Leptin Signaling in Obesity and Colorectal Cancer
Abstract
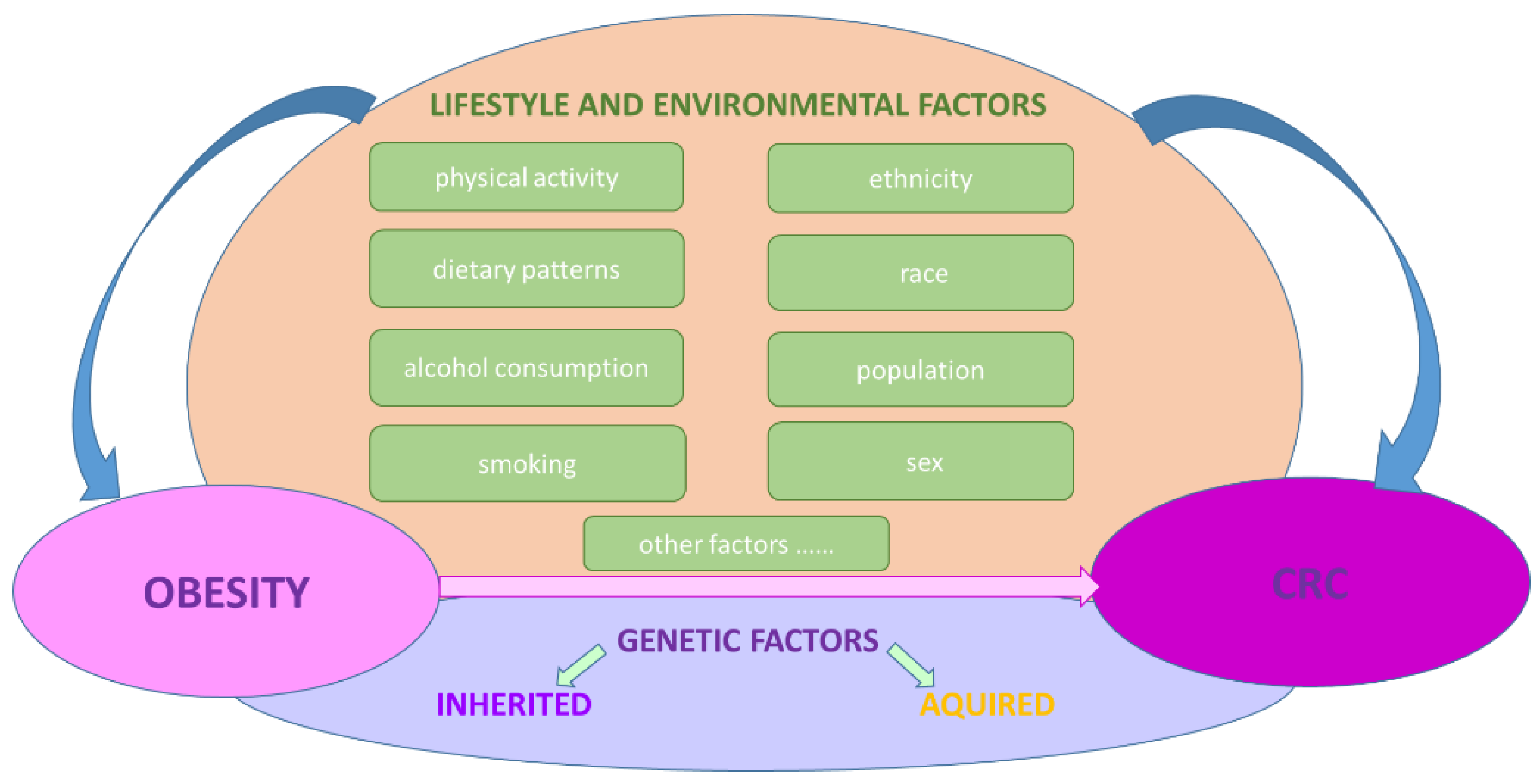
1. Obesity and Colorectal Cancer
2. Leptin Signaling, Obesity, and Colorectal Cancer
3. Genetics of Leptin in Obesity and Colorectal Cancer
3.1. Background
