The Benefits of Using Saccharose for Photocatalytic Water Disinfection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Saccharose-Modified TiO2
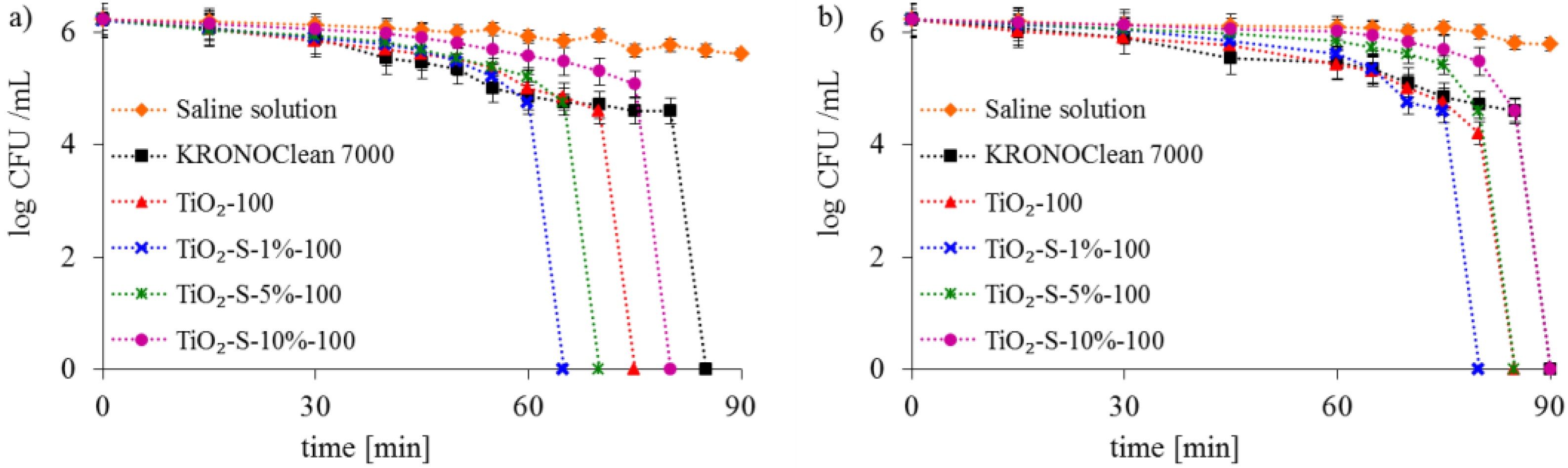
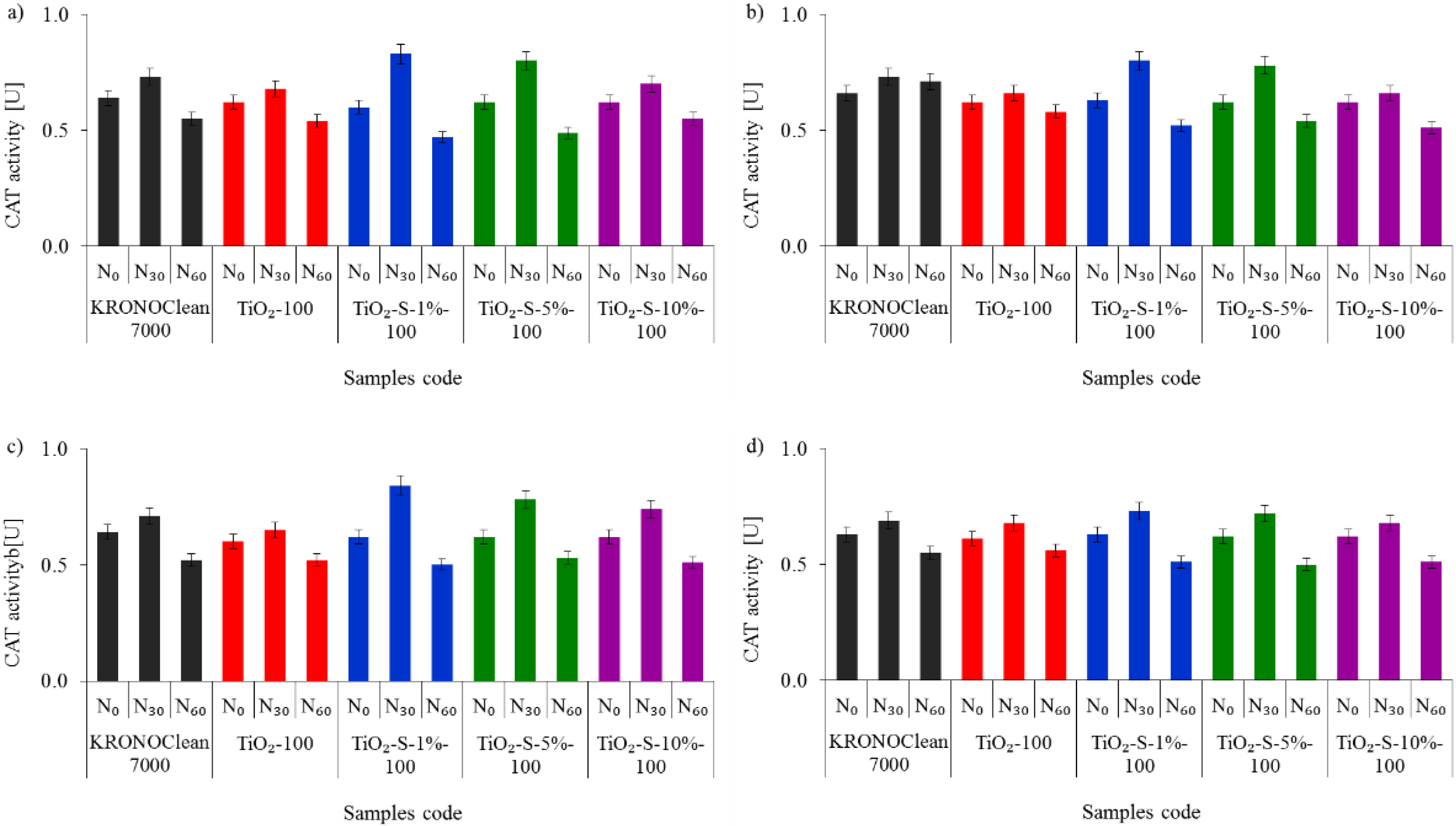
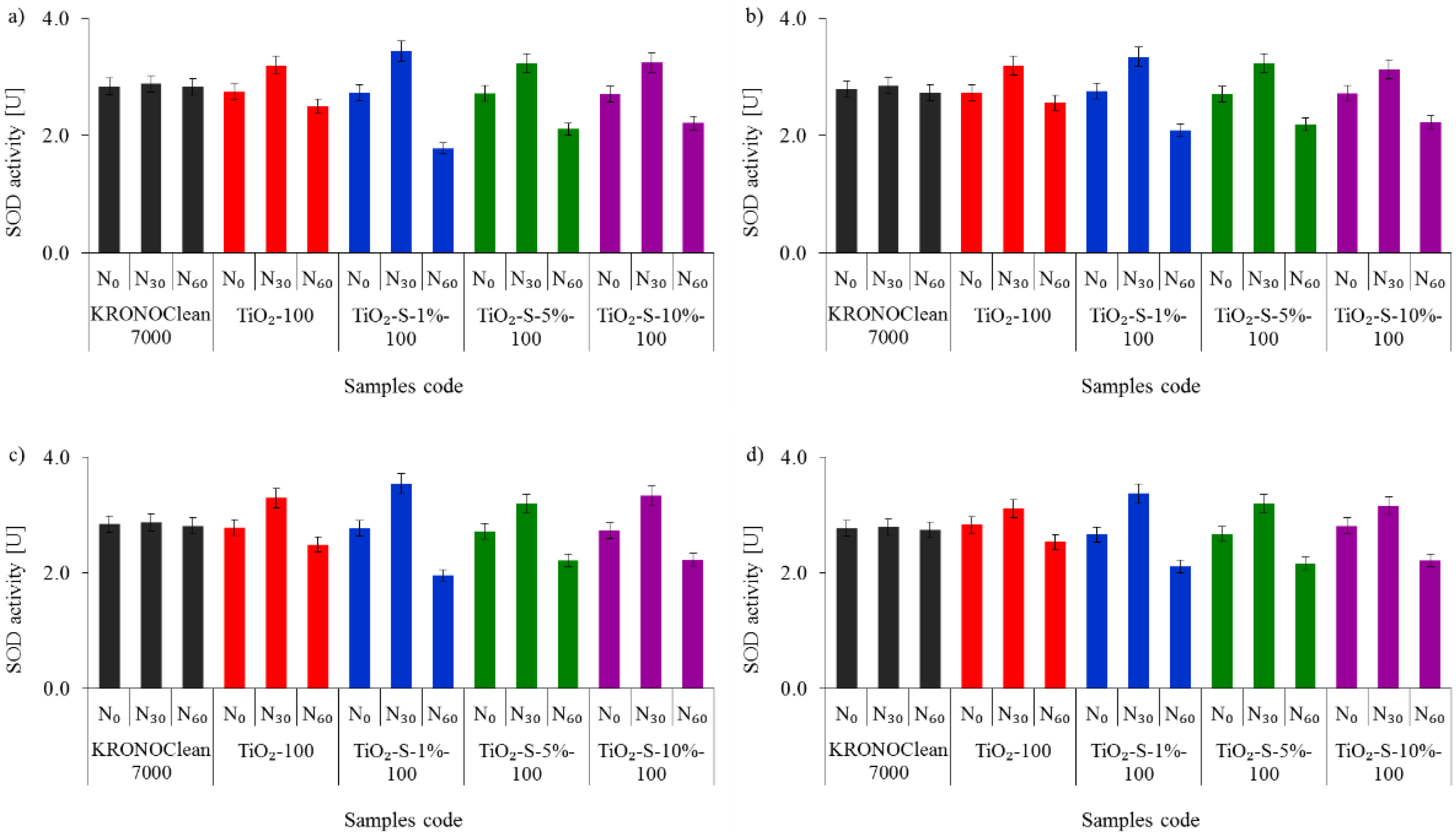
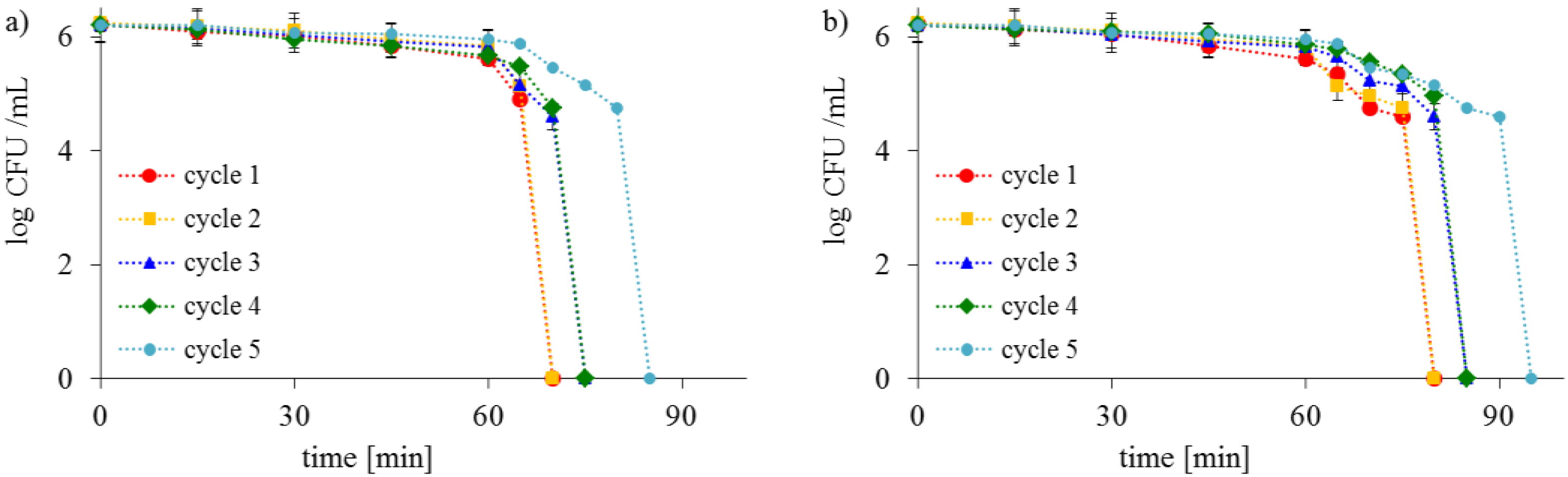
2.2. Antimicrobial Activity of Saccharose-Modified TiO2
3. Materials and Methods
3.1. Preparation of Saccharose-Modified Photocatalysts and Their Characterization
3.2. Microbiological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO/UNICEF Joint Water Supply; Sanitation Monitoring Program; World Health Organization. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241509145. [Google Scholar]
- Karim, M.R.; Khan, M.H.R.B.; Akash, M.A.S.A.; Shams, S. Effectiveness of solar disinfection for household water treatment: An experimental and modeling study. J. Water Sanit. Hyg. Dev. 2021, 11, 374–385. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Rott, M.B. Solar disinfection (SODIS) technologies as alternative for large-scale public drinking water supply: Advances and challenges. Chemosphere 2021, 281, 130754. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.Q.; Ho, S.H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar]
- Mahy, J.G.; Wolfs, C.; Vreuls, C.; Drot, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Hermans, S.; Lambert, S.D. Advanced oxidation processes for waste water treatment: From laboratory-scale model water to on-site real waste water. Environ. Technol. 2021, 42, 3974–3986. