Insight into the Role of Psychological Factors in Oral Mucosa Diseases
Abstract
1. Introduction
| Psychology Problems | Oral Symptoms | Refs. |
|---|---|---|
| Olanzapine-induced anticholinergic toxicity | Dry oral mucosa | [9] |
| Alzheimer’s disease (AD) | Tau protein in oral mucosa | [10] |
| Bulimia and anorexia nervosa | Abrasion of teeth enamel | [11] |
| Epidermoid cysts of the central nervous system | Similar to symptoms in oral mucosa | [12] |
| Neurocutaneous syndromes (and other diseases associated with DNA repair) | Teeth and oral mucosa lesions | [13] |
| Structural changes in innervations in oral cavity | Loss of sense | [14] |
| Oral Diseases | Psychological Abnormalities | Oral Manifestations | Refs. |
|---|---|---|---|
| Oral squamous cell carcinoma (OSCC) | Higher α1 adrenergic receptors | Oral ulcers and lumps, pain feeling | [15] |
| Primary Sjögren’s syndrome | Depression and anxiety | More frequent oral lesions; negative impact on life quality | [16] |
| Burning mouth syndrome (BMS) | Structural and functional deficits within the nervous system | Burning feeling without an obvious cause | [17] |
| Herpes simplex encephalitis | Fatal disease of the central nervous system | Painful blisters or open sores (ulcers) | [18] |
| Oral mucosa cancers | Antitumor drugs improve psychological symptoms | Oral pumps and lesions | [19] |
| Primary burning mouth syndrome | PNS involvement | Burning feeling in mouth | [20] |
| Recurrent aphthous stomatitis | Hypofunction of the sympathetic nervous system | Painful round shallow ulcers | [21] |
| Inflammatory stimulation of the oral mucosa | Activation of microglial cells | Oral mucosal inflammation | [22] |
| Poliovirus | Affects the anterior horn motor neurons of the spinal cord causing paralysis | [23] | |
| Xerostomia | Autonomic nervous system imbalance | Dryness of the oral mucosa | [24] |
| Burning mouth syndrome | Decreased or modified steroid synthesis | Burning feeling in mouth | [25] |
| Lingual conical papillae | Alterations of different kinds of neurons | Bumps and rough tongue | [26] |
| Lichen planus and lichenoid reactions | Loss of PNS fibers | Asymptomatic white reticular striae to painful erythema and erosions | [27] |
| Heat stimulation | Large primary neurons responding to high-threshold noxious heat are abundant in the tooth pulp | Altered pain | [28] |
| Oral mucosa continuous remodeling | Sensory nervous apparatus involvement | Leaky epithelial barrier, a fibrotic lamina propria, the release of inflammatory mediators, and the recruitment of immune infiltrate | [29] |
| Oral dysesthesia (OD) | Soft tissue grafts | Merkel cells and permanent dysesthesia in the oral mucosa | [30] |
| Diseases | Psychological Problems | Oral Manifestations | Refs. |
|---|---|---|---|
| Congenital herpes simplex | CNS infection | Oral infection | [31] |
| Enterovirus A71 (EV-A71) | Viral antigens/RNA in the CNS | Viral antigens/RNA in the squamous epithelia of the oral cavity | [32] |
| Verrucous lesions | Malocclusions in CNS | Verrucous lesions affect the oral mucosa (rare) | [33] |
| Cryptococcosis | Presentations in the CNS | Excision of nodules in the oral mucosa assists recovery | [34] |
| Paracoccidioidomycosis (PCM) | Involvement of CNS | Common infected symptoms | [35] |
| Herpes simplex virus type 1 (HSV-1) | Clinical diseases in CNS | Vesicular lesions of the oral mucosa | [36] |
| Cowden syndrome | Similar CNS symptoms with that in the oral mucosa | Multiple hamartomatous neoplasms of the oral mucosa | [37] |
| Lipoid proteinosis (LP) | Involvement of CNS | Yellow-white plaques on oral mucosa | [38] |
| Enterovirus 71 (EV71) (hand-foot-and-mouth disease (HFMD)) | brainstem encephalitis | Vesicular lesions on oral mucosa | [39] |
| Wilson disease (WD) | multi-organ manifestations involve the nervous system | Repeated oral candidiasis | [40] |
| HSV-1 | Transmission in the CNS | Infection in the oral mucosa | [41] |
| Tuberous sclerosis | Hamartoma formation in the nervous system | Hyperpigmented and hypopigmented macules affecting the oral mucosa | [42] |
| Bacillary angiomatosis | CNS | BA lesions in the oral mucosa | [43] |
| Cowden disease (CD) | CNS manifestations | Normal oral involvement | [44] |
| Dettol liquid | CNS symptoms | Oral involvement | [45] |
| Sweet’s diseases | [46] | ||
| HSV-1 infection | Vagus nerve transmission | Oral manifestations | [47] |
| Adamantiades–Behçet disease | Lesions of ulcerating systemic vasculitis in the CNS | Oral manifestations | [48] |
2. Roles of Psychological Factors in Oral Mucosal Diseases
