Regulation of Poly-E Motif Flexibility by pH, Ca2+ and the PPAK Motif
Abstract
1. Introduction
2. Results
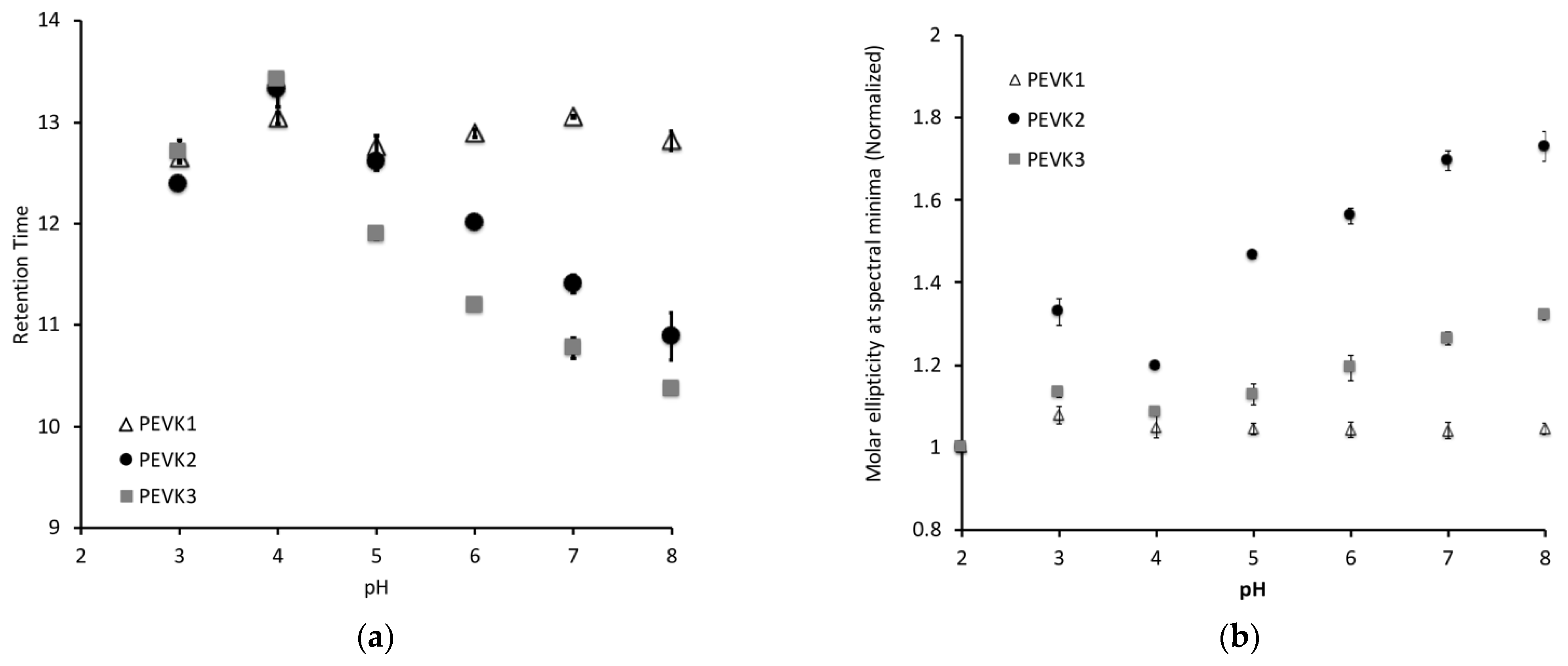
2.1. Long Poly-E and Poly-E-PPAK Construct Exhibit pH Dependent Conformational Changes
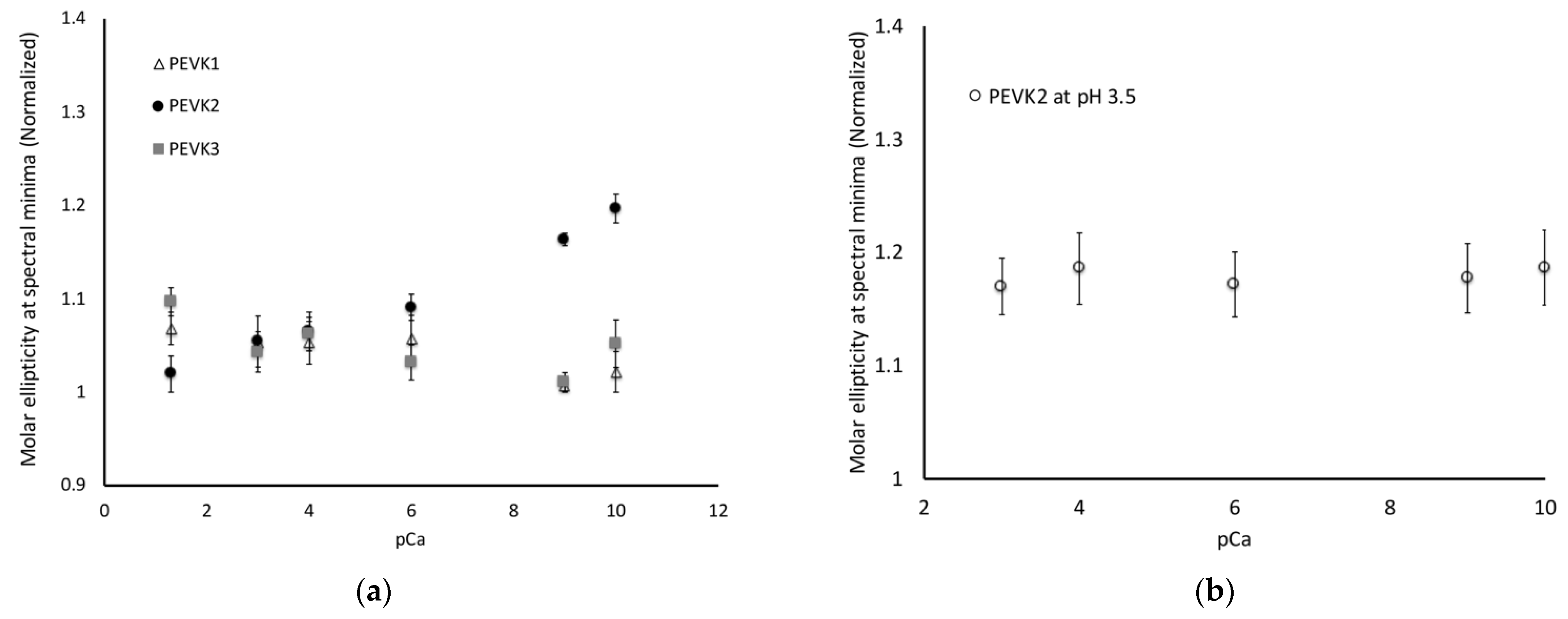
2.2. Calcium Ions Alter the Disordered Structure of Isolated Poly-E Motifs
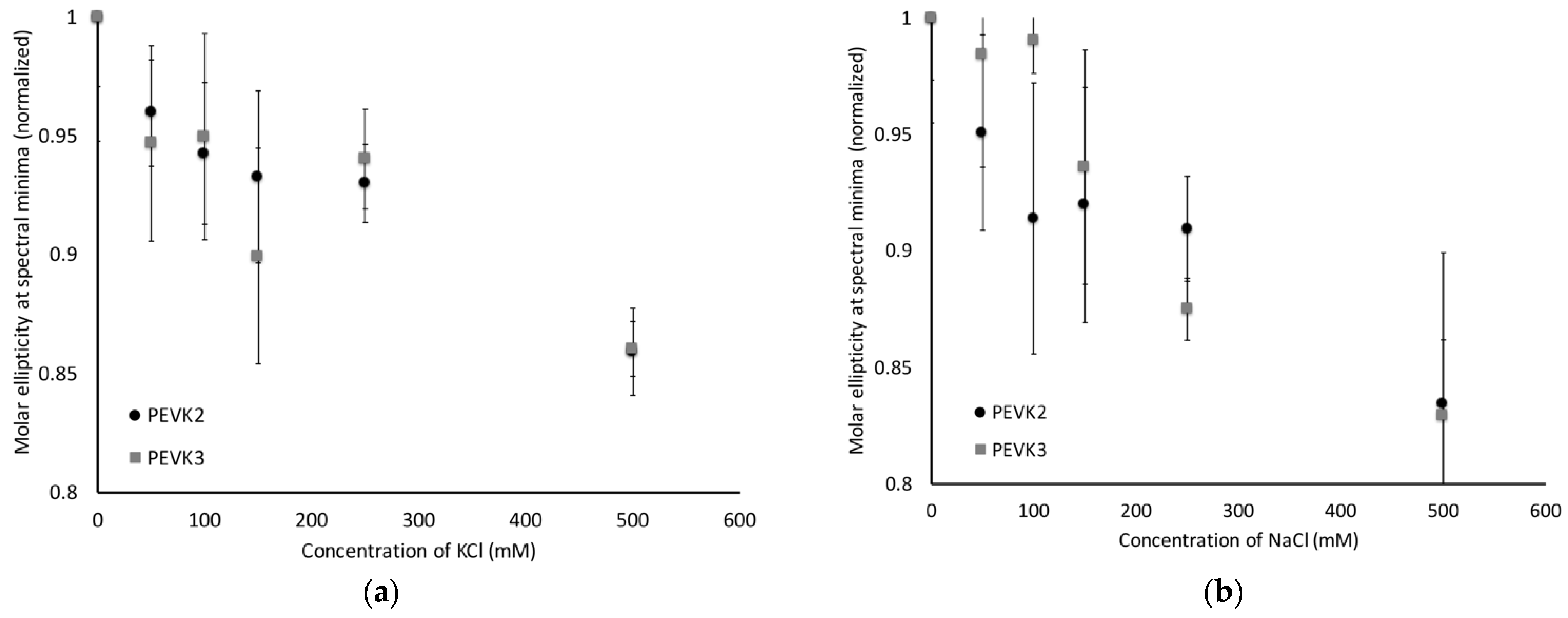
2.3. PEVK2 and PEVK3 Undergo Sodium and Potassium Dependent Structural Changes
3. Discussion
3.1. Calcium Dependency of Poly-E Regions
3.2. Impact of PPAK on Calcium Binding to Poly-E Motifs
3.3. Role of PEVK in Residual Force Enhancement
4. Materials and Methods
- PEVK1:PPAKVPEVPKKPVPEEKVPVPVPKKVEAPPAKVPEVPKKPVPEKKVPVPAPKKVEAPPAKVPEVPKKLIPEEKKPTPVPKKVEA
- PEVK2:PPPKVPKKREVPVPVALPQEEEVLFEEEIVPEEEVLPEEEEVLPEEEEVLPEEEEVLPEEEEIPPEEEEVPPEEEYVPEEEEFVPEEEVLPEVKPKVPVPAPVPEIKKKVTEKKVVIPKKEEA
- PEVK3:PPAKVPEVPKKPVPEEKVPVPVPKKVEAPPAKVPEVPKKPVPEKKVPVPAPKKVEAPPPKVPKKREPVPVPVALPQEEEVLFEEEIVPEEEVLPEEEEVLPEEEEVLPEEEEVLPEEEEIPPEEEEVPPEEEYVPEEEEFVPEEEVLPEVKPKVPVPAPVPEIKKKVTEKKVVIPKKEEAPPAKVPEVPKKVEEKRIILPKEEEVLPV
4.1. Protein Production and Purification
4.2. Circular Dichroism Measurements
4.3. Size Exclusion Chromatography
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maruyama, K.; Matsubara, S.; Natori, R.; Nonomura, Y.; Kimura, S. Connectin, an elastic protein of muscle. Characterization and Function. J. Biochem. 1977, 82, 317–337. [Google Scholar] [PubMed]
- Trinick, J.; Knight, P.; Whiting, A. Purification and properties of native titin. J. Mol. Biol. 1984, 180, 331–356. [Google Scholar] [CrossRef]
- Wang, K.; McClure, J.; Tu, A. Titin: Major myofibrillar components of striated muscle. Proc. Natl. Acad. Sci. USA 1979, 76, 3698–3702. [Google Scholar] [CrossRef]