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Wong, V.L.; Phang, S.J. Recent advances on TiO2 photocatalysis for wastewater degradation: Fundamentals, commercial TiO2 materials, and photocatalytic reactors. In Handbook of Nanotechnology Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–65. [Google Scholar]
- Magaña-López, R.; Zaragoza-Sánchez, P.I.; Jiménez-Cisneros, B.E.; Chávez-Mejía, A.C. The use of TiO2 as a disinfectant in water sanitation applications. Water 2021, 13, 1641. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Jiang, C.; Yu, J.; Zhang, P. Review on design and evaluation of environmental photocatalysts. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Silva, T.F.; Peri, P.; Fajardo, A.S.; Paulista, L.O.; Soares, P.A.; Martínez-Huitle, C.A.; Vilar, V.J. Solar-driven heterogeneous photocatalysis using a static mixer as TiO2-P25 support: Impact of reflector optics and material. Chem. Eng. J. 2022, 435, 134831. [Google Scholar] [CrossRef]
- Alkorbi, A.S.; Javed, H.M.A.; Hussain, S.; Latif, S.; Mahr, M.S.; Mustafa, M.S.; Alsaiari, R.; Alhemiary, N.A. Solar light-driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles. Opt. Mater. 2022, 127, 112259. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent advances in synthesis and applications of carbon-doped TiO2 nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N-and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Lin, X.; Rong, F.; Ji, X.; Fu, D. Carbon-doped mesoporous TiO2 film and its photocatalytic activity. Microporous Mesoporous Mater. 2011, 142, 276–281. