Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development
Abstract
:1. Introduction
2. Gibberellins in the Development of Stem/Root Tubers
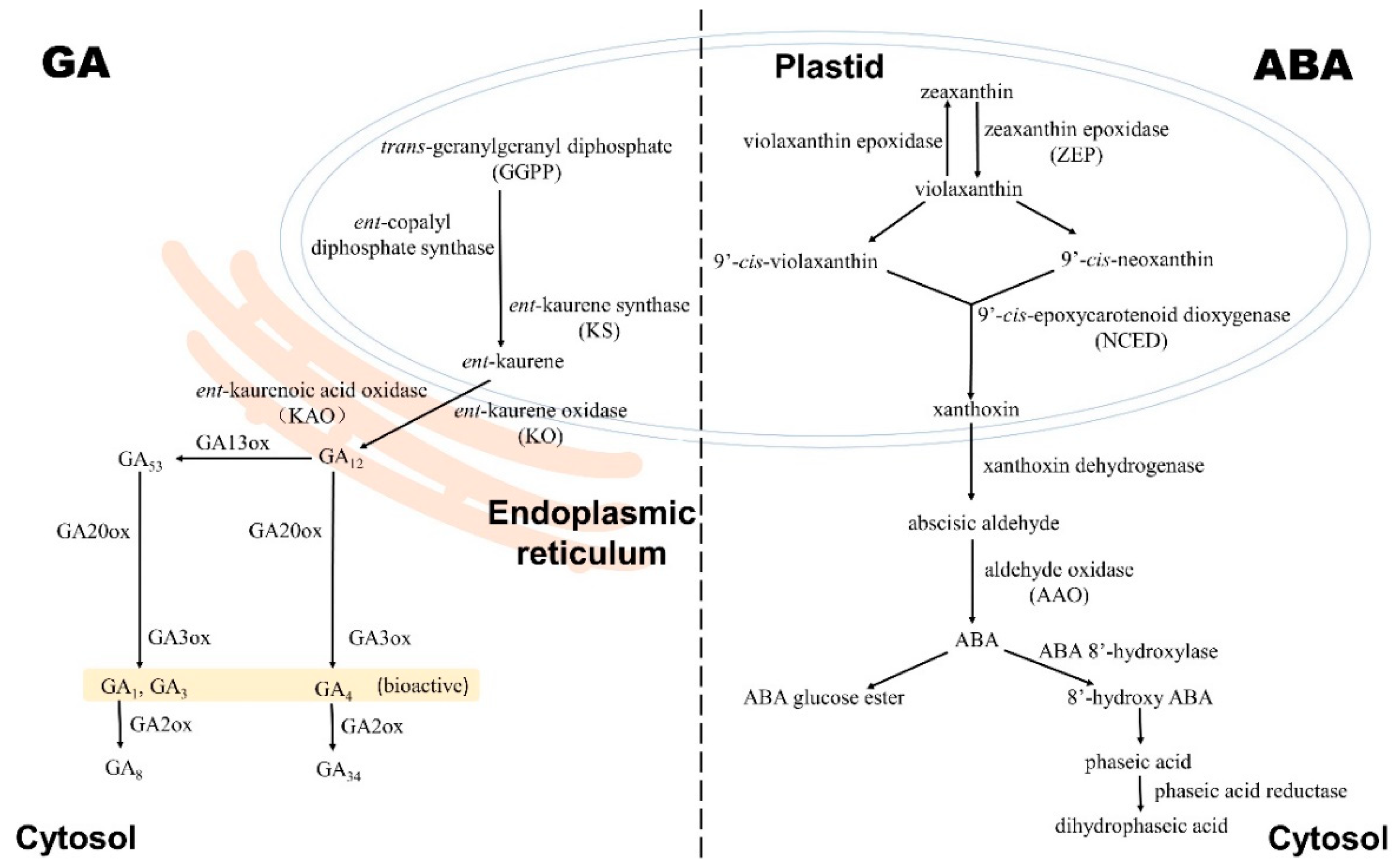
2.1. Gibberellin Biosynthesis and Deactivation
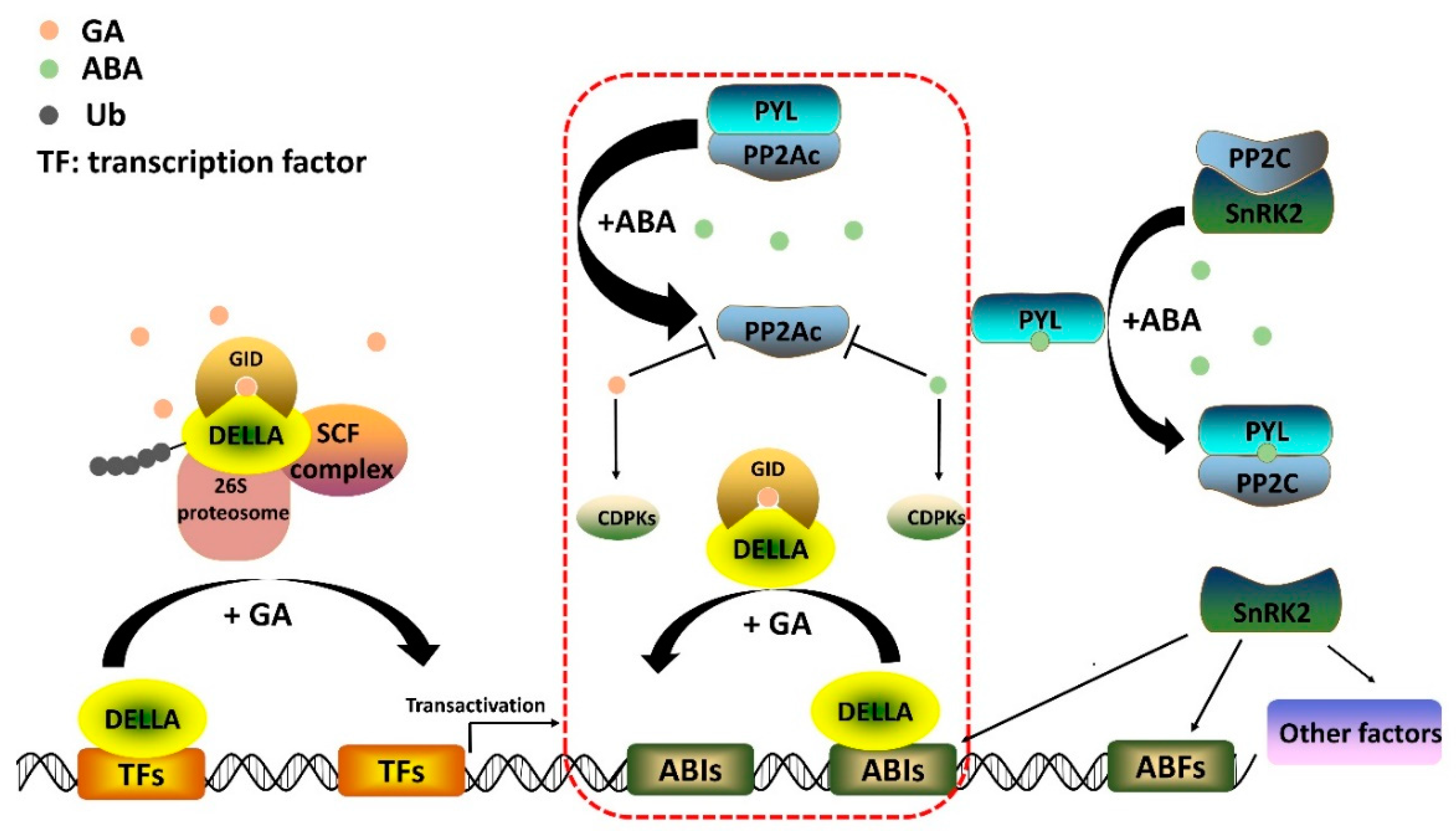
2.2. Gibberellin Signaling Pathway
3. Abscisic Acid in the Development of Stem/Root Tubers
3.1. ABA Biosynthesis, Catabolism, and Conjunction
3.2. Core ABA Signaling Pathway
4. Conclusions: The Antagonistic Role of GA and ABA in the Development of Stem/Root Tubers
| Species | Storage Organ | GA-and ABA-Related Gene Expression | GA and ABA Profile | Ref. |
|---|---|---|---|---|
| Potato (Solanum tuberosum) | Tuber (developing from the stolon) | GA20ox1: Expressed mainly in leaves. GA2ox1: Upregulated in the subapical zone of the stolon and growing tuber before tuber expansion. CDPK: Mainly located in the plasma membrane of swelling stolons and sprouting tubers. GA3ox2: Increased in the aerial parts and repressed in the stolons under the short-day condition. | GA3: The content in the tuber dropped by around 30%. | [18,29,30,31,32,33,34,41,42,43,44,45,49,50,86,98] |
| ABA: Around 30% increase detected in the tuber. | [18,41,61,78,86,87,88] | |||
| Chinese arrowhead Sagittaria trifolia | Corm (developing from the tips of the stolon) | KS and KO: Enhanced during stolon elongation and decreased when the corm swells. CDPK: Upregulated. | [28] | |
| ABA 8-hydroxylase: Decreased at the initial swelling stage. | [28] | |||
| Lotus (Nelumbo nucifera) | Rhizome (developing from the stem) | GAI: Downregulated during rhizome development. | [42] | |
| ABFs, PP2C, PYL, and SnRK2: Different expression patterns, with some upregulated, some downregulated, and two PP2Cs expressed high in the middle stage. | [42] | |||
| Yams (Dioscorea opposita) | Underground tuber (developing from the hypocotyl); and aerial tubers (bulbils) | GA20ox1, GA3ox1, and four GA2oxs: Significantly abundant in the early expansion stage and gradually declined along with tuber growth. Three GIDs and three DELLAs: Different expression patterns in the early expansion stage and gradually declined along with tuber growth. | GA3 and GA4: Reached a peak of around 150 ng/g at 90 days after field planting and then decreased. | [23,97] |
| NCED, ZEP, and ABF: Increased at the final stage. PP2Cs: Upregulated in the early microtuber formation stage. | ABA (during tuber development): Reached a maximum of over 600 ng/g at 90 days after field planting and then decreased. ABA (in bulbil tissues): Continuously increased by around 22-fold. | [24,73,97] | ||
| Turnip (Brassica rapa var. rapa) | Taproot (developing from the hypocotyl and a part of the root) | GA20ox, GA3ox, and GA13ox: Increased in the early growth stage but declined during the late developmental stage. KS: Decreased. GA2oxs (except for GA2ox8-4): Downregulated. | GA: Most abundant active GA is GA3, and all the active GAs decreased by more than 50%. | [35] |
| Sugar beet (Beta vulgaris L.) | Taproot | GA20-ox and GA3-ox: Preferentially expressed during late development. GA2-ox: Preferentially expressed during early development. | GA/ABA ratio: Decreased from 1358.5 to 18.8. | [54,55,56] |