2.1. Psychological Factors in the Progress of OSCC
2.2. Burning Mouth Syndrome (BMS)
2.3. Recurrent Aphthous Stomatitis (RAS)
3. Psychological Factors Affected by Oral Diseases and Dietary Habits
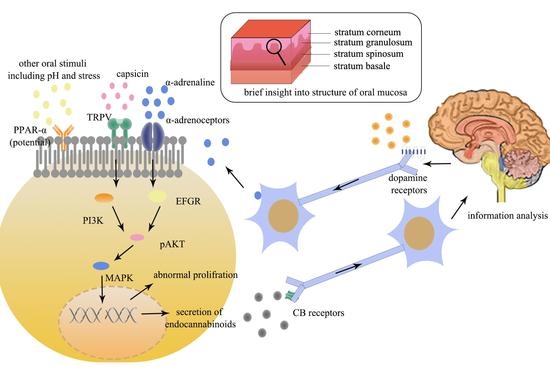
4. Information Is Analyzed in the Brain and Sent Back to Oral Mucosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shibahara, T. Oral cancer—Diagnosis and therapy. Clin. Calcium 2017, 27, 1427–1433. [Google Scholar] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- Costacurta, M.; Benavoli, D.; Arcudi, G.; Docimo, R. Oral and dental signs of child abuse and neglect. ORAL Implantol. 2015, 8, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.D.; Follmann, M.; Nast, A. The diagnosis and treatment of oral cavity cancer. Dtsch. Arztebl. Int. 2012, 109, 829–835. [Google Scholar] [CrossRef]
- Shrivastava, A.; Boylan, J.; Bureau, Y.; Sousa, A.D.; Shah, N. Biological Trajectory for Psychosocial Risk Factors in Psychiatric Disorders—A Concept Based Review. Open J. Psychiatry 2015, 5, 7–18. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Guo, L.W.; Zhang, S.K.; Liu, S.Z.; Yang, F.N.; Zheng, L.Y.; Chen, Q.; Cao, X.Q.; Sun, X.B.; Zhang, J.G. Analysis of endoscopic screening compliance and related factors among high risk population of upper gastrointestinal cancer in urban areas of Henan Province from 2013 to 2017. Zhonghua Yu Fang Yi Xue Za Zhi (Chin. J. Prev. Med.) 2020, 54, 523–528. [Google Scholar] [CrossRef]
- Jelvehzadeh, F.; Dogaheh, E.R.; Bernstein, C.; Shakiba, S.; Ranjbar, H. The effect of a group cognitive behavioral therapy on the quality of life and emotional disturbance of women with breast cancer. Support. Care Cancer 2021, 30, 305–312. [Google Scholar] [CrossRef]
- Serrano, W.C.; Maldonado, J. The Use of Physostigmine in the Diagnosis and Treatment of Anticholinergic Toxicity after Olanzapine Overdose: Literature Review and Case Report. J. Acad. Consult. Liaison Psychiatry 2021, 62, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, L.F.; Aranda-Romo, S.; Rodríguez-Leyva, I.; Chi-Ahumada, E.; Saikaly, S.K.; Portales-Pérez, D.P.; González-Amaro, R.; Salgado-Bustamante, M.; Enriquez-Macias, L.; Eng, W.; et al. Tau Protein in Oral Mucosa and Cognitive State: A Cross-Sectional Study. Front. Neurol. 2017, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.; Azevedo, Á.; Brandão, I.; Gomes, P.S. Orofacial manifestations in outpatients with anorexia nervosa and bulimia nervosa focusing on the vomiting behavior. Clin. Oral Investig. 2018, 22, 1915–1922. [Google Scholar] [CrossRef]
- Nakasu, S.; Matsumura, K.; Nioka, H.; Handa, J. Lectin histochemistry of dermoid and epidermoid cysts of the central nervous system. Noshuyo Byori (Brain Tumor Pathol.) 1993, 10, 11–17. [Google Scholar]
- Giannetti, L.; Bambini, F.; Consolo, U. Teeth and oral mucosa in neurocutaneous syndromes, metabolic diseases and in diseases with defects of DNA repair. Minerva Stomatol. 2003, 52, 175–180. [Google Scholar]
- Gemonov, V.V.; Kutvitskaia, S.A.; Cherep, O.E.; Chebotareva, T.L. The reactivity characteristics of the organs of the oral cavity in an with different types of nervous systems. Stomatologiia 1996, 75, 12–14. [Google Scholar]
- Gholizadeh, N.; Mohebbi, A.H.; Mirzaii-Dizgah, I.; Sheykhbahaei, N. α1 adrenergic receptors in serum and saliva of patients with oral squamous cell carcinoma. Clin. Transl. Oncol. 2021, 23, 1705–1710. [Google Scholar] [CrossRef]
- Fernández Castro, M.; López-Pintor, R.M.; Serrano, J.; Ramírez, L.; Sanz, M.; Andreu, J.L.; Muñoz Fernández, S. Protocolised odontological assessment of patients with primary Sjögren’s syndrome. Reumatol. Clin. 2021, 17, 25–31. [Google Scholar] [CrossRef]
- Ritchie, A.; Kramer, J.M. Recent Advances in the Etiology and Treatment of Burning Mouth Syndrome. J. Dent. Res. 2018, 97, 1193–1199. [Google Scholar] [CrossRef]
- Mancini, M.; Vidal, S.M. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mamm. Genome 2018, 29, 425–445. [Google Scholar] [CrossRef]
- Baig, S.; Rubab, Z.; Farooq, W. Molecular Pathogenesis of Chewable Tobacco. J. Coll. Phys. Surg. Pak. 2018, 28, 381–385. [Google Scholar] [CrossRef]
- Puhakka, A.; Forssell, H.; Soinila, S.; Virtanen, A.; Röyttä, M.; Laine, M.; Tenovuo, O.; Teerijoki-Oksa, T.; Jääskeläinen, S.K. Peripheral nervous system involvement in primary burning mouth syndrome—Results of a pilot study. Oral Dis. 2016, 22, 338–344. [Google Scholar] [CrossRef]