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lewis, J.P. Second-generation photocatalytic materials: Anion-doped TiO2. J. Phys. Condens. Matter 2006, 18, 421–434. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Rokicka, P.; Wang, K.; Endo, M.; Morawski, A.W.; Kowalska, E. Photocatalytic water disinfection under solar irradiation by D-glucose-modified titania. Catalysts 2018, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Kusiak-Nejman, E.; Morawski, A.W. Photocatalytic water disinfection under the artificial solar light by fructose-modified TiO2. Chem. Eng. J. 2019, 372, 203–215. [Google Scholar] [CrossRef]
- Shintani, T. Food Industrial production of monosaccharides using microbial, enzymatic, and chemical methods. Fermentation 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Hamal, D.; Yang, X.; Kwen, H.; Jones, D.; Rajagopalan, S.; Klabunde, K.J. Defining reactivity of solid sorbents: What is the most appropriate metric? Chem. Mater. 2009, 21, 2367–2374. [Google Scholar] [CrossRef]
- Maira, A.J.; Coronado, J.M.; Augugliaro, V.; Yeung, K.L.; Conesa, J.C.; Soria, J.J. Fourier transform infrared study of the performance of nanostructured TiO2 particles for the photocatalytic oxidation of gaseous toluene. J. Catal. 2001, 202, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Hadjiivanov, K. FTIR study of CO and NH3 co-adsorption on TiO2 (rutile). Appl. Surf. Sci. 1998, 135, 331–338. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.H.; Choi, W. Glucose-TiO2 charge transfer complex-mediated photocatalysis under visible light. Appl. Catal. B Environ. 2015, 162, 463–469. [Google Scholar] [CrossRef]
