Early Growth Response 1 Strengthens Pol-III-Directed Transcription and Transformed Cell Proliferation by Controlling PTEN/AKT Signalling Activity
Abstract
:1. Introduction
2. Results
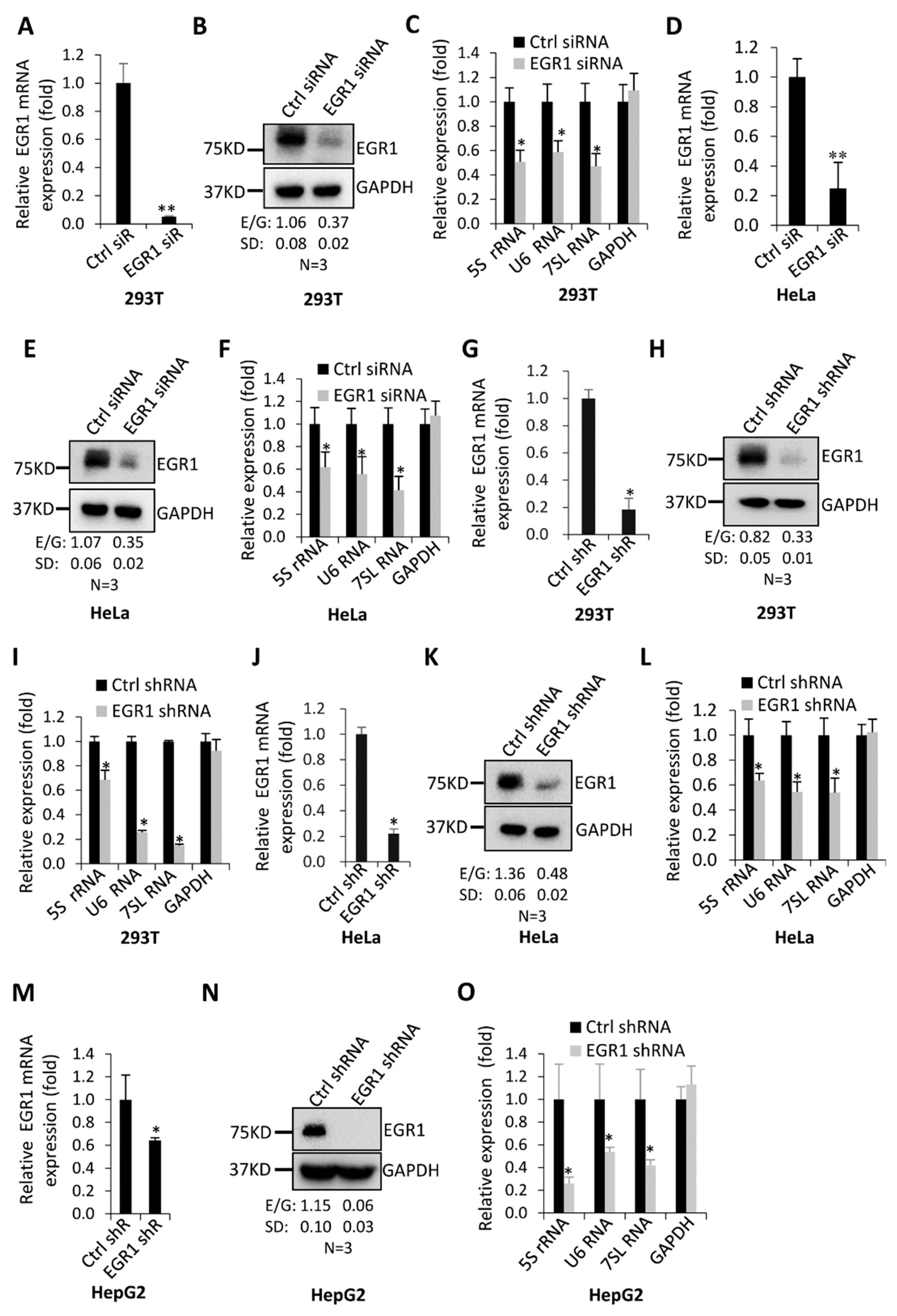
2.1. FLNA Downregulation Stimulates EGR1 Expression in Transformed Cell Lines
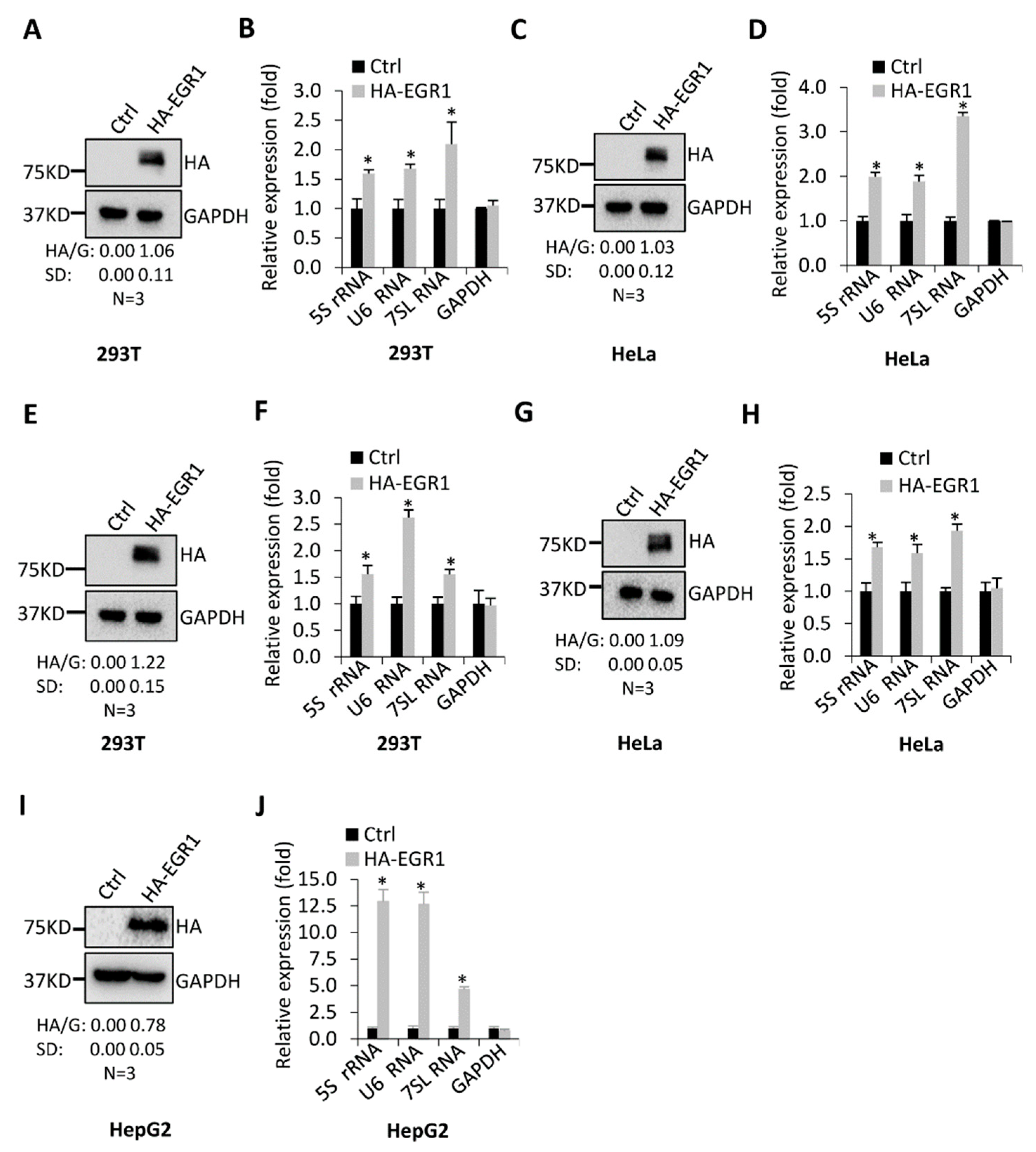
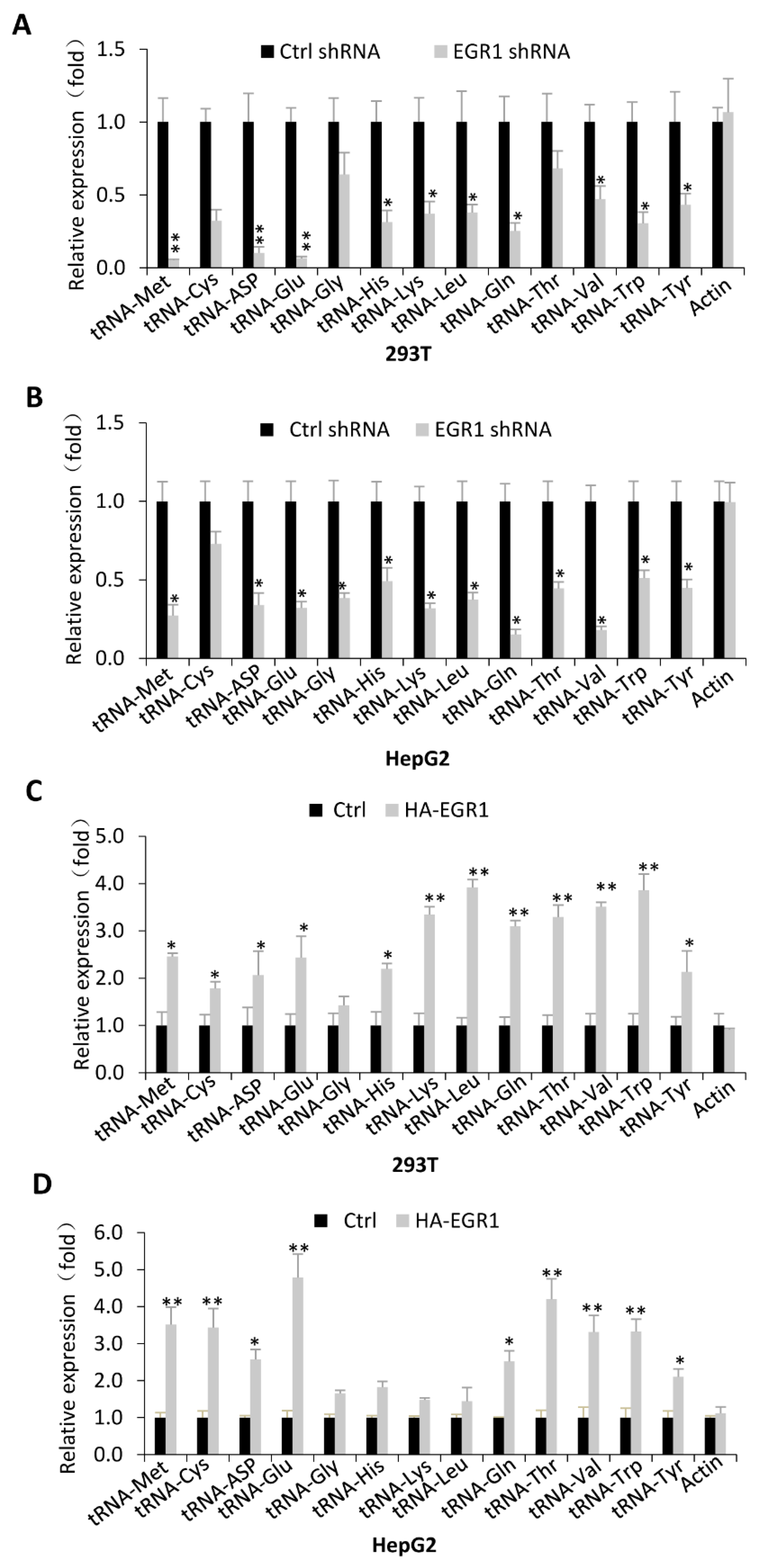
2.2. EGR1 Acts as an Activator to Regulate Transcription of Most Pol III Target Genes Tested in This Study
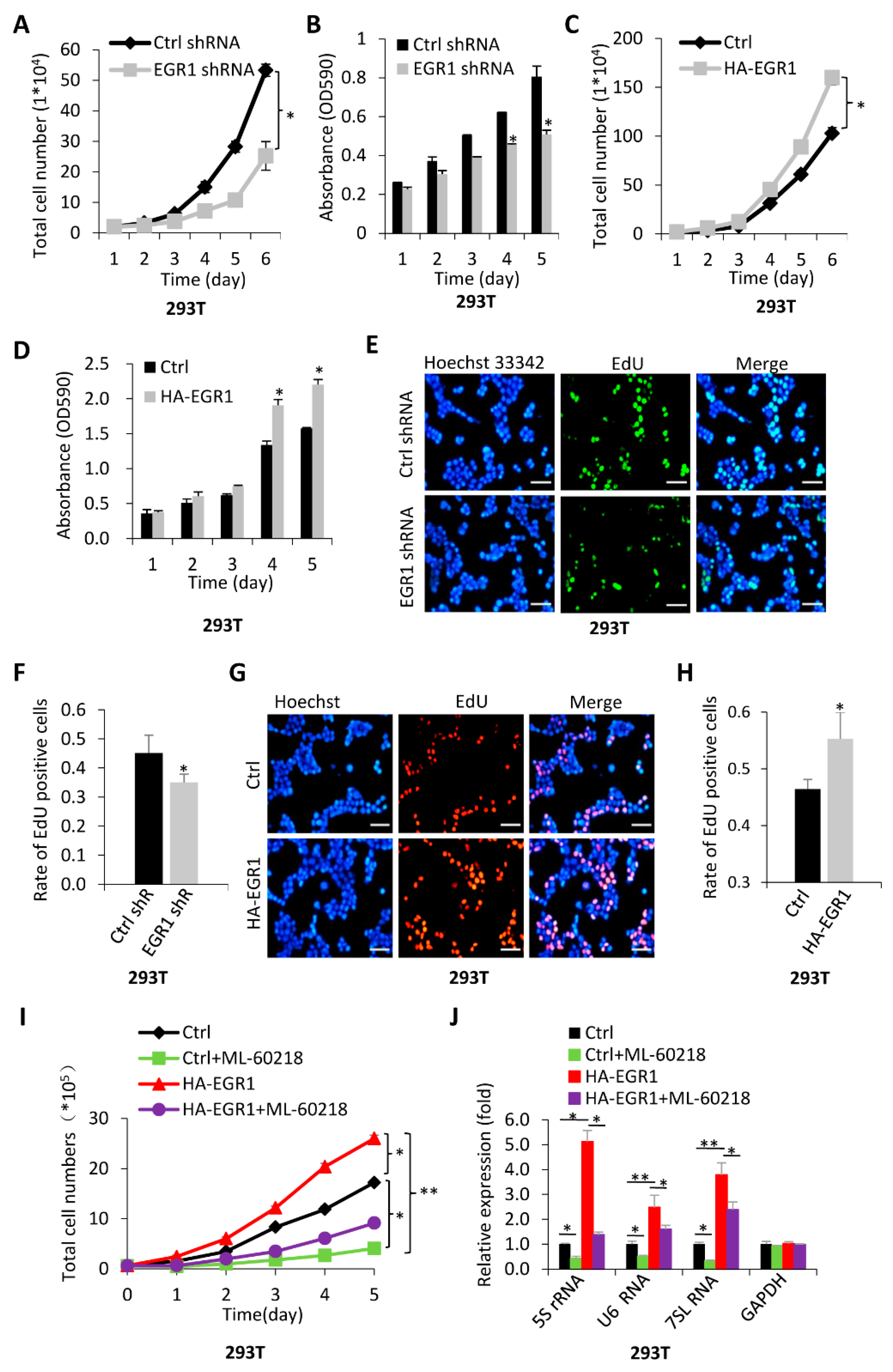
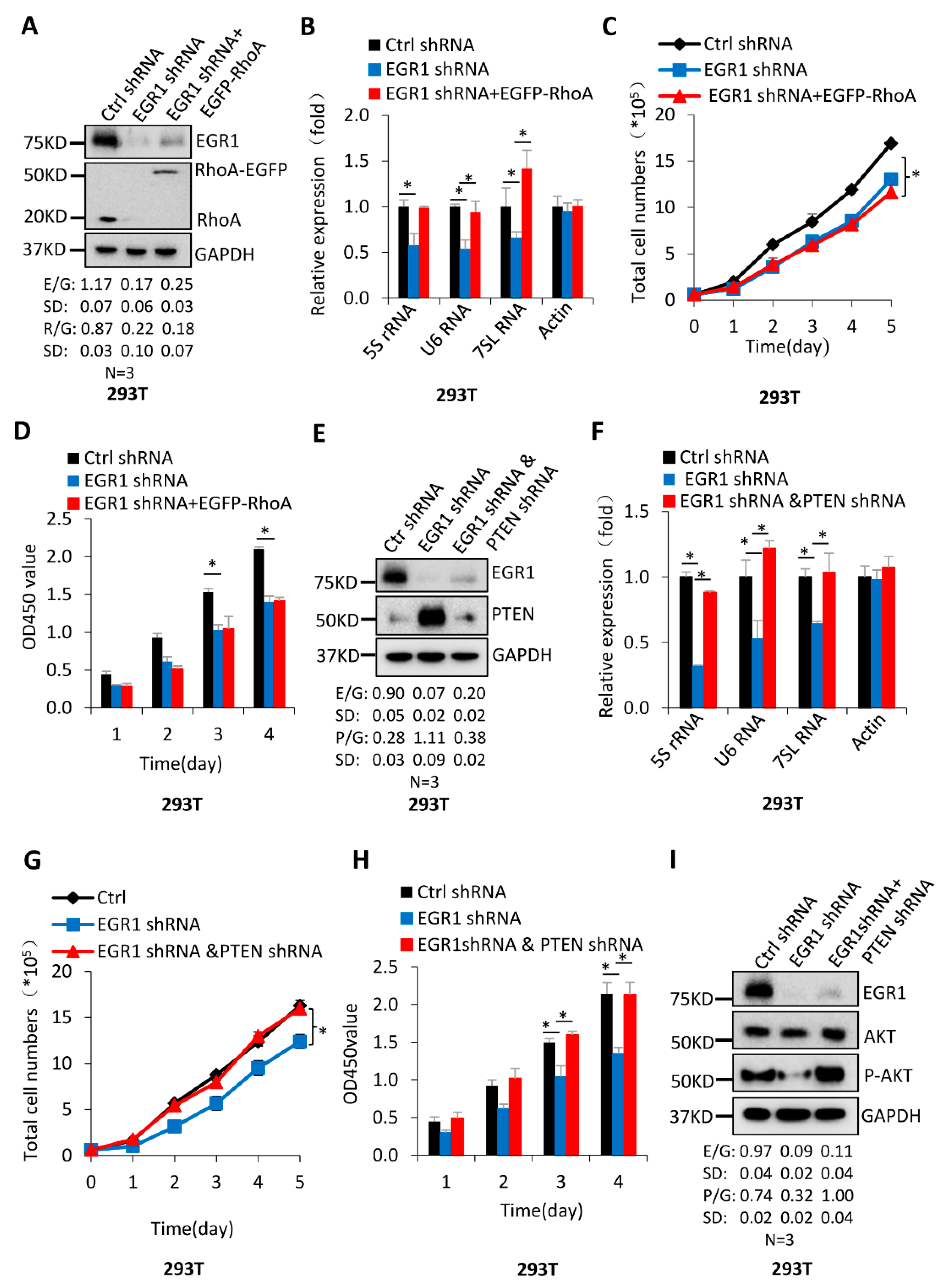
2.3. The Activation of Pol-III-Directed Transcription Mediated by EGR1 Contributes to the Promotion of Cell Proliferation
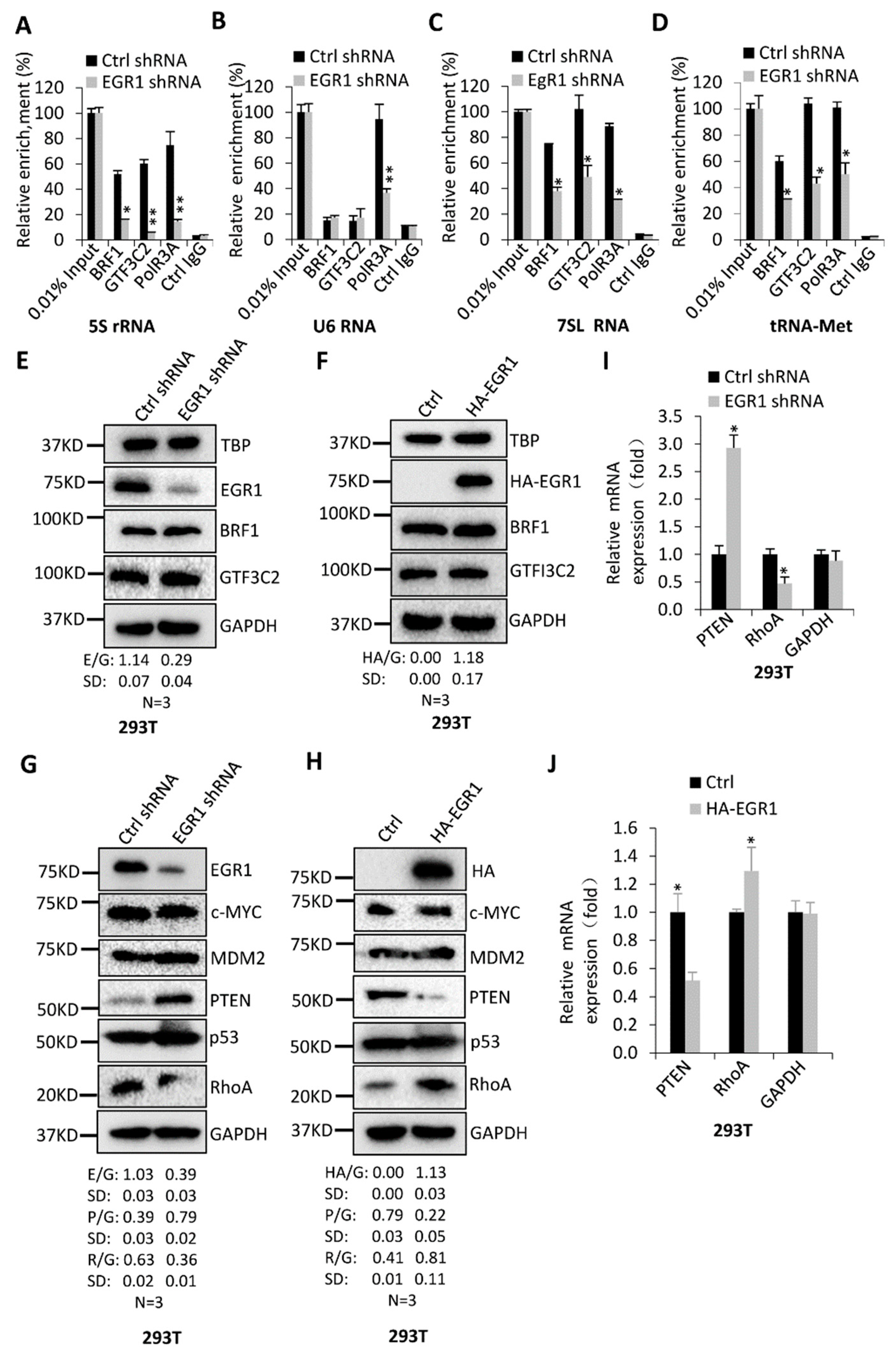
2.4. EGR1 Regulates PTEN and RhoA Expression but Does Not Affect the Expression of Pol III Transcription Factors
2.5. The PTEN/AKT Pathway Participates in the Regulation of Pol-III-Directed Transcription Mediated by EGR1
2.6. EGR1 Is Required for the Inhibition of Pol-III-Directed Transcription Mediated FLNA
3. Discussion
4. Materials and Methods
4.1. Plasmids, Cells and Reagents
4.2. Gene Cloning
4.3. Transfection and Generation of Stable Cell Lines
4.4. RT-qPCR and Detection of Pol III Products
4.5. Western Blot Analysis
4.6. Cell Proliferation Assays
4.7. ChIP Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar] [CrossRef] [PubMed]