- Present, S.I.; Check, J.H. Hypofunction of the Sympathetic Nervous System as a Possible Etiologic Cause of Recurrent Aphthous Stomatitis. Compend. Contin. Educ. Dent. 2016, 37, 381–385. [Google Scholar] [PubMed]
- Huang, H.C.; Nakatsuka, M.; Iwai, Y. Activation of microglial cells in the trigeminal subnucleus caudalis evoked by inflammatory stimulation of the oral mucosa. Okajimas Folia Anat. Jpn. 2013, 89, 137–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Cruz Pérez, E.; Rodríguez-Sainz, C. Poliovirus immunology: Vaccines, problems for the prevention/eradication and future interventions. Rev. Esp. Salud Publica 2013, 87, 443–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, K. Xerostomia and dysphagia. Clin. Calcium 2012, 22, 59–65. [Google Scholar] [PubMed]
- Woda, A.; Dao, T.; Gremeau-Richard, C. Steroid dysregulation and stomatodynia (burning mouth syndrome). J. Orofac. Pain 2009, 23, 202–210. [Google Scholar]
- Nakatsuka, M.; Iwai, Y. Expression of TRPV4 in the stimulated rat oral mucous membrane—Nociceptive mechanisms of lingual conical papillae. Okajimas Folia Anat. Jpn. 2009, 86, 45–54. [Google Scholar] [CrossRef][Green Version]
- Nissalo, S.; Hietanen, J.; Malmström, M.; Hukkanen, M.; Polak, J.; Konttinen, Y.T. Disorder-specific changes in innervation in oral lichen planus and lichenoid reactions. J. Oral Pathol. Med. 2000, 29, 361–369. [Google Scholar] [CrossRef]
- Ichikawa, H.; Sugimoto, T. Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience 2000, 101, 719–725. [Google Scholar] [CrossRef]
- Verzé, L.; Paraninfo, A.; Ramieri, G.; Viglietti-Panzica, C.; Panzica, G.C. Immunocytochemical evidence of plasticity in the nervous structures of the rat lower lip. Cell Tissue Res. 1999, 297, 203–211. [Google Scholar] [CrossRef]
- Aimetti, M.; Romano, F.; Cricenti, L.; Perotto, S.; Gotti, S.; Panzica, G.; Graziano, A. Merkel cells and permanent disesthesia in the oral mucosa after soft tissue grafts. J. Cell. Physiol. 2010, 224, 205–209. [Google Scholar] [CrossRef]
- Fernandes, N.D.; Arya, K.; Ward, R. Congenital Herpes Simplex. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Phyu, W.K.; Ong, K.C.; Wong, K.T. Modelling person-to-person transmission in an Enterovirus A71 orally infected hamster model of hand-foot-and-mouth disease and encephalomyelitis. Emerg. Microbes Infect. 2017, 6, e62. [Google Scholar] [CrossRef]
- Colletti, G.; Allevi, F.; Moneghini, L.; Rabbiosi, D.; Bertossi, D.; Frau, I.; Biglioli, F.; Tadini, G. Epidermal nevus and ameloblastoma: A rare association. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e275–e279. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Hartmann, K.; Lloret, A.; Ferrer, L.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Cryptococcosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 611–618. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, F.; Bollela, V.R.; Da Fonseca, B.A.; Martinez, R. Endemic paracoccidioidomycosis: Relationship between clinical presentation and patients’ demographic features. Med. Mycol. 2013, 51, 313–318. [Google Scholar] [CrossRef]
- O’Connor, M.B.; Phelan, M.J. The impact of HSV for inflammatory arthropathy patients. Rheumatol. Int. 2012, 32, 489–490. [Google Scholar] [CrossRef][Green Version]
- Ha, M.; Chung, J.W.; Hahm, K.B.; Kim, Y.J.; Lee, W.; An, J.; Kim, D.K.; Kim, M.G. A case of Cowden syndrome diagnosed from multiple gastric polyposis. World J. Gastroenterol. 2012, 18, 861–864. [Google Scholar] [CrossRef]
- Gündüz, O.; Sahiner, N.; Atasoy, P.; Senyücel, C. Acitretin treatment for lipoid proteinosis. Case Rep. Dermatol. Med. 2012, 2012, 324506. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; Liu, L.; Wang, J.; Zhao, H.; Liao, Y.; Na, R.; Dong, C.; Wang, L.; Xie, Z.; et al. Pathogenesis study of enterovirus 71 infection in rhesus monkeys. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1337–1350. [Google Scholar] [CrossRef]
- Tovaru, S.; Parlatescu, I.; Dumitriu, A.S.; Bucur, A.; Kaplan, I. Oral complications associated with D-penicillamine treatment for Wilson disease: A clinicopathologic report. J. Periodontol. 2010, 81, 1231–1236. [Google Scholar] [CrossRef]
- Kastrukoff, L.F.; Lau, A.S.; Takei, F.; Smyth, M.J.; Jones, C.M.; Clarke, S.R.; Carbone, F.R. Redundancy in the immune system restricts the spread of HSV-1 in the central nervous system (CNS) of C57BL/6 mice. Virology 2010, 400, 248–258. [Google Scholar] [CrossRef]
- Binitha, M.P.; Thomas, D.; Asha, L.K. Tuberous sclerosis complex associated with dyschromatosis universalis hereditaria. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 300–302. [Google Scholar] [CrossRef]