| ZEP: Preferentially expressed during late development. | [55,56] | |||
| Carrot (Daucus carota L.) | Taproot | GA20oxs and GA3oxs: Different expression patterns, with some upregulated in the middle stage and then downregulated and some downregulated from the early development stage. GA2ox, KO, and KS: Upregulated and then downregulated. KAO: Downregulated and then upregulated. | [36,37,38] | |
| NCED and AAO: Upregulated in the final growth stage. ABAH, PYL and SnRK2: Decreased. PP2c: Upregulated in the early stage and then downregulated. | [36] | |||
| Radish (Raphanus sativus L.) | Taproot | PLYs and PP2Cs: Upregulated. | [74] | |
| San qi (Panax notoginseng) | Taproot | GAI and GID: Downregulated and then upregulated. | [80] | |
| PYL: Downregulated and then upregulated. PP2Cs: Different expression patterns, with some downregulated and then upregulated and some upregulated at the final stage. Most of SnRK2: Upregulated. | [80] | |||
| Sweet potato (Ipomoea batatas) | Tuberous root (developing from the adventitious root) | GA3ox: Downregulated. | GAs: Around 2.5-fold decrease in the storage root. | [51,52,70] |
| AAO: Enhanced in the early stage and then sharply decreased until the final stage. | ABA: Increased to 6.5 nmol/g in the early stage and finally decreased to around 3 nmol/g. | [70,81] | ||
| Cassava (Manihot esculenta) | Tuberous root | KS: Mainly detected in the cortex and parenchyma of fibrous root and significantly downregulated. CDPK1: Upregulated during the initial stage and gradually downregulated. | ABA: Decreased in fibrous roots compared with the pretuberous roots. | [27,92] |
| Chinese foxglove Rehmannia glutinosa | Tuberous root | GA20ox and GA 3-beta-dioxygenase: Downregulated. Most of GA 2-beta-dioxygenase: Upregulated and then downregulated. | GA: Decreased by approximately 30%. | [33,75] |
| ZEP, AAO, PYL, and most of the NCEDs: Upregulated. Most of ABA 8′-hydroxylase 3: Downregulated. | ABA: Increased 2-fold. | [33,75] | ||
| Tai zi shen Pseudostellaria heterophylla | Tuberous root | ZEP: Upregulated. | [69] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | Aldehyde oxidase |
| ABA | Abscisic acid |
| ABEBs/ABFs | ABA-responsive element binding proteins/factors |
| ABI5 | ABA INSENSITIVE 5 |
| bZIP | Basic leucine zipper domain |
| CDPKs | Ca2+-dependent protein kinases |
| GA | Gibberellin |
| GGPP | trans-Geranylgeranyl diphosphate |
| GID1 | Gibberellin-insensitive dwarf 1 |
| KAO | ent-Kaurenoic acid oxidase |
| KO | ent-Kaurene oxidase |
| KS | ent-Kaurene synthase |
| NCEDs | 9′-cis-Epoxycarotenoid dioxygenases |
| ODD | Oxoglutarate-dependent dioxygenase |
| PP2C | Clade A protein phosphatases of type 2C |
| PYL | Pyrabactin resistance-like protein |
| SLY1 | F-box proteins SLEEPY1 |
| SnRK2 | Sucrose non-fermenting-1 (SNF1)-related protein kinase 2 |
| ZEP | Zeaxanthin epoxidase |
References