- Gitenay, D.; Baron, V.T. Is EGR1 a potential target for prostate cancer therapy? Future Oncol. 2009, 5, 993–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, V.; Adamson, E.D.; Calogero, A.; Ragona, G.; Mercola, D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006, 13, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.J.; Ning, H.; Ishida, W.; Sodin-Semrl, S.; Takagawa, S.; Mori, Y.; Varga, J. The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J. Biol. Chem. 2006, 281, 21183–21197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongartz, H.; Seiß, E.A.; Bock, J.; Schaper, F. Glucocorticoids attenuate interleukin-6-induced c-Fos and Egr1 expression and impair neuritogenesis in PC12 cells. J. Neurochem. 2021, 157, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Rubin, E.; Sukhatme, V.; Qureshi, S.; Hallahan, D.; Weichselbaum, R.R.; Kufe, D.W. Ionizing radiation activates transcription of the EGR1 gene via CArG elements. Proc. Natl. Acad. Sci. USA 1992, 89, 10149–10153. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chen, J.; Mohagheghian, E.; Wang, N. Force-induced gene up-regulation does not follow the weak power law but depends on H3K9 demethylation. Sci. Adv. 2020, 6, eaay9095. [Google Scholar] [CrossRef] [Green Version]
- Mora-Garcia, P.; Sakamoto, K.M. Granulocyte colony-stimulating factor induces Egr-1 up-regulation through interaction of serum response element-binding proteins. J. Biol. Chem. 2000, 275, 22418–22426. [Google Scholar] [CrossRef] [Green Version]
- Maronde, E. Influence of Phosphodiesterase Inhibition on CRE- and EGR1-Dependent Transcription in a Mouse Hippocampal Cell Line. Int. J. Mol. Sci. 2020, 21, 8658. [Google Scholar] [CrossRef]