- Improta, S.; Politou, A.S.; Pastore, A. Immunoglobulin-like modules from titin I-band: Extensible components of muscle elasticity. Structure 1996, 4, 323–337. [Google Scholar] [CrossRef]
- Labeit, S.; Kolmerer, B.; Linke, W.A. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ. Res. 1997, 80, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A.; Ivemeyer, M.; Olivieri, N.; Kolmerer, B.; Ruegg, J.C.; Labeit, S. Towards a molecular understanding of the elasticity of titin. J. Mol. Biol. 1996, 261, 62–71. [Google Scholar] [CrossRef]
- Pfuhl, M.; Gautel, M.; Politou, A.S.; Joseph, C.; Pastore, A. Secondary structure determination by NMR spectroscopy of an immunoglobulin-like domain from the giant muscle protein titin. J. Biomol. NMR 1995, 6, 48–58. [Google Scholar] [CrossRef]
- Pfuhl, M.; Pastore, A. Tertiary structure of an immunoglobulin-like domain from the giant muscle protein titin: A new member of the I set. Structure 1995, 3, 391–401. [Google Scholar] [CrossRef]
- Forbes, J.G.; Jin, A.J.; Ma, K.; Gutierrez-Cruz, G.; Tsai, W.L.; Wang, K. Titin PEVK segment: Charge-driven elasticity of the open and flexible polyampholyte. J. Muscle Res. Cell Motil. 2005, 26, 291–301. [Google Scholar] [CrossRef]
- Linke, W.A.; Ivemeyer, M.; Mundel, P.; Stockmeier, M.R.; Kolmerer, B. Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA 1998, 95, 8052–8057. [Google Scholar] [CrossRef]
- Linke, W.A.; Kulke, M.; Li, H.; Fujita-Becker, S.; Neagoe, C.; Manstein, D.J.; Gautel, M.; Fernandez, J.M. PEVK domain of titin: An entropic spring with actin-binding properties. J. Struct. Biol. 2002, 137, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Labeit, D.; Watanabe, K.; Witt, C.; Fujita, H.; Wu, Y.; Lahmers, S.; Funck, T.; Labeit, S.; Granzier, H. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl. Acad. Sci. USA 2003, 100, 13716–13721. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; DeKeyser, J.G.; Damodaran, S.; Greaser, M.L. Studies on titin PEVK peptides and their interaction. Arch. Biochem. Biophys 2006, 454, 16–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greaser, M. Identification of new repeating motifs in titin. Proteins 2001, 43, 145–149. [Google Scholar] [CrossRef]
- Nagy, A.; Grama, L.; Huber, T.; Bianco, P.; Trombitas, K.; Granzier, H.L.; Kellermayer, M.S. Hierarchical extensibility in the PEVK domain of skeletal-muscle titin. Biophys. J. 2005, 89, 329–336. [Google Scholar] [CrossRef]
- Sudarshi Premawardhana, D.M.; Zhang, F.; Xu, J.; Gage, M.J. The Poly-E motif in Titin’s PEVK region undergoes pH dependent conformational changes. Biochem. Biophys. Rep. 2020, 24, 100859. [Google Scholar] [CrossRef]
- Maughan, D.W.; Godt, R.E. Equilibrium distribution of ions in a muscle fiber. Biophys. J. 1989, 56, 717–722. [Google Scholar] [CrossRef]
- Previs, M.J.; Prosser, B.L.; Mun, J.Y.; Previs, S.B.; Gulick, J.; Lee, K.; Robbins, J.; Craig, R.; Lederer, W.J.; Warshaw, D.M. Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling. Sci. Adv. 2015, 1, e1400205. [Google Scholar] [CrossRef]
- Chu, W.T.; Shammas, S.L.; Wang, J. Charge Interactions Modulate the Encounter Complex Ensemble of Two Differently Charged Disordered Protein Partners of KIX. J. Chem. Theory Comput. 2020, 16, 3856–3868. [Google Scholar] [CrossRef]
- Cook, E.C.; Creamer, T.P. Influence of electrostatic forces on the association kinetics and conformational ensemble of an intrinsically disordered protein. Proteins 2020, 88, 1607–1619. [Google Scholar] [CrossRef]