- Zierer, W.; Ruscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and Tuberous Root Development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wei, X.; Qi, Q.; Jia, W.; Zhao, M.; Wang, H.; Zhou, Y.; Duan, H. Study of Terpenoid Synthesis and Prenyltransferase in Roots of Rehmannia glutinosa Based on iTRAQ Quantitative Proteomics. Front. Plant Sci. 2021, 12, 693758. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2021, 56, 1093–1114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, J.; Zhang, P.; He, S. Root and Tuber Crops. In Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, MA, USA, 2014; pp. 46–61. [Google Scholar]
- Villordon, A.Q.; Ginzberg, I.; Firon, N. Root architecture and root and tuber crop productivity. Trends Plant Sci. 2014, 19, 419–425. [Google Scholar] [CrossRef]
- Padhan, B.; Panda, D. Potential of Neglected and Underutilized Yams (Dioscorea spp.) for Improving Nutritional Security and Health Benefits. Front. Pharmacol. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, G.; Wang, X.; Ni, G.; Cui, Z.; Yan, Z. Sagittaria trifolia tuber: Bioconstituents, processing, products, and health benefits. J. Sci. Food Agric. 2021, 101, 3085–3098. [Google Scholar] [CrossRef]
- Caetano, B.F.; de Moura, N.A.; Almeida, A.P.; Dias, M.C.; Sivieri, K.; Barbisan, L.F. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 436. [Google Scholar] [CrossRef]
- Yu, B.; Chen, M.; Grin, I.; Ma, C. Mechanisms of Sugar Beet Response to Biotic and Abiotic Stresses. Adv. Exp. Med. Biol. 2020, 1241, 167–194. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wojciechowski, T. Root systems of major tropical root and tuber crops: Root architecture, size, and growth and initiation of storage organs. Adv. Agron. 2020, 161, 1–25. [Google Scholar]
- Kondhare, K.R.; Natarajan, B.; Banerjee, A.K. Molecular signals that govern tuber development in potato. Int. J. Dev. Biol. 2020, 64, 133–140. [Google Scholar] [CrossRef]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop-Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, S.; Hameed, S.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Integrated mRNA and miRNA transcriptome analysis reveals a regulatory network for tuber expansion in Chinese yam (Dioscorea opposita). BMC Genom. 2020, 21, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, E.E.; Struik, P.C. Tuber formation in the potato: Induction, initiation and growth. In Horticultural Reviews; Janick, J., Ed.; Wiley: New York, NY, USA, 1992; Volume 14, pp. 89–198. [Google Scholar]
- Liu, L.; Huang, Y.; Huang, X.; Yang, J.; Wu, W.; Xu, Y.; Cong, Z.; Xie, J.; Xia, W.; Huang, D. Characterization of the Dioscorin Gene Family in Dioscorea alata Reveals a Role in Tuber Development and Environmental Response. Int. J. Mol. Sci. 2017, 18, 1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Kohlen, W.; Bouwmeester, H.J.; Visser, R.G.; Bachem, C.W. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; van Lammeren, A.A.; Vermeer, E.; Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Cerezo, S.; Martinez-Montiel, N.; Garcia-Sanchez, J.; Perez, Y.T.R.; Martinez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, Y.; Gong, M.; Qin, F.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Regulatory mechanism of GA3 on tuber growth by DELLA-dependent pathway in yam (Dioscorea opposita). Plant Mol. Biol. 2021, 106, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.G.; Jiang, W.; Tao, Z.M.; Pan, X.J.; Yu, W.H.; Huang, H.L. Morphological and stage-specific transcriptome analyses reveal distinct regulatory programs underlying yam (Dioscorea alata L.) bulbil growth. J. Exp. Bot. 2020, 71, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.; Davière, J.M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Lopez Diaz, I.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants 2015, 1, 15073. [Google Scholar] [CrossRef] [PubMed]