- Saha, S.K.; Islam, S.M.R.; Saha, T.; Nishat, A.; Biswas, P.K.; Gil, M.; Nkenyereye, L.; El-Sappagh, S.; Islam, M.S.; Cho, S.G. Prognostic role of EGR1 in breast cancer: A systematic review. BMB Rep. 2021, 7, 5402. [Google Scholar] [CrossRef]
- Yu, J.; Baron, V.; Mercola, D.; Mustelin, T.; Adamson, E.D. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell. Death Differ. 2007, 14, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.N.; Qi, Y.; Li, Z.; Hann, S.R. Egr1 mediates p53-independent c-Myc-induced apoptosis via a noncanonical ARF-dependent transcriptional mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 632–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, B.; Bepler, G.; Sharma, S.; Cantor, A.; Haura, E.B. EGR1 predicts PTEN and survival in patients with non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yang, X.; Zhu, Y.; Xu, F.; Zhang, H.; Qiu, Z. Knockdown of plasmacytoma variant translocation 1 (PVT1) inhibits high glucose-induced proliferation and renal fibrosis in HRMCs by regulating miR-23b-3p/early growth response factor 1 (EGR1). Endocr. J. 2021, 68, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.G.; Agarwal, S.; et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef]
- Zagurovskaya, M.; Shareef, M.M.; Das, A.; Reeves, A.; Gupta, S.; Sudol, M.; Bedford, M.T.; Prichard, J.; Mohiuddin, M.; Ahmed, M.M. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene 2009, 28, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Ock, J.; Lee, H.J.; Lee, Y.J.; Kwon, B.M.; Hong, S.H. Early growth response protein 1 upregulation and nuclear translocation by 2′-benzoyloxycinnamaldehyde induces prostate cancer cell death. Cancer Lett. 2013, 329, 217–227. [Google Scholar] [CrossRef]
- Meng, X.; Liu, K.; Xiang, Z.; Yu, X.; Wang, P.; Ma, Q. MiR-125b-2-3p associates with prognosis of ccRCC through promoting tumor metastasis via targeting EGR1. Am. J. Transl. Res. 2020, 12, 5575–5585. [Google Scholar]
- Liu, H.T.; Liu, S.; Liu, L.; Ma, R.R.; Gao, P. EGR1-Mediated Transcription of lncRNA-HNF1A-AS1 Promotes Cell-Cycle Progression in Gastric Cancer. Cancer Res. 2018, 78, 5877–5890. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.; Carroll, J.S.; Yee, E.; Thomas, D.D.; Wert-Lamas, L.; Neier, S.C.; Sheynkman, G.; Ritz, J.; Novina, C.D. The lncRNA SLNCR Recruits the Androgen Receptor to EGR1-Bound Genes in Melanoma and Inhibits Expression of Tumor Suppressor p21. Cell Rep. 2019, 27, 2493–2507. [Google Scholar] [CrossRef] [Green Version]
- Moir, R.D.; Willis, I.M. Regulation of pol III transcription by nutrient and stress signaling pathways. Biochim. Biophys. Acta 2013, 1829, 361–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieci, G.; Bosio, M.C.; Fermi, B.; Ferrari, R. Transcription reinitiation by RNA polymerase III. Biochim. Biophys. Acta 2013, 1829, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, M.; Hernandez, N. RNA polymerase III transcription as a disease factor. Genes Dev. 2020, 34, 865–882. [Google Scholar] [CrossRef]