- Huihui, J.; Firman, T.; Ghosh, K. Modulating charge patterning and ionic strength as a strategy to induce conformational changes in intrinsically disordered proteins. J. Chem. Phys. 2018, 149, 085101. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.C.; Monroy, J.A.; Uyeno, T.E.; Yeo, S.H.; Pai, D.K.; Lindstedt, S.L. Is titin a ‘winding filament’? A new twist on muscle contraction. Proc. Biol. Sci. 2012, 279, 981–990. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J. The roles of ionic processes in muscular fatigue during intense exercise. Sports Med. 1992, 13, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Grama, L.; Hetenyi, C.; Schay, G.; Fulop, L.; Penke, B.; Kellermayer, M.S. Conformational dynamics of titin PEVK explored with FRET spectroscopy. Biophys. J. 2012, 103, 1480–1489. [Google Scholar] [CrossRef]
- Tatsumi, R.; Maeda, K.; Hattori, A.; Takahashi, K. Calcium binding to an elastic portion of connectin/titin filaments. J. Muscle Res. Cell Motil. 2001, 22, 149–162. [Google Scholar] [CrossRef]
- DuVall, M.M.; Gifford, J.L.; Amrein, M.; Herzog, W. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur. Biophys. J. 2013, 42, 301–307. [Google Scholar] [CrossRef]
- Kelly, C.; Pace, N.; Gage, M.; Pfuhl, M. Solution NMR Structure of Titin N2A Region Ig Domain I83 and Its Interaction with Metal Ions. J. Mol. Biol. 2021, 433, 166977. [Google Scholar] [CrossRef]
- Kelly, C.M.; Manukian, S.; Kim, E.; Gage, M.J. Differences in stability and calcium sensitivity of the Ig domains in titin’s N2A region. Protein Sci. 2020, 29, 1160–1171. [Google Scholar] [CrossRef]
- Herzog, W.; Schappacher, G.; DuVall, M.; Leonard, T.R.; Herzog, J.A. Residual Force Enhancement Following Eccentric Contractions: A New Mechanism Involving Titin. Physiology 2016, 31, 300–312. [Google Scholar] [CrossRef]
