi, F.; Busceti, C.L.; Fornai, F. Locus Coeruleus and neurovascular unit: From its role in physiology to its potential role in Alzheimer’s disease pathogenesis. J. Neurosci. Res. 2020, 98, 2406–2434. [Google Scholar] [CrossRef] [PubMed]
- Bekar, L.K.; Wei, H.S.; Nedergaard, M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J. Cereb. Blood Flow Metab. 2012, 32, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Toussay, X.; Basu, K.; Lacoste, B.; Hamel, E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci. 2013, 33, 3390–3401. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.; Zeppenfeld, D.; McConnell, E.; Pena, S.; Nedergaard, M. Norepinephrine: A neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 2012, 37, 2496–2512. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Hou, S.S.; Calvo-Rodriguez, M.; Arbel-Ornath, M.; Snyder, A.C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 2020, 105, 549–561. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Fukunaga, M.; de Zwart, J.A.; van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum. Brain Mapp. 2008, 29, 671–682. [Google Scholar] [CrossRef]
- Fukunaga, M.; Horovitz, S.G.; van Gelderen, P.; de Zwart, J.A.; Jansma, J.M.; Ikonomidou, V.N.; Chu, R.; Deckers, R.H.; Leopold, D.A.; Duyn, J.H. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn. Res. Imag. 2006, 24, 979–992. [Google Scholar] [CrossRef]
- Larson-Prior, L.J.; Zempel, J.M.; Nolan, T.S.; Prior, F.W.; Snyder, A.Z.; Raichle, M.E. Cortical network functional connectivity in the descent to sleep. Proc. Natl. Acad. Sci. USA 2009, 106, 4489–4494. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and human aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Gagnon, J.F.; Lafrenière, A.; Rauchs, G.; Petit, D.; Carrier, J. Sleep in normal aging, Alzheimer’s disease, and mild cognitive impairment. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 677–692. [Google Scholar]
- Li, S.B.; Damonte, V.M.; Chen, C.; Wang, G.X.; Kebschull, J.M.; Yamaguchi, H.; Bian, W.J.; Purmann, C.; Pattni, R.; Urban, A.E.; et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science 2022, 375, eabh3021. [Google Scholar] [CrossRef]
- Lew, C.H.; Petersen, C.; Neylan, T.C.; Grinberg, L.T. Tau-driven degeneration of sleep-and wake-regulating neurons in Alzheimer’s disease. Sleep Med. Rev. 2021, 60, 101541. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef]
- Jacobs, H.I.; Becker, J.A.; Kwong, K.; Engels-Domínguez, N.; Prokopiou, P.C.; Papp, K.V.; Properzi, M.; Hampton, O.L.; d’Oleire Uquillas, F.; Sanchez, J.S.; et al. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci. Transl. Med. 2021, 13, eabj2511. [Google Scholar] [CrossRef]
- Rorabaugh, J.M.; Chalermpalanupap, T.; Botz-Zapp, C.A.; Fu, V.M.; Lembeck, N.A.; Cohen, R.M.; Weinshenker, D. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 2017, 140, 3023–3038. [Google Scholar] [CrossRef]
- Chylinski, D.O.; Van Egroo, M.; Narbutas, J.; Grignard, M.; Koshmanova, E.; Berthomier, C.; Berthomier, P.; Brandewinder, M.; Salmon, E.; Bahri, M.A.; et al. Heterogeneity in the links between sleep arousals, amyloid-β, and cognition. JCI Insight 2021, 6, e152858. [Google Scholar] [CrossRef]
- Cano, G.; Mochizuki, T.; Saper, C.B. Neural circuitry of stress-induced insomnia in rats. J. Neurosci. 2008, 28, 10167–10184. [Google Scholar] [CrossRef]
- Berridge, C.W.; Foote, S.L. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J. Neurosci. 1991, 11, 3135–3145. [Google Scholar] [CrossRef]
- Marzo, A.; Totah, N.K.; Neves, R.M.; Logothetis, N.K.; Eschenko, O. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J. Neurophysiol. 2014, 111, 2570–2588. [Google Scholar] [CrossRef]
- Vazey, E.M.; Aston-Jones, G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA 2014, 111, 3859–3864. [Google Scholar] [CrossRef]
- Ploner, M.; Sorg, C.; Gross, J. Brain rhythms of pain. Trends Cogn. Sci. 2017, 21, 100–110. [Google Scholar] [CrossRef]
- Van Someren, E.J. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Kobayashi, I.; Boarts, J.M.; Delahanty, D.L. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiol. 2007, 44, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Wassing, R.; Lakbila-Kamal, O.; Ramautar, J.R.; Stoffers, D.; Schalkwijk, F.; Van Someren, E.J. Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol. 2019, 29, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- de Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Dampney, R.A. Central control of cardiovascular function during sleep. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1683–H1692. [Google Scholar] [CrossRef]
- Chouchou, F.; Desseilles, M. Heart rate variability: A tool to explore the sleeping brain? Front. Neurosci. 2014, 8, 402. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Forero, A.; Cherrad, N.; Banterle, L.; Fernandez, L.M.J.; Lüthi, A. When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives. Int. J. Mol. Sci. 2022, 23, 5028. https://doi.org/10.3390/ijms23095028
Osorio-Forero A, Cherrad N, Banterle L, Fernandez LMJ, Lüthi A. When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives. International Journal of Molecular Sciences. 2022; 23(9):5028. https://doi.org/10.3390/ijms23095028
Chicago/Turabian StyleOsorio-Forero, Alejandro, Najma Cherrad, Lila Banterle, Laura M. J. Fernandez, and Anita Lüthi. 2022. "When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives" International Journal of Molecular Sciences 23, no. 9: 5028. https://doi.org/10.3390/ijms23095028
APA StyleOsorio-Forero, A., Cherrad, N., Banterle, L., Fernandez, L. M. J., & Lüthi, A. (2022). When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives. International Journal of Molecular Sciences, 23(9), 5028. https://doi.org/10.3390/ijms23095028