- López de Blanc, S.; Sambuelli, R.; Femopase, F.; Luna, N.; Gravotta, M.; David, D.; Bistoni, A.; Criscuolo, M.I. Bacillary angiomatosis affecting the oral cavity. Report of two cases and review. J. Oral Pathol. Med. 2000, 29, 91–96. [Google Scholar] [CrossRef]
- Nelen, M.R.; Padberg, G.W.; Peeters, E.A.; Lin, A.Y.; van den Helm, B.; Frants, R.R.; Coulon, V.; Goldstein, A.M.; van Reen, M.M.; Easton, D.F.; et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat. Genet. 1996, 13, 114–116. [Google Scholar] [CrossRef]
- Chan, T.Y.; Critchley, J.A. Pulmonary aspiration following Dettol poisoning: The scope for prevention. Hum. Exp. Toxicol. 1996, 15, 843–846. [Google Scholar] [CrossRef]
- von den Driesch, P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J. Am. Acad. Dermatol. 1994, 31, 535–556; quiz 557–560. [Google Scholar] [CrossRef]
- Gesser, R.M.; Valyi-Nagy, T.; Altschuler, S.M.; Fraser, N.W. Oral-oesophageal inoculation of mice with herpes simplex virus type 1 causes latent infection of the vagal sensory ganglia (nodose ganglia). J. Gen. Virol. 1994, 75 Pt 9, 2379–2386. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Kurz, K.; Bratzke, B.; Orfanos, C.E. Adamantiades-Behçet disease: Necrotizing systemic vasculitis with a fatal outcome. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 1991, 42, 451–454. [Google Scholar]
- Molinoff, P.B. α- and β-adrenergic receptor subtypes properties, distribution and regulation. Drugs 1984, 28 (Suppl. S2), 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moretti-Rojas, I.; Ezrailson, E.G.; Birnbaumer, L.; Entman, M.L.; Garber, A.J. Serotonergic and adrenergic regulation of skeletal muscle metabolism in the rat. II. The use of [125I]iodolysergic acid diethylamide and [125I]iodopindolol as probes of sarcolemmal receptor function and specificity. J. Biol. Chem. 1983, 258, 12499–12508. [Google Scholar] [CrossRef]
- Lüthy, I.A.; Bruzzone, A.; Piñero, C.P.; Castillo, L.F.; Chiesa, I.J.; Vázquez, S.M.; Sarappa, M.G. Adrenoceptors: Non conventional target for breast cancer? Curr. Med. Chem. 2009, 16, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, C.; Groh, L.; Keating, S.T.; Kaffa, C.; Noz, M.P.; Kersten, S.; van Herwaarden, A.E.; Hoischen, A.; Joosten, L.A.B.; Timmers, H.; et al. Catecholamines Induce Trained Immunity in Monocytes In Vitro and In Vivo. Circ. Res. 2020, 127, 269–283. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, C.; Chen, X.; Fei, Y.; Jiang, L.; Wang, G. Vitamin C Promotes Apoptosis and Cell Cycle Arrest in Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 976. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Sarode, S.C.; Hedaoo, A.; Dasgupta, S.; Sharma, B.; Sharma, A.; Yuwanati, M.; Gondivkar, R.S.; Gaikwad, R.N.; et al. Oral Psychosomatic Disorders in Family Caregivers of Oral Squamous Cell Carcinoma Patients. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 477–483. [Google Scholar] [CrossRef]
- Morse, D.E.; Psoter, W.J.; Cleveland, D.; Cohen, D.; Mohit-Tabatabai, M.; Kosis, D.L.; Eisenberg, E. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Contr. 2007, 18, 919–929. [Google Scholar] [CrossRef]
- Schell, J.T.; Petermann-Meyer, A.; Kloss-Brandstätter, A.; Bartella, A.K.; Kamal, M.; Hölzle, F.; Lethaus, B.; Teichmann, J. Distress thermometer for preoperative screening of patients with oral squamous cell carcinoma. J. Cranio-Maxillo-Facial Surg. 2018, 46, 1111–1116. [Google Scholar] [CrossRef]
- Almangush, A.; Leivo, I.; Mäkitie, A.A. Biomarkers for Immunotherapy of Oral Squamous Cell Carcinoma: Current Status and Challenges. Front. Oncol. 2021, 11, 616629. [Google Scholar] [CrossRef]
- D’Addazio, G.; Artese, L.; Traini, T.; Rubini, C.; Caputi, S.; Sinjari, B. Immunohistochemical study of osteopontin in oral squamous cell carcinoma allied to fractal dimension. J. Biol. Regul. Homeost. Agents 2018, 32, 1033–1038. [Google Scholar]
- Nishioka, T.; Tada, H.; Ibaragi, S.; Chen, C.; Sasano, T. Nicotine exposure induces the proliferation of oral cancer cells through the α7 subunit of the nicotinic acetylcholine receptor. Biochem. Biophys. Res. Commun. 2019, 509, 514–520. [Google Scholar] [CrossRef]
- Catassi, A.; Servent, D.; Paleari, L.; Cesario, A.; Russo, P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: Implications on lung carcinogenesis. Mutat. Res. Rev. Mutat. Res. 2008, 659, 221–231. [Google Scholar] [CrossRef]
- Hartwig, C.L.; Sprick, J.D.; Jeong, J.; Hu, Y.; Morison, D.G.; Stein, C.M.; Paranjape, S.; Park, J. Increased vascular α1-adrenergic receptor sensitivity in older adults with posttraumatic stress disorder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R611–R616. [Google Scholar] [CrossRef]