- Rassier, D.E. The mechanisms of the residual force enhancement after stretch of skeletal muscle: Non-uniformity in half-sarcomeres and stiffness of titin. Proc. Biol. Sci. 2012, 279, 2705–2713. [Google Scholar] [CrossRef]
- Shalabi, N.; Cornachione, A.; de Souza Leite, F.; Vengallatore, S.; Rassier, D.E. Residual force enhancement is regulated by titin in skeletal and cardiac myofibrils. J. Physiol. 2017, 595, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Fukutani, A.; Herzog, W. Current Understanding of Residual Force Enhancement: Cross-Bridge Component and Non-Cross-Bridge Component. Int. J. Mol. Sci. 2019, 20, 5479. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W. The multiple roles of titin in muscle contraction and force production. Biophys. Rev. 2018, 10, 1187–1199. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dassanayake Mudiyanselage, S.P.; Gage, M.J. Regulation of Poly-E Motif Flexibility by pH, Ca2+ and the PPAK Motif. Int. J. Mol. Sci. 2022, 23, 4779. https://doi.org/10.3390/ijms23094779
Dassanayake Mudiyanselage SP, Gage MJ. Regulation of Poly-E Motif Flexibility by pH, Ca2+ and the PPAK Motif. International Journal of Molecular Sciences. 2022; 23(9):4779. https://doi.org/10.3390/ijms23094779
Chicago/Turabian StyleDassanayake Mudiyanselage, Sudarshi Premawardhana, and Matthew J. Gage. 2022. "Regulation of Poly-E Motif Flexibility by pH, Ca2+ and the PPAK Motif" International Journal of Molecular Sciences 23, no. 9: 4779. https://doi.org/10.3390/ijms23094779
APA StyleDassanayake Mudiyanselage, S. P., & Gage, M. J. (2022). Regulation of Poly-E Motif Flexibility by pH, Ca2+ and the PPAK Motif. International Journal of Molecular Sciences, 23(9), 4779. https://doi.org/10.3390/ijms23094779