- Sojikul, P.; Kongsawadworakul, P.; Viboonjun, U.; Thaiprasit, J.; Intawong, B.; Narangajavana, J.; Svasti, M.R. AFLP-based transcript profiling for cassava genome-wide expression analysis in the onset of storage root formation. Physiol. Plant 2010, 140, 189–198. [Google Scholar] [CrossRef]
- Cheng, L.; Li, S.; Xu, X.; Hussain, J.; Yin, J.; Zhang, Y.; Li, L.; Chen, X. Identification of differentially expressed genes relevant to corm formation in Sagittaria trifolia. PLoS ONE 2013, 8, e54573. [Google Scholar] [CrossRef]
- Jackson, S.D.; James, P.E.; Carrera, E.; Prat, S.; Thomas, B. Regulation of transcript levels of a potato gibberellin 20-oxidase gene by light and phytochrome B. Plant Physiol. 2000, 124, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Carrera, E.; Bou, J.; García-Martínez, J.L.; Prat, S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 2000, 22, 247–256. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Lange, T.; Visser, R.G.; Bachem, C.W. Down regulation of StGA3ox genes in potato results in altered GA content and affect plant and tuber growth characteristics. J. Plant Physiol. 2013, 170, 1228–1234. [Google Scholar] [CrossRef]
- Bou-Torrent, J.; Martinez-Garcia, J.F.; Garcia-Martinez, J.L.; Prat, S. Gibberellin A1 metabolism contributes to the control of photoperiod-mediated tuberization in potato. PLoS ONE 2011, 6, e24458. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Xiao, X.; Duan, L.; Guo, Y.; Qi, J.; Liao, D.; Zhao, C.; Liu, Y.; Zhou, L.; Li, X. Dynamic transcriptional profiling provides insights into tuberous root development in Rehmannia glutinosa. Front. Plant Sci. 2015, 6, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloosterman, B.; Navarro, C.; Bijsterbosch, G.; Lange, T.; Prat, S.; Visser, R.G.; Bachem, C.W. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J. 2007, 52, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, J.; Ke, X.; Zhang, J.; Sun, X.; Wang, C.; Yang, Y. Gibberellin inhibition of taproot formation by modulation of DELLA-NAC complex activity in turnip (Brassica rapa var. rapa). Protoplasma 2021, 258, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jia, X.L.; Xu, Z.S.; Wang, F.; Xiong, A.S. Sequencing, assembly, annotation, and gene expression: Novel insights into the hormonal control of carrot root development revealed by a high-throughput transcriptome. Mol. Genet. Genom. 2015, 290, 1379–1391. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 2017, 254, 839–848. [Google Scholar] [CrossRef]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Daviere, J.M.; Achard, P. A Pivotal Role of DELLAs in Regulating Multiple Hormone Signals. Mol. Plant 2016, 9, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Mao, Z.; Wang, Q.; Song, J.; Nie, X.; Wang, T.; Zhang, H.; Guo, H. Comprehensive transcriptome profiling and phenotyping of rootstock and scion in a tomato/potato heterografting system. Physiol. Plant 2019, 166, 833–847. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Pan, C.; Xu, L.; Liu, Y.; Ke, W.; Yang, P. Transcriptomic Analysis of the Regulation of Rhizome Formation in Temperate and Tropical Lotus (Nelumbo nucifera). Sci. Rep. 2015, 5, 13059. [Google Scholar] [CrossRef]