- Aykac, A.; Şehirli, A.; Gören, M.Z. Evaluation of the Effect of Prazosin Treatment on α-2c Adrenoceptor and Apoptosis Protein Levels in the Predator Scent-Induced Rat Model of Post-Traumatic Stress Disorder. J. Mol. Neurosci. 2020, 70, 1120–1129. [Google Scholar] [CrossRef]
- Sharma, P.; Sandhu, S.V.; Bhandari, R.; Verma, I.; Bhullar, R.K.; Khangura, R.K. Estimation of cortisol levels in patients with premalignant disorders and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2018, 22, 27–34. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, D.-W.; Stein, M.A. Increased Cortisol after Stress is Associated with Variability in Response Time in ADHD Children. Yonsei Med. J. 2010, 51, 206–211. [Google Scholar] [CrossRef][Green Version]
- Suleyman, H.; Halici, Z.; Cadirci, E.; Hacimuftuoglu, A.; Bilen, H. Indirect role of beta2-adrenergic receptors in the mechanism of anti-inflammatory action of NSAIDS. J. Physiol. Pharmacol. 2008, 59, 661–672. [Google Scholar]
- Myslivecek, J.; Rícný, J.; Kolár, F.; Tucek, S. The effects of hydrocortisone on rat heart muscarinic and adrenergic alpha 1, beta 1 and beta 2 receptors, propranolol-resistant binding sites and on some subsequent steps in intracellular signalling. Naunyn-Schmiedebergs Arch. Pharmacol. 2003, 368, 366–376. [Google Scholar] [CrossRef]
- Hoen, S.; Mazoit, J.X.; Asehnoune, K.; Brailly-Tabard, S.; Benhamou, D.; Moine, P.; Edouard, A.R. Hydrocortisone increases the sensitivity to alpha1-adrenoceptor stimulation in humans following hemorrhagic shock. Crit. Care Med. 2005, 33, 2737–2743. [Google Scholar] [CrossRef]
- Maeda, H.; Watanabe, Y.; Lai, R.T.; Yoshida, H. Effect of glucocorticoids on alpha-2 adrenoceptors in vas deferens of reserpinized rat in organ culture. Life Sci. 1983, 33, 39–46. [Google Scholar] [CrossRef]
- Cuneo, R.C.; Livesey, J.H.; Nicholls, M.G.; Espiner, E.A.; Donald, R.A. Effects of alpha-1 adrenergic blockade on the hormonal response to hypoglycaemic stress in normal man. Clin. Endocrinol. 1987, 26, 1–8. [Google Scholar] [CrossRef]
- Obata, K.; Shimo, T.; Okui, T.; Matsumoto, K.; Takada, H.; Takabatake, K.; Kunisada, Y.; Ibaragi, S.; Yoshioka, N.; Kishimoto, K.; et al. Role of Neurokinin 3 Receptor Signaling in Oral Squamous Cell Carcinoma. Anticancer Res. 2017, 37, 6119–6123. [Google Scholar] [CrossRef]
- Obata, K.; Shimo, T.; Okui, T.; Matsumoto, K.; Takada, H.; Takabatake, K.; Kunisada, Y.; Ibaragi, S.; Nagatsuka, H.; Sasaki, A. Tachykinin Receptor 3 Distribution in Human Oral Squamous Cell Carcinoma. Anticancer Res. 2016, 36, 6335–6341. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Zhang, Y.; Wang, J.; Zhao, T.; Ji, P.; Song, J.; Zhang, H.; Luo, W. Proliferative effects of gamma-amino butyric acid on oral squamous cell carcinoma cells are associated with mitogen—Activated protein kinase signaling pathways. Int. J. Mol. Med. 2016, 38, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Kasamatsu, A.; Koyama, T.; Fukumoto, C.; Kouzu, Y.; Higo, M.; Endo-Sakamoto, Y.; Ogawara, K.; Shiiba, M.; Tanzawa, H.; et al. Glutamate acid decarboxylase 1 promotes metastasis of human oral cancer by β-catenin translocation and MMP7 activation. BMC Cancer 2013, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.H.; Say, Y.H. Resistance against tumour necrosis factor α apoptosis by the cellular prion protein is cell-specific for oral, colon and kidney cancer cell lines. Cell Biol. Int. 2012, 36, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.H.; Say, Y.H. Resistance against apoptosis by the cellular prion protein is dependent on its glycosylation status in oral HSC-2 and colon LS 174T cancer cells. Cancer Lett. 2011, 306, 111–119. [Google Scholar] [CrossRef]
- Saito, T.; Kasamatsu, A.; Ogawara, K.; Miyamoto, I.; Saito, K.; Iyoda, M.; Suzuki, T.; Endo-Sakamoto, Y.; Shiiba, M.; Tanzawa, H.; et al. Semaphorin7A Promotion of Tumoral Growth and Metastasis in Human Oral Cancer by Regulation of G1 Cell Cycle and Matrix Metalloproteases: Possible Contribution to Tumoral Angiogenesis. PLoS ONE 2015, 10, e0137923. [Google Scholar] [CrossRef]
- Ahmed, A.; Gulino, A.; Amayo, S.; Arancio, W.; Florena, A.M.; Belmonte, B.; Jurjus, A.; Leone, A.; Miletich, I. Natriuretic peptide system expression in murine and human submandibular salivary glands: A study of the spatial localisation of ANB, BNP, CNP and their receptors. J. Mol. Histol. 2020, 51, 3–13. [Google Scholar] [CrossRef]