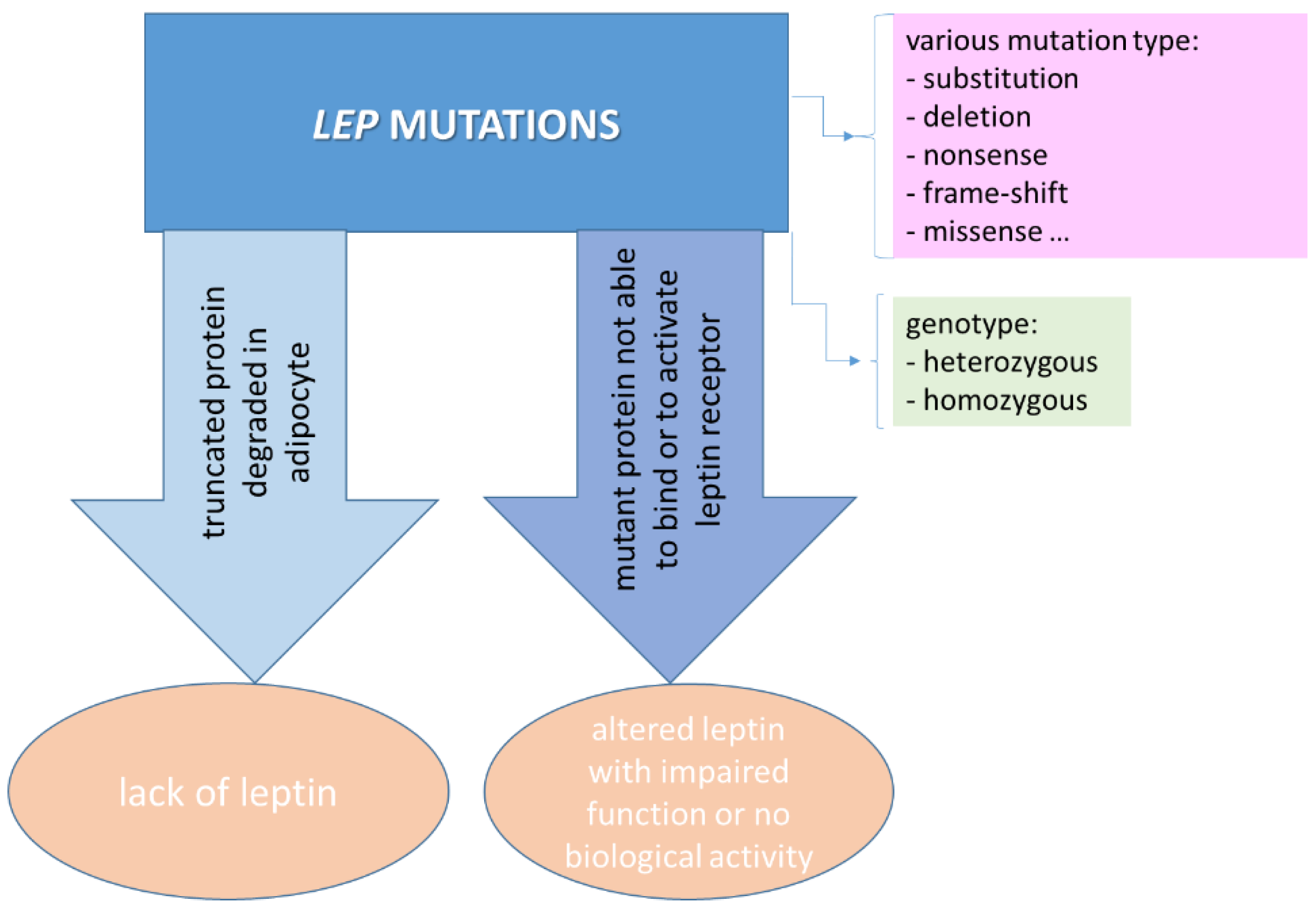
3.2. Rare Mutations in LEP and LEPR
3.3. Relevant SNPs in LEP and LEPR
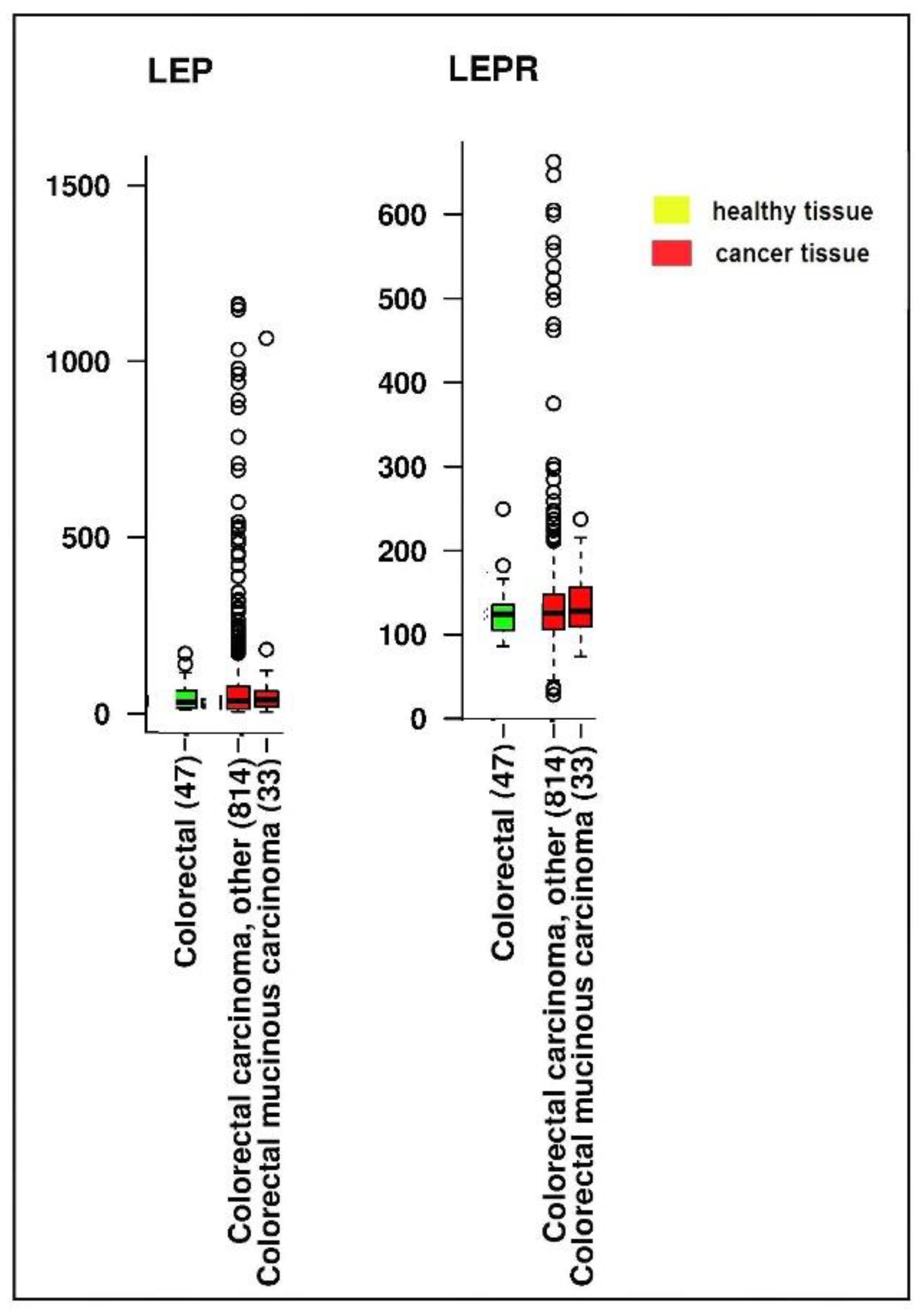
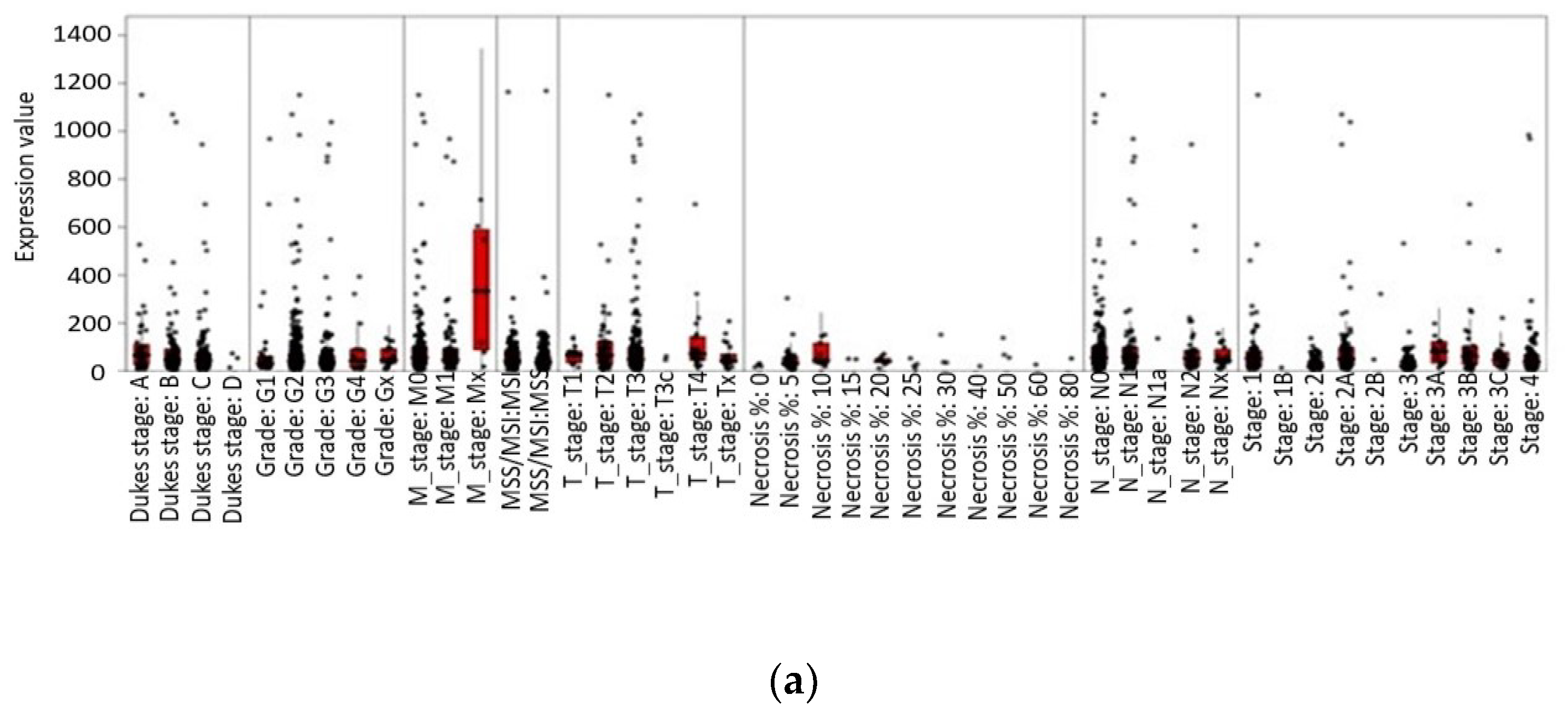
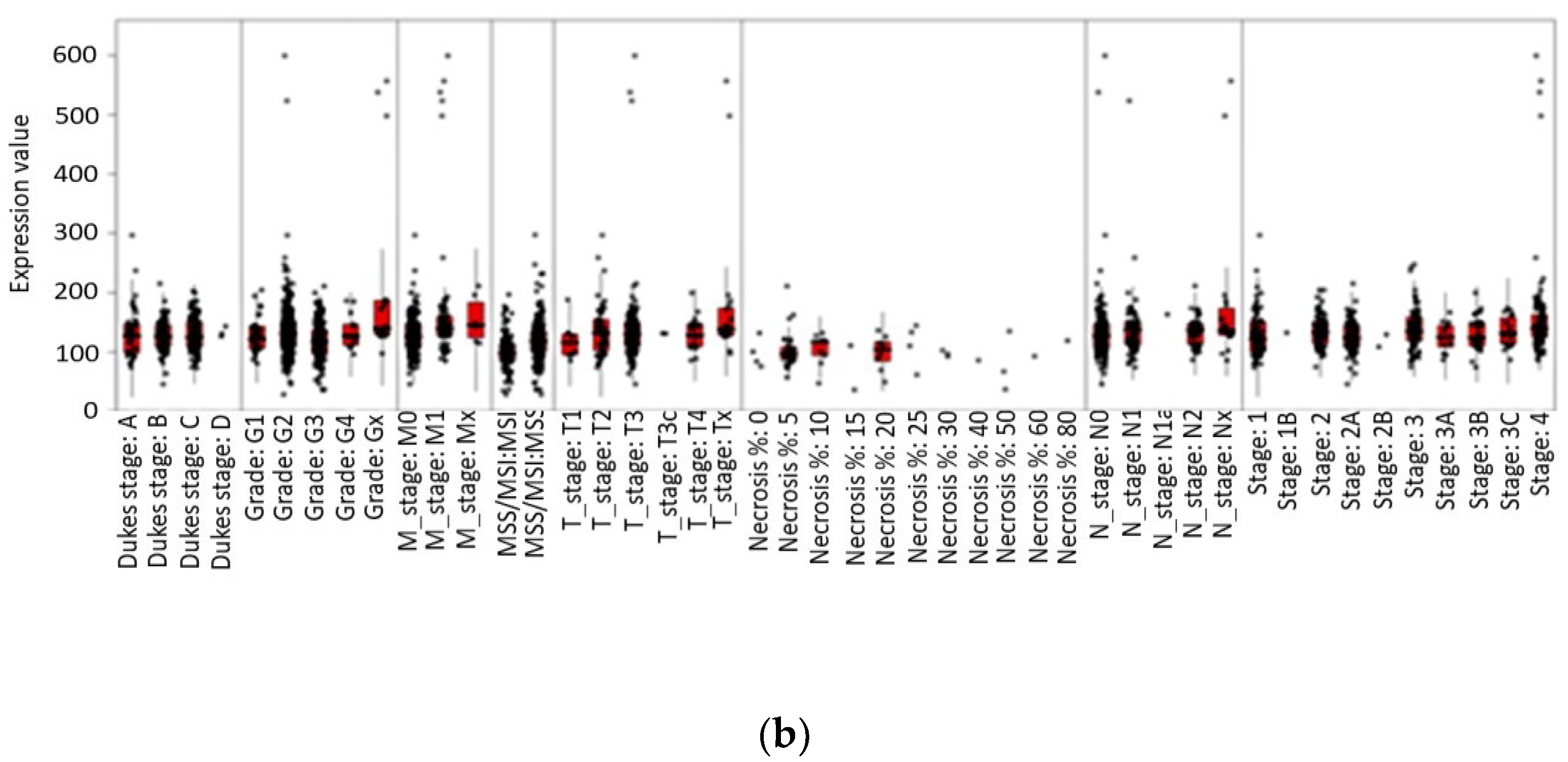
3.4. Impact of Leptin Genetic Variation on Obesity and Colorectal Cancer
4. Interplay of Obesity, Gut Microbiota, and Leptin Signaling in Colorectal Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 July 2021).
- Shabana; Ullah Shahid, S.; Wah Li, K.; Acharya, J.; Cooper, J.A.; Hasnain, S.; Humphries, S.E. Effect of six type II diabetes susceptibility loci and an FTO variant on obesity in Pakistani subjects. Eur. J. Hum. Genet. 2015, 24, 903–910. [Google Scholar] [CrossRef][Green Version]
- Soltani, G.; Poursheikhani, A.; Yassi, M.; Hayatbakhsh, A.; Kerachian, M.; Kerachian, M.A. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr. Disord. 2019, 19, 113. [Google Scholar] [CrossRef]
- World Obesity. Obesity Classification. Available online: https://www.worldobesity.org/about/about-obesity/obesity-classification (accessed on 19 July 2021).
