The Role of Hydrolases in Biology and Xenobiotics Metabolism
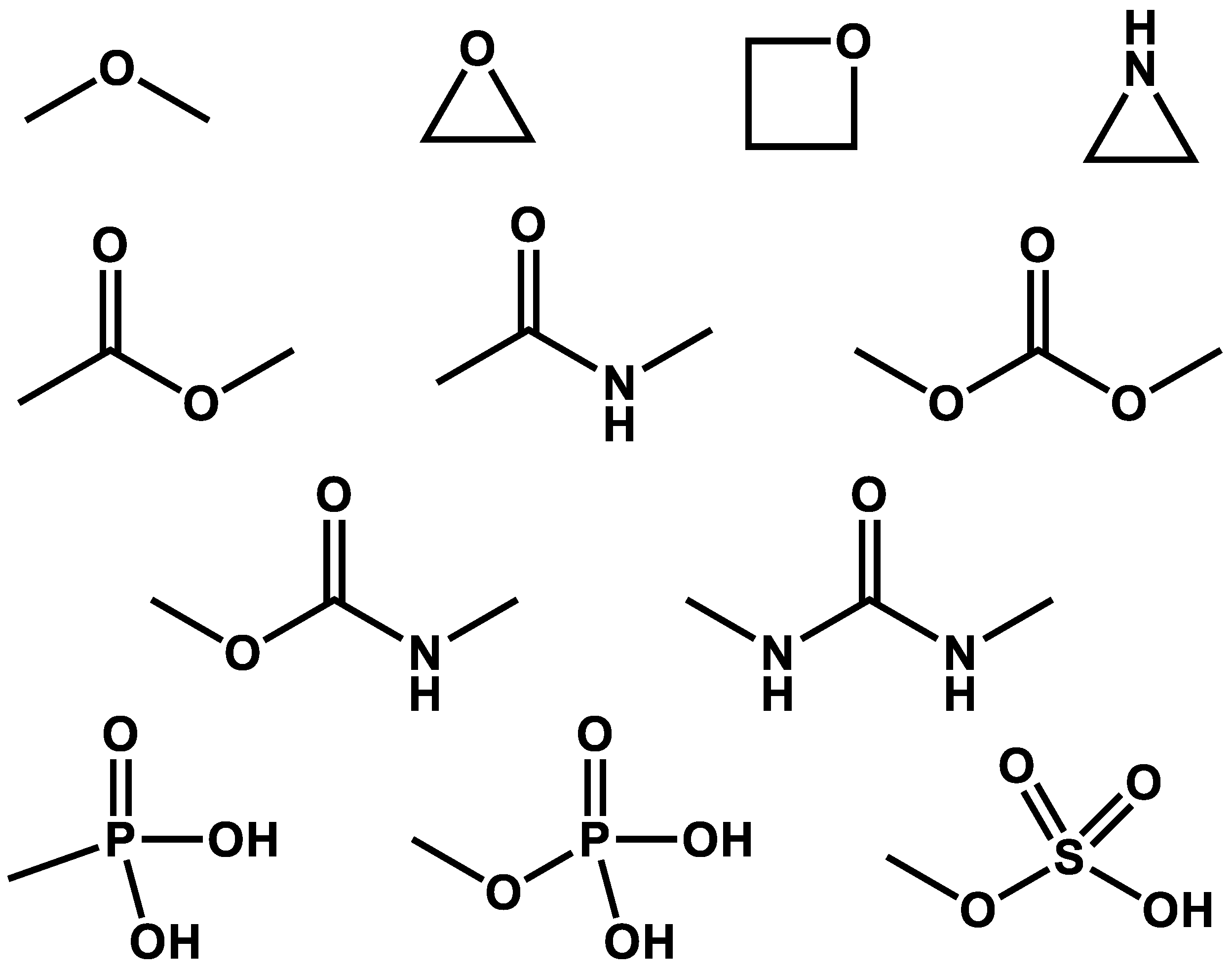
1. Epoxide Hydrolase
2. Glycoside Hydrolase
3. Phosphatase
4. Proteosome
5. Amyloid Hydrolase
6. Conclusions
Funding
Conflicts of Interest
References
- Guengerich, F.P. P450 Catalysis Mechanisms. Arch. Biochem. Biophys. 2011, 507, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Boverhof, D.R.; Chamberlain, M.P.; Elcombe, C.R.; Gonzalez, F.J.; Heflich, R.H.; Hernández, L.G.; Jacobs, A.C.; Jacobson-Kram, D.; Luijten, M.; Maggi, A.; et al. Transgenic animal models in toxicology: Historical perspectives and future outlook. Toxicol Sci. 2011, 121, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, M.; Arand, M.; Cronin, A. Mammalian epoxide hydrolases in xenobiotic metabolism and signaling. Arch. Toxicol. 2009, 83, 297–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta 2013, 1831, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautheron, J.; Jérum, I. The Multifaceted Role of Epoxide Hydrolases in Human Health and Disease. Int. J. Mol. Sci. 2020, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Morisseau, C.; Kodani, S.D.; Kamita, S.G.; Yang, J.; Lee, K.S.S.; Hammock, B.D. Relative Importance of Soluble and Microsomal Epoxide Hydrolases for the Hydrolysis of Epoxy-Fatty Acids in Human Tissues. Int. J. Mol. Sci. 2021, 22, 4993. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Abdelkawy, K.; Moshirian, N.; Abdel-Megied, A.M. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int. J. Mol. Sci. 2020, 21, 6554. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, K.L.; Darwesh, A.M.; Sosnowski, D.K.; Zhang, H.; Shah, S.; Zhabyeyev, P.; Yang, J.; Hammock, B.D.; Edin, M.L.; Zeldin, D.C.; et al. Soluble Epoxide Hydrolase in Aged Female Mice and Human Explanted Hearts Following Ischemic Injury. Int. J. Mol. Sci. 2021, 22, 1691. [Google Scholar] [CrossRef] [PubMed]
- Overby, H.; Yang, Y.; Xu, X.; Graham, K.; Hildreth, K.; Choi, S.; Wan, D.; Morisseau, C.; Zeldin, D.C.; Hammock, B.D.; et al. Soluble Epoxide Hydrolase Inhibition by t-TUCB Promotes Brown Adipogenesis and Reduces Serum Triglycerides in Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 7039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, X.; Wu, H.; Yang, J.; Chen, J.; Morisseau, C.; Hammock, B.D.; Bettaieb, A.; Zhao, L. Differential Effects of 17,18-EEQ and 19,20-EDP Combined with Soluble Epoxide Hydrolase Inhibitor t-TUCB on Diet-Induced Obesity in Mice. Int. J. Mol. Sci. 2021, 22, 8267. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Zhang, W.H.; Allen, E.M.; Bah, T.M.; Shangraw, R.E.; Alkayed, N.J. Soluble Epoxide Hydrolase Blockade after Stroke Onset Protects Normal but Not Diabetic Mice. Int. J. Mol. Sci. 2021, 22, 5419. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, R.; Frömel, T.; Zukunft, S.; Brüne, B.; Weigert, A.; Wittig, I.; Popp, R.; Fleming, I. The Consequences of Soluble Epoxide Hydrolase Deletion on Tumorigenesis and Metastasis in a Mouse Model of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7120. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, V.; Vélëz, H.; Tzelepis, G. The Role of Glycoside Hydrolases in Phytopathogenic Fungi and Oomycetes Virulence. Int. J. Mol. Sci. 2021, 22, 9359. [Google Scholar] [CrossRef] [PubMed]
- Chrabąszczewska, M.; Winiewska-Szajewska, M.; Ostrowska, N.; Bojarska, E.; Stępiński, J.; Mancewicz, Ł.; Łukaszewicz, M.; Trylska, J.; Taube, M.; Kozak, M.; et al. Insight into the Binding and Hydrolytic Preferences of hNudt16 Based on Nucleotide Diphosphate Substrates. Int. J. Mol. Sci. 2021, 22, 10929. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, K.; Hegde, R.S. The Eyes Absent Proteins: Unusual HAD Family Tyrosine Phosphatases. Int. J. Mol. Sci. 2021, 22, 3925. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Y.; Fan, R.A.; Kirk, C.J. Proteasome Inhibitors and Their Pharmacokinetics, Pharmacodynamics, and Metabolism. Int. J. Mol. Sci. 2021, 22, 11595. [Google Scholar] [CrossRef] [PubMed]
- Duran-Meza, E.; Diaz-Espinoza, R. Catalytic Amyloids as Novel Synthetic Hydrolases. Int. J. Mol. Sci. 2021, 22, 9166. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morisseau, C. The Role of Hydrolases in Biology and Xenobiotics Metabolism. Int. J. Mol. Sci. 2022, 23, 4870. https://doi.org/10.3390/ijms23094870
Morisseau C. The Role of Hydrolases in Biology and Xenobiotics Metabolism. International Journal of Molecular Sciences. 2022; 23(9):4870. https://doi.org/10.3390/ijms23094870
Chicago/Turabian StyleMorisseau, Christophe. 2022. "The Role of Hydrolases in Biology and Xenobiotics Metabolism" International Journal of Molecular Sciences 23, no. 9: 4870. https://doi.org/10.3390/ijms23094870
APA StyleMorisseau, C. (2022). The Role of Hydrolases in Biology and Xenobiotics Metabolism. International Journal of Molecular Sciences, 23(9), 4870. https://doi.org/10.3390/ijms23094870