- Willis, I.M.; Moir, R.D. Signaling to and from the RNA polymerase III transcription and processing machinery. Annu. Rev. Biochem. 2018, 87, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Acker, J.; Conesa, C.; Lefebvre, O. Yeast RNA polymerase III transcription factors and effectors. Biochim. Biophys. Acta 2013, 1829, 283–295. [Google Scholar] [CrossRef]
- Marshall, L.; White, R.J. Non-coding RNA production by RNA polymerase III is implicated in cancer. Nat. Rev. Cancer 2008, 8, 911–914. [Google Scholar] [CrossRef]
- Lei, J.; Chen, S.; Zhong, S. Abnormal expression of TFIIIB subunits and RNA Pol III genes is associated with hepatocellular carcinoma. Liver Res. 2017, 1, 112–120. [Google Scholar] [CrossRef]
- Santos, M.; Fidalgo, A.; Varanda, A.S.; Oliveira, C.; Santos, M.A.S. tRNA Deregulation and Its Consequences in Cancer. Trends Mol. Med. 2019, 25, 853–865. [Google Scholar] [CrossRef]
- Park, J.L.; Lee, Y.S.; Kunkeaw, N.; Kim, S.Y.; Kim, I.H.; Lee, Y.S. Epigenetic regulation of noncoding RNA transcription by mammalian RNA polymerase III. Epigenomics 2017, 9, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Tsang, C.K.; Zheng, X.F. Mechanisms of regulation of RNA polymerase III-dependent transcription by TORC1. EMBO J. 2009, 28, 2220–2230. [Google Scholar] [CrossRef]