- Dong, F.; Wang, H.; Wu, Z.J. One-step “green” synthetic approach for mesoporous C-doped titanium dioxide with efficient visible light photocatalytic activity. J. Phys. Chem. C 2009, 113, 16717–16723. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Tul’chinsky, V.M.; Zurabyan, S.E.; Asankozhoev, K.A.; Kogan, G.A.; Khorlin, A.Y. Study of the infrared spectra of oligosaccharides in the region 1,000–40 cm−1. Carbohydr. Res. 1976, 51, 1–8. [Google Scholar] [CrossRef]
- Brizuela, A.B.; Bichara, L.C.; Romano, E.; Yurquina, A.; Locatelli, S.; Brandán, S.A. A complete characterization of the vibrational spectra of sucrose. Carbohydr. Res. 2012, 361, 212–218. [Google Scholar] [CrossRef]
- Garg, H.; Cowman, M.K.; Hales, C. Carbohydrate Chemistry Biology and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ząbek, P.; Eberl, J.; Kisch, H. On the origin of visible light activity in carbon-modified titania. Photochem. Photobiol. Sci. 2009, 8, 264–269. [Google Scholar] [CrossRef]
- Colón, G.; Sanchez-Espana, J.M.; Hidalgo, M.C.; Navío, J.A. Effect of TiO2 acidic pre-treatment on the photocatalytic properties for phenol degradation. J. Photochem. Photobiol. A Chem. 2006, 179, 20–27. [Google Scholar] [CrossRef]
- Morawski, A.W.; Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Wróbel, R.J.; Ohtani, B.; Lipińska, L. Photocatalytic degradation of acetic acid in the presence of visible light-active TiO2-reduced graphene oxide photocatalysts. Catal. Today 2017, 280, 108–113. [Google Scholar] [CrossRef]
- Available online: https://kronostio2.com/en/products/photocatalysts/kronosclean-7000 (accessed on 12 January 2022).