- Binmadi, N.O.; Basile, J.R. Perineural invasion in oral squamous cell carcinoma: A discussion of significance and review of the literature. Oral Oncol. 2011, 47, 1005–1010. [Google Scholar] [CrossRef]
- Ide, F.; Ito, Y.; Matsuoka, K.; Muramatsu, T.; Saito, I. Re-excision perineural invasion in oral squamous cell carcinoma. Oral Dis. 2014, 20, 219–220. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, W.; Wu, B.; Fang, F. Long non-coding RNAs are novel players in oral inflammatory disorders, potentially premalignant oral epithelial lesions and oral squamous cell carcinoma (Review). Int. J. Mol. Med. 2020, 46, 535–545. [Google Scholar] [CrossRef]
- Pereira, C.M.; Sehnem, D.; da Fonseca, E.O.; Barboza, H.F.G.; de Carvalho, A.C.P.; DaSilva, A.F.M.; Moura-Neto, V.; DosSantos, M.F. miRNAs: Important Targets for Oral Cancer Pain Research. BioMed Res. Int. 2017, 2017, 4043516. [Google Scholar] [CrossRef]
- Xie, S.F.; Li, C.C.; He, H.; Xia, X.Y.; Wang, X.T. Current advances in research of burning mouth syndrome: Biological nerve and social psychology patterns. Zhonghua Kou Qiang Yi Xue Za Zhi 2020, 55, 123–128. [Google Scholar] [CrossRef]
- Galli, F.; Lodi, G.; Sardella, A.; Vegni, E. Role of psychological factors in burning mouth syndrome: A systematic review and meta-analysis. Ceph. Int. J. Headache 2017, 37, 265–277. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, J.; Fan, Y.; Svensson, P.; Wang, K. Thermal and mechanical quantitative sensory testing in Chinese patients with burning mouth syndrome—A probable neuropathic pain condition? J. Headache Pain 2015, 16, 84. [Google Scholar] [CrossRef]
- Lauria, G.; Majorana, A.; Borgna, M.; Lombardi, R.; Penza, P.; Padovani, A.; Sapelli, P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 2005, 115, 332–337. [Google Scholar] [CrossRef]
- Feller, L.; Fourie, J.; Bouckaert, M.; Khammissa, R.A.G.; Ballyram, R.; Lemmer, J. Burning Mouth Syndrome: Aetiopathogenesis and Principles of Management. Pain Res. Manag. 2017, 2017, 1926269. [Google Scholar] [CrossRef]
- Lin, M.; Li, B.; Gu, F.; Yue, Y.; Huang, Y.; Chen, Q.; Zeng, G.; Xia, J. Study on psychoprophylaxis and monoamines neurotransmitter of patients with burning mouth syndrome. Hua Xi Yi Ke Da Xue Xue Bao 2001, 32, 576–578. [Google Scholar]
- Sinjari, B.; Feragalli, B.; Cornelli, U.; Belcaro, G.; Vitacolonna, E.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Zuccari, F.; Caputi, S. Artificial Saliva in Diabetic Xerostomia (ASDIX): Double Blind Trial of Aldiamed((R)) Versus Placebo. J. Clin. Med. 2020, 9, 2196. [Google Scholar] [CrossRef]
- Wada, A.; Shizukuishi, T.; Kikuta, J.; Yamada, H.; Watanabe, Y.; Imamura, Y.; Shinozaki, T.; Dezawa, K.; Haradome, H.; Abe, O. Altered structural connectivity of pain-related brain network in burning mouth syndrome-investigation by graph analysis of probabilistic tractography. Neuroradiology 2017, 59, 525–532. [Google Scholar] [CrossRef]
- Sinding, C.; Gransjøen, A.M.; Schlumberger, G.; Grushka, M.; Frasnelli, J.; Singh, P.B. Grey matter changes of the pain matrix in patients with burning mouth syndrome. Eur. J. Neurosci. 2016, 43, 997–1005. [Google Scholar] [CrossRef]
- Lee, Y.C.; Jahng, G.H.; Ryu, C.W.; Byun, J.Y. Change in gray matter volume and cerebral blood flow in patients with burning mouth syndrome. J. Oral Pathol. Med. 2019, 48, 335–342. [Google Scholar] [CrossRef]
- Jääskeläinen, S.K. Pathophysiology of primary burning mouth syndrome. Clin. Neurophysiol. 2012, 123, 71–77. [Google Scholar] [CrossRef]
- Martikainen, I.K.; Hagelberg, N.; Jääskeläinen, S.K.; Hietala, J.; Pertovaara, A. Dopaminergic and serotonergic mechanisms in the modulation of pain: In vivo studies in human brain. Eur. J. Pharmacol. 2018, 834, 337–345. [Google Scholar] [CrossRef]
- Guarneri, F.; Guarneri, C.; Marini, H. Contribution of neuroinflammation in burning mouth syndrome: Indications from benzodiazepine use. Dermatol. Ther. 2008, 21, S21–S24. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Gallelli, L.; Marigliano, N.M.; Orlando, P.; De Sarro, G. Use of topiramate for glossodynia. Pain Med. 2007, 8, 531–534. [Google Scholar] [CrossRef][Green Version]
- Mendak-Ziółko, M.; Konopka, T.; Bogucki, Z.A. Evaluation of select neurophysiological, clinical and psychological tests for burning mouth syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 325–332. [Google Scholar] [CrossRef]
- Momota, Y.; Takano, H.; Kani, K.; Matsumoto, F.; Motegi, K.; Aota, K.; Yamamura, Y.; Omori, M.; Tomioka, S.; Azuma, M. Frequency analysis of heart rate variability: A useful assessment tool of linearly polarized near-infrared irradiation to stellate ganglion area for burning mouth syndrome. Pain Med. 2013, 14, 351–357. [Google Scholar] [CrossRef][Green Version]