- Sriskanthadevan-Pirahas, S.; Deshpande, R.; Lee, B.; Grewal, S.S. Ras/ERK-signalling promotes tRNA synthesis and growth via the RNA polymerase III repressor Maf1 in Drosophila. PLoS Genet. 2018, 14, e1007202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Johnson, D.L. The JNKs differentially regulate RNA polymerase III transcription by coordinately modulating the expression of all TFIIIB subunits. Proc. Natl. Acad. Sci. USA 2009, 106, 12682–12687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woiwode, A.; Johnson, S.A.; Zhong, S.; Zhang, C.; Roeder, R.G.; Teichmann, M.; Johnson, D.L. PTEN represses RNA polymerase III-dependent transcription by targeting the TFIIIB complex. Mol. Cell Biol. 2008, 28, 4204–4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chymkowitch, P.; Nguéa, P.A.; Aanes, H.; Robertson, J.; Klungland, A.; Enserink, J.M. TORC1-dependent sumoylation of Rpc82 promotes RNA polymerase III assembly and activity. Proc. Natl. Acad. Sci. USA 2017, 114, 1039–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tsang, C.K.; Wang, S.; Li, X.X.; Yang, Y.; Fu, L.; Huang, W.; Li, M.; Wang, H.Y.; Zheng, X.F. MAF1 suppresses AKT-mTOR signaling and liver cancer through activation of PTEN transcription. Hepatology 2016, 63, 1928–1942. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.X.; Hartwig, J.H.; Akyürek, L.M. Filamins in cell signaling, transcription and organ development. Trends Cell. Biol. 2010, 20, 113–123. [Google Scholar] [CrossRef]