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Kowalczyk, Ł.; Kapica-Kozar, J.; Ohtani, B.; Morawski, A.W. Synthesis and characterization of TiO2/graphitic carbon nanocomposites with enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 437, 441–450. [Google Scholar] [CrossRef]
- Orsi, F.J. Kinetic studies on thermal-decomposition of glucose and fructose. Therm. Anal. 1973, 5, 329–335. [Google Scholar]
- Woo, K.S.; Kim, H.; Hwang, I.G.; Lee, S.H.; Jeong, H.S. Characteristics of the thermal degradation of glucose and maltose solutions. Prev. Nutr. Food Sci. 2015, 20, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (·OH) generation processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Crestani, C.E.; Bernardo, A.; Costa, C.B.B.; Giulietti, M. Experimental data and estimation of sucrose solubility in impure solutions. J. Food Eng. 2018, 218, 14–23. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modelling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Zimbone, M.; Buccheri, M.A.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Grimaldi, M.G. Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B Environ. 2015, 165, 487–494. [Google Scholar] [CrossRef]
- Janus, M.; Markowska-Szczupak, A.; Kusiak-Nejman, E.; Morawski, A.W. Disinfection of E. coli by carbon modified TiO2 photocatalysts. Environ. Prot. Eng. 2012, 38, 89–97. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Markowska-Szczupak, A.; Morawski, A.W. TiO2/glucose nanomaterials with enhanced antibacterial properties. Mater. Lett. 2016, 185, 264–267. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Morawski, A.W. Photocatalytic performance of thermally prepared TiO2/C photocatalysts under artificial solar light. Micro Nano Lett. 2016, 11, 202–206. [Google Scholar] [CrossRef]