- Cao, D.; Lin, Z.; Huang, L.; Damaris, R.N.; Li, M.; Yang, P. A CONSTANS-LIKE gene of Nelumbo nucifera could promote potato tuberization. Planta 2021, 253, 65. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Mori, M.; Watanabe, S.; Miyamoto, C.; Ito, T.; Takahashi, Y. DELLA-GAF1 Complex Is a Main Component in Gibberellin Feedback Regulation of GA20 Oxidase 2. Plant Physiol. 2017, 175, 1395–1406. [Google Scholar] [CrossRef] [Green Version]
- Carrera, E.; Jackson, S.D.; Prat, S. Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol. 1999, 119, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Okada, K.; Fukazawa, J.; Takahashi, Y. DELLA-dependent and -independent gibberellin signaling. Plant Signal. Behav. 2018, 13, e1445933. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New insights into gibberellin signaling in regulating plant growth-metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef]
- Gilroy, S.; Jones, R.L. Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc. Natl. Acad. Sci. USA 1992, 89, 3591–3595. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wang, Y.; Liu, Y.; Zhang, Q.; Gao, H.; Zhang, F. Comparative proteomics illustrates the molecular mechanism of potato (Solanum tuberosum L.) tuberization inhibited by exogenous gibberellins in vitro. Physiol. Plant 2018, 163, 103–123. [Google Scholar] [CrossRef]
- Gargantini, P.R.; Giammaria, V.; Grandellis, C.; Feingold, S.E.; Maldonado, S.; Ulloa, R.M. Genomic and functional characterization of StCDPK1. Plant Mol. Biol. 2009, 70, 153–172. [Google Scholar] [CrossRef]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin Promotes Sweetpotato Root Vascular Lignification and Reduces Storage-Root Formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Firon, N.; LaBonte, D.; Villordon, A.; Kfir, Y.; Solis, J.; Lapis, E.; Perlman, T.S.; Doron-Faigenboim, A.; Hetzroni, A.; Althan, L.; et al. Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genom. 2013, 14, 460. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Huang, Y.; Zhang, X.Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Transcriptome-based identification of genes revealed differential expression profiles and lignin accumulation during root development in cultivated and wild carrots. Plant Cell Rep. 2016, 35, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Li, G.L.; Wang, X.F.; Sun, Y.Q.; Zhang, S.Y. Transcriptomic profiling of taproot growth and sucrose accumulation in sugar beet (Beta vulgaris L.) at different developmental stages. PLoS ONE 2017, 12, e0175454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellin, D.; Schulz, B.; Soerensen, T.R.; Salamini, F.; Schneider, K. Transcript profiles at different growth stages and tap-root zones identify correlated developmental and metabolic pathways of sugar beet. J. Exp. Bot. 2007, 58, 699–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozolina, N.V.; Pradedova, E.V.; Saliaev, R.K. The dynamics of hormonal status of developing red beet root (Beta vulgaris L.) in correlation with the dynamics of sugar accumulation. Biol. Bull. 2005, 32, 22–26. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA-Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]