- Varvat, J.; Thomas-Anterion, C.; Decousus, M.; Perret-Liaudet, A.; Laurent, B. Atypical Lewy body disease revealed by burning mouth syndrome and a pseudo-psychiatric syndrome. Rev. Neurol. 2010, 166, 547–549. [Google Scholar] [CrossRef]
- Koszewicz, M.; Mendak, M.; Konopka, T.; Koziorowska-Gawron, E.; Budrewicz, S. The characteristics of autonomic nervous system disorders in burning mouth syndrome and Parkinson disease. J. Orofac. Pain 2012, 26, 315–320. [Google Scholar] [PubMed]
- Mignogna, M.D.; Adamo, D.; Schiavone, V.; Ravel, M.G.; Fortuna, G. Burning mouth syndrome responsive to duloxetine: A case report. Pain Med. 2011, 12, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Shinozaki, T.; Hara, K.; Noma, N.; Okada-Ogawa, A.; Asano, M.; Shinoda, M.; Eliav, E.; Gracely, R.H.; Iwata, K.; et al. Immune and endocrine function in patients with burning mouth syndrome. Clin. J. Pain 2014, 30, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tokura, T.; Yoshida, K.; Nagashima, W.; Kimura, H.; Umemura, E.; Tachibana, M.; Miyauchi, T.; Kobayashi, Y.; Arao, M.; et al. Five Patients with Burning Mouth Syndrome in Whom an Antidepressant (Serotonin-Noradrenaline Reuptake Inhibitor) Was Not Effective, but Pregabalin Markedly Relieved Pain. Clin. Neuropharmacol. 2015, 38, 158–161. [Google Scholar] [CrossRef]
- Eccleston, C.; Hearn, L.; Williams, A.C. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, 2015, CD011259. [Google Scholar] [CrossRef]
- Imamura, Y.; Okada-Ogawa, A.; Noma, N.; Shinozaki, T.; Watanabe, K.; Kohashi, R.; Shinoda, M.; Wada, A.; Abe, O.; Iwata, K. A perspective from experimental studies of burning mouth syndrome. J. Oral Sci. 2020, 62, 165–169. [Google Scholar] [CrossRef]
- de Pedro, M.; López-Pintor, R.M.; Casañas, E.; Hernández, G. General health status of a sample of patients with burning mouth syndrome: A case-control study. Oral Dis. 2020, 26, 1020–1031. [Google Scholar] [CrossRef]
- Adler, C.J.; Dobney, K.; Weyrich, L.S.; Kaidonis, J.; Walker, A.W.; Haak, W.; Bradshaw, C.J.; Townsend, G.; Sołtysiak, A.; Alt, K.W.; et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 2013, 45, 450–455. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef]
- Gall-Troselj, K.; Mravak-Stipetić, M.; Jurak, I.; Ragland, W.L.; Pavelić, J. Helicobacter pylori colonization of tongue mucosa—Increased incidence in atrophic glossitis and burning mouth syndrome (BMS). J. Oral Pathol. Med. 2001, 30, 560–563. [Google Scholar] [CrossRef]
- Brailo, V.; Vuéiaeeviae-Boras, V.; Alajbeg, I.Z.; Alajbeg, I.; Lukenda, J.; Aeurkoviae, M. Oral burning symptoms and burning mouth syndrome-significance of different variables in 150 patients. Med. Oral Patol. Oral Y Cir. Bucal 2006, 11, E252–E255. [Google Scholar]
- Chung, M.K.; Wang, S.; Oh, S.L.; Kim, Y.S. Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment. Int. J. Mol. Sci. 2021, 22, 5810. [Google Scholar] [CrossRef]
- Porter, S.R.; Scully, C.; Pedersen, A. Recurrent Aphthous Stomatitis. Crit. Rev. Oral Biol. Med. 1998, 9, 306–321. [Google Scholar] [CrossRef]
- Altenburg, A.; El-Haj, N.; Micheli, C.; Puttkammer, M.; Abdel-Naser, M.B.; Zouboulis, C.C. The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int. 2014, 111, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Dhopte, A.; Naidu, G.; Singh-Makkad, R.; Nagi, R.; Bagde, H.; Jain, S. Psychometric analysis of stress, anxiety and depression in patients with recurrent aphthous Stomatitis—A cross-sectional survey based study. J. Clin. Exp. Dent. 2018, 10, e1109–e1114. [Google Scholar] [CrossRef] [PubMed]
- Nadendla, L.K.; Meduri, V.; Paramkusam, G.; Pachava, K.R. Relationship of salivary cortisol and anxiety in recurrent aphthous stomatitis. Indian J. Endocrinol. Metab. 2015, 19, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Aminian, M.; Raygani, A.V. Evaluation of Salivary Cortisol Changes and Psychological Profiles in Patients with Recurrent Aphthous Stomatitis. Contemp. Clin. Dent. 2017, 8, 259–263. [Google Scholar] [CrossRef]
- Eguia-del Valle, A.; Martínez-Conde-Llamosas, R.; López-Vicente, J.; Uribarri-Etxebarria, A.; Aguirre-Urizar, J.M. Salivary cortisol determination in patients from the Basque Country with recurrent aphthous stomatitis. A pilot study. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e207–e211. [Google Scholar] [CrossRef]
- McCartan, B.E.; Lamey, P.J.; Wallace, A.M. Salivary cortisol and anxiety in recurrent aphthous stomatitis. J. Oral Pathol. Med. 1996, 25, 357–359. [Google Scholar] [CrossRef]