- Cui, Y.; Li, H.; Hong, W.; Fan, S.; Zhu, L. The effect of carbon content on the structure and photocatalytic activity of nano-Bi2WO6 powder. Powder Technol. 2013, 247, 151–160. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Huang, X.; Yu, J. Carbon-modified Bi2WO6 nanostructures with improved photocatalytic activity under visible light. Dalton Trans. 2010, 39, 3420–3425. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.M.; Schellhorn, H.E. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 2012, 525, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hoerter, J.D.; Arnold, A.A.; Kuczynska, D.A.; Shibuya, A.; Ward, C.S.; Sauer, M.G.; Gizahev, A.; Hotchkiss, T.M.; Fleming, T.J.; Johnson, S.J. Effects of sublethal UVA irradiation on activity levels of oxidative defense enzymes and protein oxidation in Escherichia coli. J. Photochem. Photobiol. B 2005, 81, 171–180. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Wanag, A.; Sienkiewicz, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of APTES modified TiO2 on antioxidant enzymes activity secreted by Escherichia coli and Staphylococcus epidermidis. Biochem. Biophys. Res. Commun. 2021, 534, 1064–1068. [Google Scholar] [CrossRef]
- Koizumi, Y.; Yamada, R.; Nishioka, M.; Matsumura, Y.; Tsuchido, T.; Taya, M.J. Deactivation kinetics of Escherichia coli cells correlated with intracellular superoxide dismutase activity in photoreaction with titanium dioxide particles. J. Chem. Technol. Biotechnol. 2002, 77, 671–677. [Google Scholar] [CrossRef]