- Rosa, J.P.; Raslova, H.; Bryckaert, M. Filamin A: Key actor in platelet biology. Blood 2019, 134, 1279–1288. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Wei, Y.; Zhou, Y.; Shore, P.; Deng, W. Cytoskeletal Filamin a Differentially Modulates RNA Polymerase III Gene Transcription in Transformed Cell Lines. J. Biol. Chem. 2016, 291, 25239–25246. [Google Scholar] [CrossRef] [Green Version]
- Crighton, D.; Woiwode, A.; Zhang, C.; Mandavia, N.; Morton, J.P.; Warnock, L.J.; Milner, J.; White, R.J.; Johnson, D.L. p53 represses RNA polymerase III transcription by targeting TBP and inhibiting promoter occupancy by TFIIIB. EMBO J. 2003, 22, 2810–2820. [Google Scholar] [CrossRef] [Green Version]
- Ao, H.; Liu, B.; Li, H.; Lu, L. Egr1 mediates retinal vascular dysfunction in diabetes mellitus via promoting p53 transcription. J. Cell Mol. Med. 2019, 23, 3345–3356. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, S.S.; Saito, K.; Williams, S.; Arimura, Y.; Ma, Y.; Ke, Y.; Baron, V.; Mercola, D.; Feng, G.S.; et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 2009, 28, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Zhou, Y.; Wang, J.; Guo, B.; Wei, Y.; Deng, H.; Wu, Z.; Zhang, C.; Shi, K.; Li, Y.; et al. The transcription factor Sp1 modulates RNA polymerase III gene transcription by controlling BRF1 and GTF3C2 expression in human cells. J. Biol. Chem. 2020, 295, 4617–4630. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, K.; Wang, J.; Zhou, X.; Zhang, C.; Song, X.; Wu, Z.; Du, J.; Chen, Q.; Zhang, S.; et al. RNA polymerase I subunit 12 plays opposite roles in cell proliferation and migration. Biochem. Biophys. Res. Commun. 2021, 560, 112–118. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Huang, L.; Zhao, S.; Wang, J.; Zhang, C.; Song, X.; Chen, Q.; Du, J.; Yu, D.; Sun, X.; et al. Early Growth Response 1 Strengthens Pol-III-Directed Transcription and Transformed Cell Proliferation by Controlling PTEN/AKT Signalling Activity. Int. J. Mol. Sci. 2022, 23, 4930. https://doi.org/10.3390/ijms23094930
Wu Z, Huang L, Zhao S, Wang J, Zhang C, Song X, Chen Q, Du J, Yu D, Sun X, et al. Early Growth Response 1 Strengthens Pol-III-Directed Transcription and Transformed Cell Proliferation by Controlling PTEN/AKT Signalling Activity. International Journal of Molecular Sciences. 2022; 23(9):4930. https://doi.org/10.3390/ijms23094930
Chicago/Turabian StyleWu, Zhongyu, Liyun Huang, Shasha Zhao, Juan Wang, Cheng Zhang, Xiaoye Song, Qiyue Chen, Jiannan Du, Deen Yu, Xiaomeng Sun, and et al. 2022. "Early Growth Response 1 Strengthens Pol-III-Directed Transcription and Transformed Cell Proliferation by Controlling PTEN/AKT Signalling Activity" International Journal of Molecular Sciences 23, no. 9: 4930. https://doi.org/10.3390/ijms23094930
APA StyleWu, Z., Huang, L., Zhao, S., Wang, J., Zhang, C., Song, X., Chen, Q., Du, J., Yu, D., Sun, X., Zhang, Y., Deng, W., Zhang, S., & Deng, H. (2022). Early Growth Response 1 Strengthens Pol-III-Directed Transcription and Transformed Cell Proliferation by Controlling PTEN/AKT Signalling Activity. International Journal of Molecular Sciences, 23(9), 4930. https://doi.org/10.3390/ijms23094930