- Albanidou-Farmaki, E.; Poulopoulos, A.K.; Epivatianos, A.; Farmakis, K.; Karamouzis, M.; Antoniades, D. Increased anxiety level and high salivary and serum cortisol concentrations in patients with recurrent aphthous stomatitis. Tohoku J. Exp. Med. 2008, 214, 291–296. [Google Scholar] [CrossRef]
- Hong, C.; Wang, W.M.; Wang, X.; Tang, W. Assessment of mental health status and plasma catecholamine levels in patients with recurrent aphthous ulcer. Shanghai Kou Qiang Yi Xue (Shanghai J. Stomatol.) 2018, 27, 48–51. [Google Scholar]
- Cardoso, J.A.; Dos Santos Junior, A.A.; Nunes, M.L.; de Figueiredo, M.A.; Cherubini, K.; Salum, F.G. Salivary Alpha-Amylase Enzyme, Psychological Disorders, and Life Quality in Patients with Recurrent Aphthous Stomatitis. Int. J. Dent. 2017, 2017, 5269856. [Google Scholar] [CrossRef]
- Broche, N., Jr.; Takeshita, R.S.C.; Mouri, K.; Bercovitch, F.B.; Huffman, M.A. Salivary alpha-amylase enzyme is a non-invasive biomarker of acute stress in Japanese macaques (Macaca fuscata). Primates J. Primatol. 2019, 60, 547–558. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Yamaguchi, T.; Sato, S.; Oho, T. Oral Fusobacterium nucleatum subsp. polymorphum binds to human salivary α-amylase. Mol. Oral Microbiol. 2013, 28, 425–434. [Google Scholar] [CrossRef]
- Victoria, J.M.; Correia-Silva Jde, F.; Pimenta, F.J.; Kalapothakis, E.; Gomez, R.S. Serotonin transporter gene polymorphism (5-HTTLPR) in patients with recurrent aphthous stomatitis. J. Oral Pathol. Med. 2005, 34, 494–497. [Google Scholar] [CrossRef]
- Ni Riordain, R.; Meaney, S.; McCreary, C. Impact of chronic oral mucosal disease on daily life: Preliminary observations from a qualitative study. Oral Dis. 2011, 17, 265–269. [Google Scholar] [CrossRef]
- Frid, A.H.; Kreugel, G.; Grassi, G.; Halimi, S.; Hicks, D.; Hirsch, L.J.; Smith, M.J.; Wellhoener, R.; Bode, B.W.; Hirsch, I.B.; et al. New Insulin Delivery Recommendations. Mayo Clin. Proc. 2016, 91, 1231–1255. [Google Scholar] [CrossRef]
- Lee, H.; Macpherson, L.J.; Parada, C.A.; Zuker, C.S.; Ryba, N.J.P. Rewiring the taste system. Nature 2017, 548, 330–333. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ou-Yang, Q.; Xia, B.; Liu, L.N.; Gu, F.; Zhou, K.F.; Mei, Q.; Shi, R.H.; Ran, Z.H.; Wang, X.D.; et al. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J. Gastroenterol. 2013, 19, 1827–1833. [Google Scholar] [CrossRef]
- Motoki, K.; Suzuki, S. Extrinsic Factors Underlying Food Valuation in the Human Brain. Front. Behav. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef]
- Winsor, A.M.; Ihle, M.; Taylor, L.A. Methods for independently manipulating palatability and color in small insect prey. PLoS ONE 2020, 15, e0231205. [Google Scholar] [CrossRef]
- Bowen, D.J.; Grunberg, N.E. Variations in food preference and consumption across the menstrual cycle. Physiol. Behav. 1990, 47, 287–291. [Google Scholar] [CrossRef]
- Potvin Kent, M.; Pauzé, E.; Roy, E.A.; de Billy, N.; Czoli, C. Children and adolescents’ exposure to food and beverage marketing in social media apps. Pediatr. Obes. 2019, 14, e12508. [Google Scholar] [CrossRef]
- Jensterle, M.; DeVries, J.H.; Battelino, T.; Battelino, S.; Yildiz, B.; Janez, A. Glucagon-like peptide-1, a matter of taste? Rev. Endocr. Metab. Disord. 2021, 22, 763–775. [Google Scholar] [CrossRef]
- Ghonghadze, M.; Pachkoria, K.; Okujava, M.; Antelava, N.; Gongadze, N. Endocannabinoids receptors mediated central and peripheral effects (review). Georgian Med. News 2020, 298, 137–143. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Gao, L.; Du, Y.; Xu, J. Insight into the Role of Psychological Factors in Oral Mucosa Diseases. Int. J. Mol. Sci. 2022, 23, 4760. https://doi.org/10.3390/ijms23094760
Guo Y, Wang B, Gao H, He C, Hua R, Gao L, Du Y, Xu J. Insight into the Role of Psychological Factors in Oral Mucosa Diseases. International Journal of Molecular Sciences. 2022; 23(9):4760. https://doi.org/10.3390/ijms23094760
Chicago/Turabian StyleGuo, Yuexin, Boya Wang, Han Gao, Chengwei He, Rongxuan Hua, Lei Gao, Yixuan Du, and Jingdong Xu. 2022. "Insight into the Role of Psychological Factors in Oral Mucosa Diseases" International Journal of Molecular Sciences 23, no. 9: 4760. https://doi.org/10.3390/ijms23094760
APA StyleGuo, Y., Wang, B., Gao, H., He, C., Hua, R., Gao, L., Du, Y., & Xu, J. (2022). Insight into the Role of Psychological Factors in Oral Mucosa Diseases. International Journal of Molecular Sciences, 23(9), 4760. https://doi.org/10.3390/ijms23094760