- Zhang, H.M.; Cao, J.; Tang, B.P.; Wang, Y.Q. Effect of TiO2 nanoparticles on the structure and activity of catalase. Chem. Biol. Interact. 2014, 219, 168–174. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Saint, C.P. Bacterial inactivation kinetics of a photo-disinfection system using novel titania-impregnated kaolinite photocatalyst. Chem. Eng. J. 2011, 171, 16–23. [Google Scholar] [CrossRef]
- Marugán, J.; Van Grieken, R.; Sordo, C.; Cruz, C. Kinetics of the photocatalytic disinfection of Escherichia coli suspensions. Appl. Catal. B Environ. 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Shen, T.T.; Guan, K.; Sun, J.; Wang, C.; Wang, Y.-N.; Wang, X.-K. Glucose modification of titania for enhanced photodegradation of organic azo dye. Sci. Adv. Mater. 2019, 11, 540–546. [Google Scholar] [CrossRef]
- Chen, S.-H.; Hsiao, Y.-C.; Chiu, Y.-J.; Tseng, Y.-H. A simple route in fabricating carbon-modified titania films with glucose and their visible-light-responsive photocatalytic activity. Catalysts 2018, 8, 178. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.; Chung, H.; Yoon, J. Disinfection of water containing natural organic matter by using ozone-initiated radical reactions. Appl. Environ. Microbiol. 2003, 69, 2284–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]

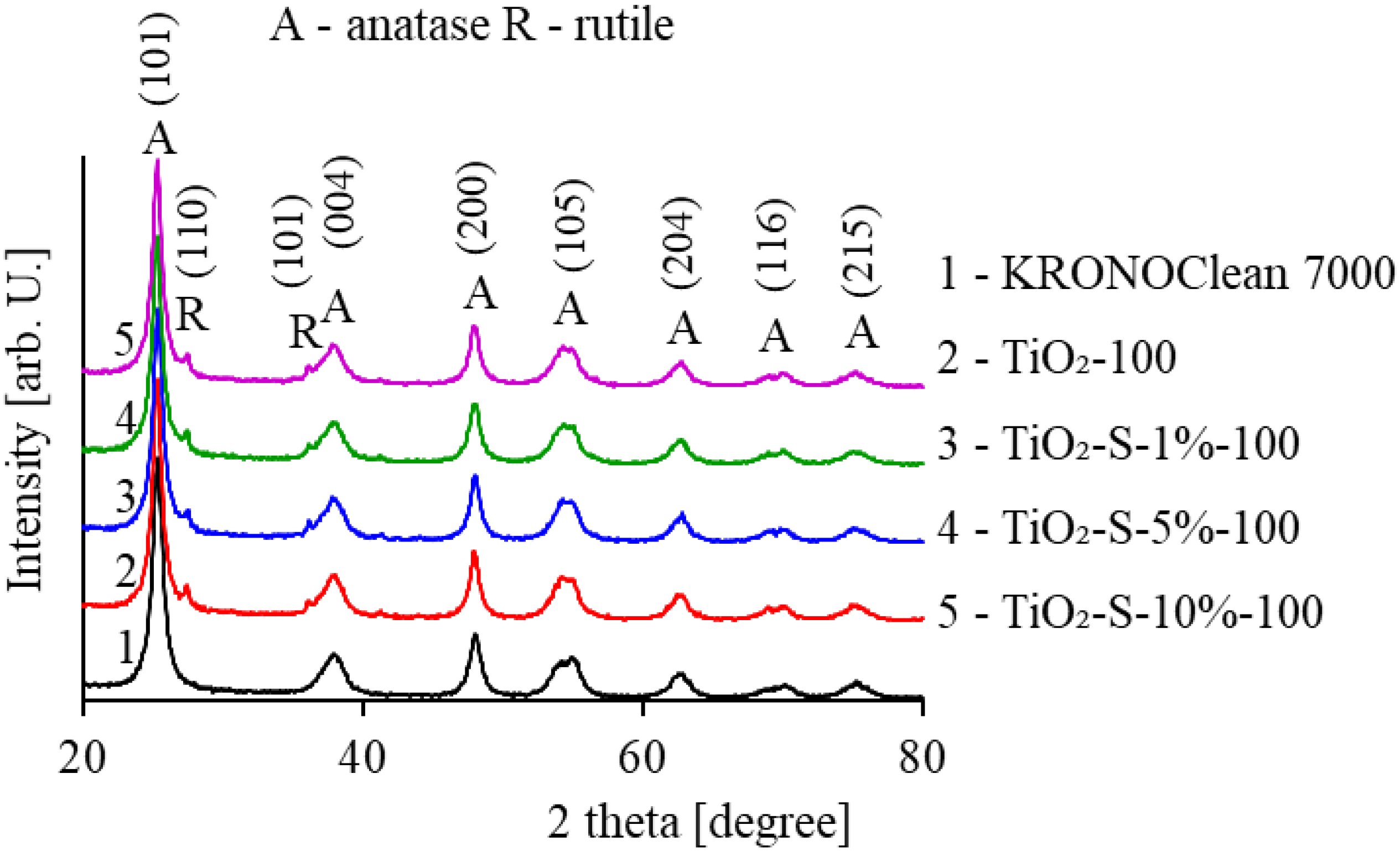

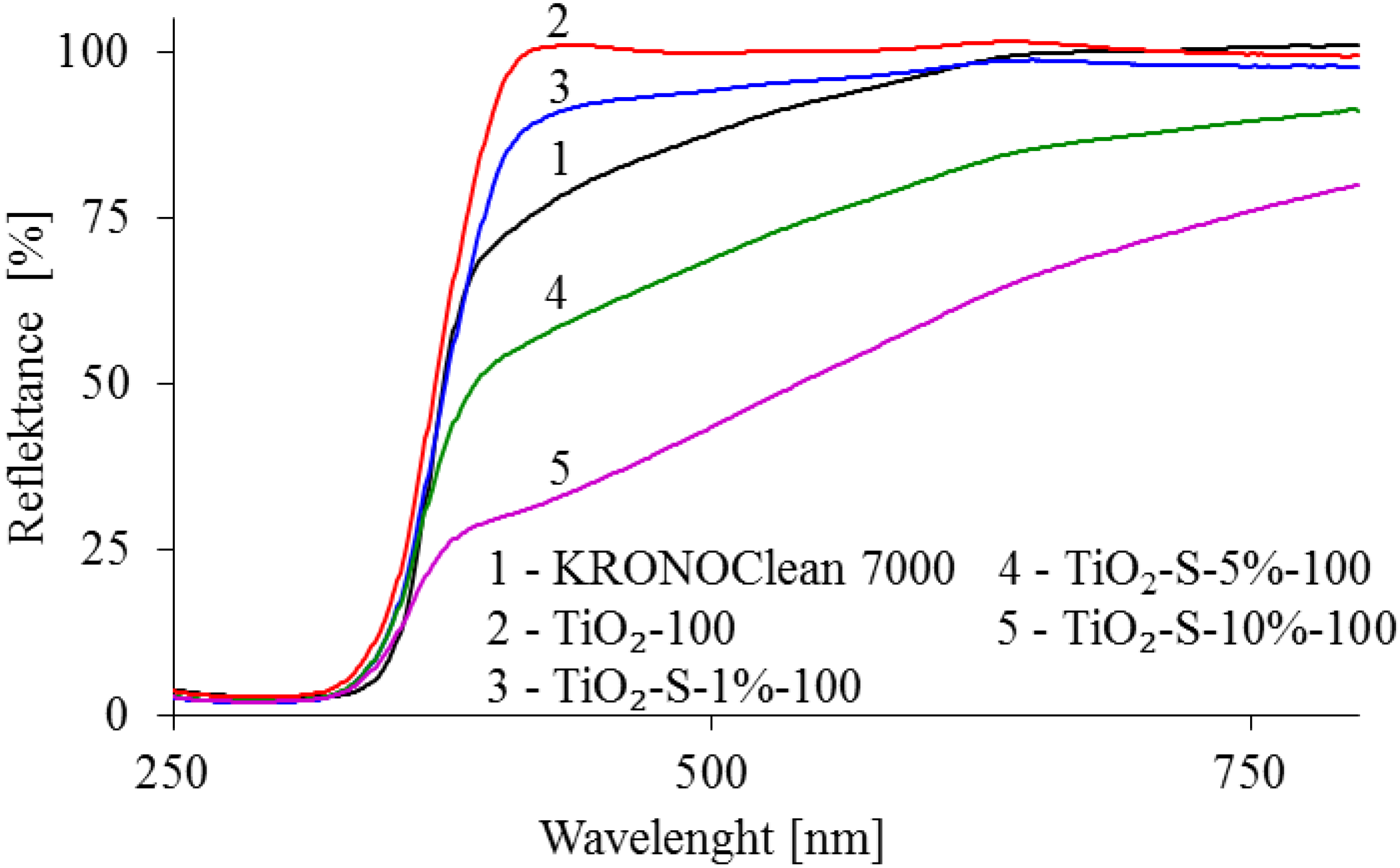

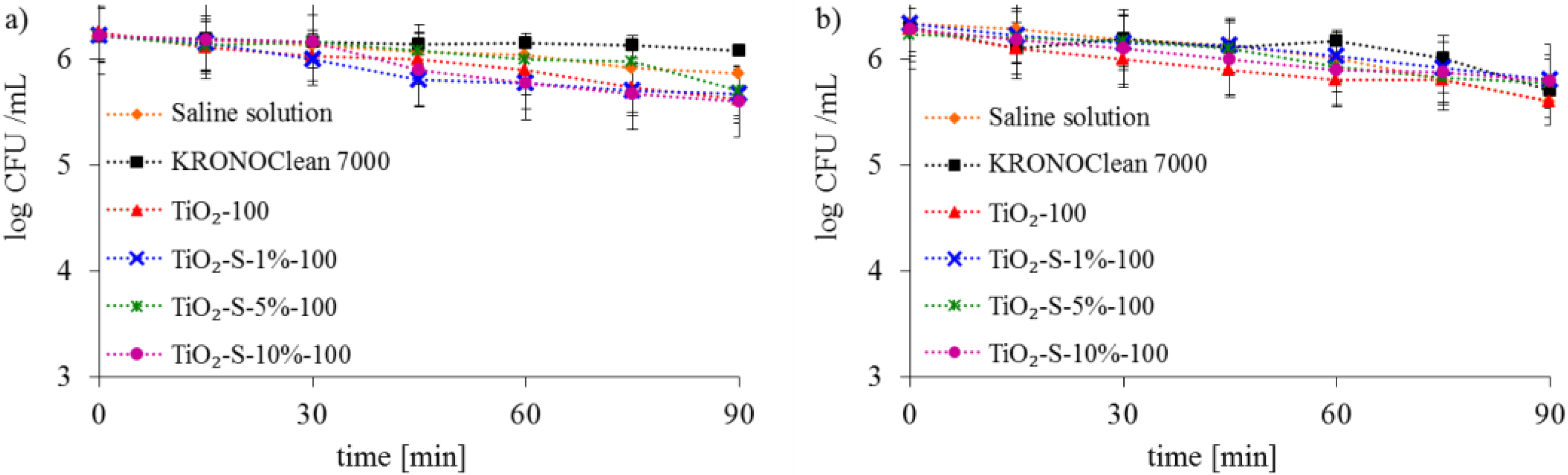
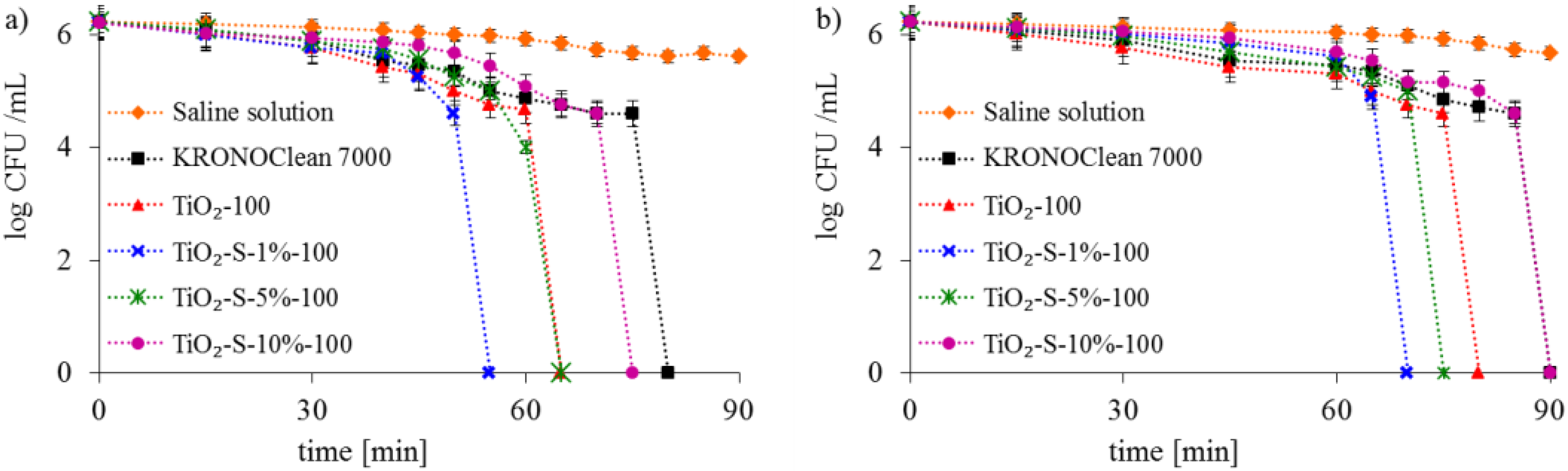



| Sample Code | TiO2 Crystalline Phase Participation [%] | Mean Crystallite Size [nm] | ||
|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | |
| KRONOClean 7000 | 100 | - | 11.0 | - |