- Forstner, S.; Rusu, A. Development of personalised food for the nutrition of elderly consumers. In Know Your Food. Food Ethics and Innovation; Dumitras, D.E., Jitea, I.M., Aerts, S., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 24–28. [Google Scholar]
- Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings 2020, 10, 302. [Google Scholar] [CrossRef]
- Murphy, N.; Jenab, M.; Gunter, M.J. Adiposity and gastrointestinal cancers: Epidemiology, mechanisms and future directions. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 659–670. [Google Scholar] [CrossRef]
- WCRF-AICR. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer. Continuous Update Project. Available online: http://www.wcrf.org/sites/default/files/Pancreatic-Cancer-2012-Report.pdf (accessed on 19 July 2021).
- WCRF-AICR. Diet, Nutrition, Physical Activity and Colorectal Cancer. Continuous Update Project. Available online: http://www.wcrf.org/sites/default/files/CUP%20Colorectal%20Report_2017_Digital.pdf (accessed on 19 July 2021).
- WCRF. Comparing More and Less Developed Countries. Available online: https://www.wcrf.org/dietandcancer/comparing-more-and-less-developed-countries/ (accessed on 19 July 2021).
- WCRF. Colorectal Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/colorectal-cancer-statistics/ (accessed on 19 July 2021).
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Parkin, E.; O’Reilly, D.A.; Sherlock, D.J.; Manoharan, P.; Renehan, A.G. Excess adiposity and survival in patients with colorectal cancer: A systematic review. Obes. Rev. 2014, 15, 434–451. [Google Scholar] [CrossRef]
- Daniel, C.R.; Shu, X.; Ye, Y.; Gu, J.; Raju, G.S.; Kopetz, S.; Wu, X. Severe obesity prior to diagnosis limits survival in colorectal cancer patients evaluated at a large cancer centre. Br. J. Cancer 2016, 114, 103–109. [Google Scholar] [CrossRef]
- Shah, M.S.; Fogelman, D.R.; Raghav, K.P.S.; Heymach, J.V.; Tran, H.T.; Jiang, Z.-Q.; Kopetz, S.; Daniel, C.R. Joint prognostic effect of obesity and chronic systemic inflammation in patients with metastatic colorectal cancer. Cancer 2015, 121, 2968–2975. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; López-Contreras, B.E.; Canizales-Quinteros, S. Gut Microbiota in Obesity and Metabolic Abnormalities: A Matter of Composition or Functionality? Arch. Med. Res. 2017, 48, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Tözün, N.; Vardareli, E. Gut Microbiome and Gastrointestinal Cancer: Les liaisons Dangereuses. In Proceedings of the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health Meeting, Rome, Italy, 13–15 September 2015; Volume 50, pp. S191–S196. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sobal, J.; Stunkard, A.J. Socioeconomic status and obesity: A review of the literature. Psychol. Bull. 1989, 105, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Bhurosy, T.; Jeewon, R. Overweight and Obesity Epidemic in Developing Countries: A Problem with Diet, Physical Activity, or Socioeconomic Status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide Variations in Colorectal Cancer. CA Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Liefers, S. Physiology and Genetics of Leptin in Periparturient Dairy Cows; Wageningen University: Wageningen, The Netherlands, 2004; ISBN 90-5808-998-3. Available online: https://edepot.wur.nl/121544 (accessed on 19 July 2021).