- Simko, I.; McMurry, S.; Yang, H.M.; Manschot, A.; Davies, P.J.; Ewing, E.E. Evidence from Polygene Mapping for a Causal Relationship between Potato Tuber Dormancy and Abscisic Acid Content. Plant Physiol. 1997, 115, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Da Ros, L.; Elferjani, R.; Soolanayakanahally, R.; Kagale, S.; Pahari, S.; Kulkarni, M.; Wahab, J.; Bizimungu, B. Drought-Induced Regulatory Cascades and Their Effects on the Nutritional Quality of Developing Potato Tubers. Genes 2020, 11, 864. [Google Scholar] [CrossRef]
- Vreugdenhil, D.; Bindels, P.; Reinhoud, P.; Hendriks, K.T. Use of the growth retardant tetcyclacis for potato tuber formation in vitro. Plant Growth Regul. 1994, 14, 257–265. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [Green Version]
- Sano, N.; Marion-Poll, A. ABA Metabolism and Homeostasis in Seed Dormancy and Germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular Mechanism for the Regulation of ABA Homeostasis During Plant Development and Stress Responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destefano-Beltran, L.; Knauber, D.; Huckle, L.; Suttle, J.C. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol. Biol. 2006, 61, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Destefano-Beltran, L.; Knauber, D.; Huckle, L.; Suttle, J. Chemically forced dormancy termination mimics natural dormancy progression in potato tuber meristems by reducing ABA content and modifying expression of genes involved in regulating ABA synthesis and metabolism. J. Exp. Bot. 2006, 57, 2879–2886. [Google Scholar] [CrossRef] [Green Version]
- Römer, S.; Lübeck, J.; Kauder, F.; Steiger, S.; Adomat, C.; Sandmann, G. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab. Eng. 2002, 4, 263–272. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, T.; Li, J.; Long, D.K.; Jiang, W.K.; Ding, L. Cloning and expression analysis on full length CDS of zeaxanthin epoxidase gene in Pseudostellaria heterophylla. Zhongguo Zhong Yao Za Zhi 2017, 42, 669–674. [Google Scholar] [CrossRef]
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef] [Green Version]
- Suttle, J.C.; Abrams, S.R.; De Stefano-Beltran, L.; Huckle, L.L. Chemical inhibition of potato ABA-8′-hydroxylase activity alters in vitro and in vivo ABA metabolism and endogenous ABA levels but does not affect potato microtuber dormancy duration. J. Exp. Bot. 2012, 63, 5717–5725. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, C.; Peng, J.; Li, C.; Wan, F.; Zhang, S.; Zhou, Y.; Yan, Y.; Qi, L.; Sun, K.; et al. ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol. 2019, 221, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, X.; Dong, Y.; Li, S.; Yuan, J.; Li, C.; Zhang, X.; Li, M. Transcriptome Analysis Reveals Key Pathways and Hormone Activities Involved in Early Microtuber Formation of Dioscorea opposita. Biomed. Res. Int. 2020, 2020, 8057929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Xu, L.; Wang, Y.; Fan, L.; Chen, Y.; Tang, M.; Luo, X.; Liu, L. Comparative proteomic analysis provides insight into a complex regulatory network of taproot formation in radish (Raphanus sativus L.). Hortic. Res. 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yang, Y.; Li, X.; Gu, L.; Wang, F.; Feng, F.; Tian, Y.; Wang, F.; Wang, X.; Lin, W.; et al. Analysis of integrated multiple ‘omics’ datasets reveals the mechanisms of initiation and determination in the formation of tuberous roots in Rehmannia glutinosa. J. Exp. Bot. 2015, 66, 5837–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.; Nguyen, N.H.; Cheong, J.J. Transcriptional Regulation of Protein Phosphatase 2C Genes to Modulate Abscisic Acid Signaling. Int. J. Mol. Sci. 2020, 21, 9517. [Google Scholar] [CrossRef]
- Collin, A.; Daszkowska-Golec, A.; Szarejko, I. Updates on the Role of ABSCISIC ACID INSENSITIVE 5 (ABI5) and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORs (ABFs) in ABA Signaling in Different Developmental Stages in Plants. Cells 2021, 10, 1996. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef]
- Moon, S.J.; Han, S.Y.; Kim, D.Y.; Yoon, I.S.; Shin, D.; Byun, M.O.; Kwon, H.B.; Kim, B.G. Ectopic expression of a hot pepper bZIP-like transcription factor in potato enhances drought tolerance without decreasing tuber yield. Plant Mol. Biol. 2015, 89, 421–431. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, J.L.; Hao, B.; Lu, Y.C.; Qian, Z.L.; Li, Y.; Ye, S.; Tang, J.R.; Chen, M.; Long, G.Q.; et al. Comparative transcriptome and metabolome analyses provide new insights into the molecular mechanisms underlying taproot thickening in Panax notoginseng. BMC Plant Biol. 2019, 19, 451. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, M.; Komeichi, M. Changes in the Endogenous Level of Zeatin Riboside, Abscisic Acid and Indole Acetic Acid during Formation and Thickening of Tuberous Roots in Sweet Potato. Jpn. J. Crop Sci. 2008, 60, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Jang, S.; Yoon, E.K.; Heo, J.O.; Chang, K.S.; Choi, J.W.; Dhar, S.; Kim, G.; Choe, J.E.; Heo, J.B.; et al. Interplay between ABA and GA Modulates the Timing of Asymmetric Cell Divisions in the Arabidopsis Root Ground Tissue. Mol. Plant 2016, 9, 870–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Park, J.; Lee, N.; Jeong, J.; Toh, S.; Watanabe, A.; Kim, J.; Kang, H.; Kim, D.H.; Kawakami, N.; et al. ABA-insensitive3, ABA-insensitive5, and DELLAs Interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 2013, 25, 4863–4878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Ma, Z.; Chen, H.; Liu, M. MYB Gene Family in Potato (Solanum tuberosum L.): Genome-Wide Identification of Hormone-Responsive Reveals Their Potential Functions in Growth and Development. Int. J. Mol. Sci. 2019, 20, 4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- País, S.M.; García, M.N.; Téllez-Iñón, M.T.; Capiati, D.A. Protein phosphatases type 2A mediate tuberization signaling in Solanum tuberosum L. leaves. Planta 2010, 232, 37–49. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Muro, M.C.; Mazzocchi, L.C.; País, S.M.; Stritzler, M.; Schlesinger, M.; Capiati, D.A. The protein phosphatase 2A catalytic subunit StPP2Ac2b acts as a positive regulator of tuberization induction in Solanum tuberosum L. Plant Mol. Biol. 2017, 93, 227–245. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Tan, S.; Li, Z.; Yuan, Z.; Glanc, M.; Domjan, D.; Wang, K.; Xuan, W.; Guo, Y.; et al. Root Growth Adaptation is Mediated by PYLs ABA Receptor-PP2A Protein Phosphatase Complex. Adv. Sci. 2020, 7, 1901455. [Google Scholar] [CrossRef]
- Roumeliotis, E.; Visser, R.G.; Bachem, C.W. A crosstalk of auxin and GA during tuber development. Plant Signal. Behav. 2012, 7, 1360–1363. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Utsumi, C.; Takahashi, S.; Matsui, A.; Fukushima, A.; Kobayashi, M.; Sasaki, R.; Oikawa, A.; Kusano, M.; et al. Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava. Plant Mol. Biol. 2020, 104, 1–21. [Google Scholar] [CrossRef]
- Begum, S.; Jing, S.; Yu, L.; Sun, X.; Wang, E.; Abu Kawochar, M.; Qin, J.; Liu, J.; Song, B. Modulation of JA signalling reveals the influence of StJAZ1-like on tuber initiation and tuber bulking in potato. Plant J. 2022, 109, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.; Konstantinova, T.; Golyanovskaya, S.; Sergeeva, L.; Romanov, G. Hormonal regulation of tuber formation in potato plants. Russ. J. Plant Physiol. 2012, 59, 451–466. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Sergeeva, L.I.; Getman, I.A.; Lomin, S.N.; Savelieva, E.M.; Romanov, G.A. Core features of the hormonal status in in vitro grown potato plants. Plant Signal. Behav. 2018, 13, e1467697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, A.; Hajibarat, Z. Phytohormones: Plant switchers in developmental and growth stages in potato. J. Genet. Eng. Biotechnol. 2021, 19, 89. [Google Scholar] [CrossRef]
- Gong, M.; Luo, H.; Wang, A.; Zhou, Y.; Huang, W.; Zhu, P.; He, L. Phytohormone Profiling During Tuber Development of Chinese Yam by Ultra-high performance Liquid Chromatography–Triple Quadrupole Tandem Mass Spectrometry. J. Plant Growth Regul. 2016, 36, 362–373. [Google Scholar] [CrossRef]
- Chen, H.; Banerjee, A.K.; Hannapel, D.J. The tandem complex of BEL and KNOX partners is required for transcriptional repression of ga20ox1. Plant J. 2004, 38, 276–284. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. Int. J. Mol. Sci. 2022, 23, 4955. https://doi.org/10.3390/ijms23094955
Chen P, Yang R, Bartels D, Dong T, Duan H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. International Journal of Molecular Sciences. 2022; 23(9):4955. https://doi.org/10.3390/ijms23094955
Chicago/Turabian StyleChen, Peilei, Ruixue Yang, Dorothea Bartels, Tianyu Dong, and Hongying Duan. 2022. "Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development" International Journal of Molecular Sciences 23, no. 9: 4955. https://doi.org/10.3390/ijms23094955
APA StyleChen, P., Yang, R., Bartels, D., Dong, T., & Duan, H. (2022). Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. International Journal of Molecular Sciences, 23(9), 4955. https://doi.org/10.3390/ijms23094955