| TiO2-100 | 98.1 | 1.9 | 12.0 | 52.8 |
| TiO2-S-1%-100 | 97.9 | 2.1 | 11.7 | 42.3 |
| TiO2-S-5%-100 | 97.8 | 2.2 | 11.7 | 42.3 |
| TiO2-S-10%-100 | 97.8 | 2.2 | 11.6 | 42.3 |
| Sample Code | SBET [m2/g] | Vtotal(0.99) [cm3/g] | Vmicro(DR) [cm3/g] | Vmezo [cm3/g] | Carbon Content (wt.%) | Eg [eV] |
|---|---|---|---|---|---|---|
| KRONOClean 7000 | 242 | 0.37 | 0.09 | 0.28 | 0.96 | 3.24 |
| TiO2-100 | 266 | 0.46 | 0.09 | 0.37 | - | 3.25 |
| TiO2-S-1%-100 | 264 | 0.43 | 0.09 | 0.34 | 0.53 | 3.21 |
| TiO2-S-5%-100 | 261 | 0.45 | 0.08 | 0.37 | 2.42 | 3.20 |
| TiO2-S-10%-100 | 217 | 0.39 | 0.04 | 0.34 | 4.40 | 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Kusiak-Nejman, E.; Morawski, A.W. The Benefits of Using Saccharose for Photocatalytic Water Disinfection. Int. J. Mol. Sci. 2022, 23, 4719. https://doi.org/10.3390/ijms23094719
Rokicka-Konieczna P, Markowska-Szczupak A, Kusiak-Nejman E, Morawski AW. The Benefits of Using Saccharose for Photocatalytic Water Disinfection. International Journal of Molecular Sciences. 2022; 23(9):4719. https://doi.org/10.3390/ijms23094719
Chicago/Turabian StyleRokicka-Konieczna, Paulina, Agata Markowska-Szczupak, Ewelina Kusiak-Nejman, and Antoni W. Morawski. 2022. "The Benefits of Using Saccharose for Photocatalytic Water Disinfection" International Journal of Molecular Sciences 23, no. 9: 4719. https://doi.org/10.3390/ijms23094719
APA StyleRokicka-Konieczna, P., Markowska-Szczupak, A., Kusiak-Nejman, E., & Morawski, A. W. (2022). The Benefits of Using Saccharose for Photocatalytic Water Disinfection. International Journal of Molecular Sciences, 23(9), 4719. https://doi.org/10.3390/ijms23094719