- La Cava, A.; Alviggi, C.; Matarese, G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J. Mol. Med. 2004, 82, 4–11. [Google Scholar] [CrossRef]
- Madej, T.; Boguski, M.S.; Bryant, S.H. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995, 373, 13–18. [Google Scholar] [CrossRef]
- Rock, F.L.; Altmann, S.W.; van Heek, M.; Kastelein, R.A.; Bazan, J.F. The Liptin Haemopoietic Cytokine Fold is Stabilized by an Intrachain Disulfide Bond. Horm. Metab. Res. 1996, 28, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Hiroike, T.; Higo, J.; Jingami, H.; Toh, H. Homology Modeling of Human Leptin/ Leptin Receptor Complex. Biochem. Biophys. Res. Commun. 2000, 275, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.-P.; Bortoluzzi, M.-N.; Moizo, L.; Lehy, T.; Guerre-Millo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790–793. [Google Scholar] [CrossRef]
- Smith-Kirwin, S.M.; O’Connor, D.M.; Johnston, J.; de Lancy, E.; Hassink, S.G.; Funanage, V.L. Leptin Expression in Human Mammary Epithelial Cells and Breast Milk. J. Clin. Endocrinol. Metab. 1998, 83, 1810. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Ż.; Niezgoda, M.; Łebkowski, W. Differences in leptin biosynthesis in the stomach and in serum leptin level between men and women. J. Gastroenterol. Hepatol. 2019, 34, 1922–1928. [Google Scholar] [CrossRef]
- Masuzaki, H.; Ogawa, Y.; Sagawa, N.; Hosoda, K.; Matsumoto, T.; Mise, H.; Nishimura, H.; Yoshimasa, Y.; Tanaka, I.; Mori, T.; et al. Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997, 3, 1029–1033. [Google Scholar] [CrossRef]
- Hoggard, N.; Hunter, L.; Duncan, J.S.; Williams, L.M.; Trayhurn, P.; Mercer, J.G. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl. Acad. Sci. USA 1997, 94, 11073–11078. [Google Scholar] [CrossRef]
- Henson, M.C.; Swan, K.F.; O’Neil, J.S. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet. Gynecol. 1998, 92, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, R.; Hawkins, M.; Barzilai, N.; Rossetti, L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998, 393, 684–688. [Google Scholar] [CrossRef]
- Morash, B.; Li, A.; Murphy, P.R.; Wilkinson, M.; Ur, E. Leptin Gene Expression in the Brain and Pituitary Gland. Endocrinology 1999, 140, 5995–5998. [Google Scholar] [CrossRef]
- Shklyaev, S.; Aslanidi, G.; Tennant, M.; Prima, V.; Kohlbrenner, E.; Kroutov, V.; Campbell-Thompson, M.; Crawford, J.; Shek, E.W.; Scarpace, P.J.; et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 14217–14222. [Google Scholar] [CrossRef]
- Unger, R.H.; Zhou, Y.-T.; Orci, L. Regulation of fatty acid homeostasis in cells: Novel role of leptin. Proc. Natl. Acad. Sci. USA 1999, 96, 2327–2332. [Google Scholar] [CrossRef]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and me-tastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Xiong, Y.; McDonald, L.; Russell, D.L.; Kelly, R.R.; Wilson, K.R.; Mehrotra, M.; Soloff, A.C.; LaRue, A.C. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment. World J. Stem Cells 2015, 7, 253–265. [Google Scholar] [CrossRef]
- Duquenne, M.; Folgueira, C.; Bourouh, C.; Millet, M.; Silva, A.; Clasadonte, J.; Imbernon, M.; Fernandois, D.; Martinez-Corral, I.; Kusumakshi, S.; et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat. Metab. 2021, 3, 1071–1090. [Google Scholar] [CrossRef]
- Prevot, V.; Dehouck, B.; Sharif, A.; Ciofi, P.; Giacobini, P.; Clasadonte, J. The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr. Rev. 2018, 39, 333–368. [Google Scholar] [CrossRef]
- Yuan, X.; Caron, A.; Wu, H.; Gautron, L. Leptin Receptor Expression in Mouse Intracranial Perivascular Cells. Front. Neuroanat. 2018, 12, 4. [Google Scholar] [CrossRef]
- Yoo, S.; Cha, D.; Kim, D.W.; Hoang, T.V.; Blackshaw, S. Tanycyte-Independent Control of Hypothalamic Leptin Signaling. Front. Neurosci. 2019, 13, 240. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef]
- Ghasemi, A.; Saeidi, J.; Azimi-Nejad, M.; Hashemy, S.I. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell. Oncol. 2019, 42, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, V.S.; Vukmirovic, F.C.; Krstic, M.S.; Zindovic, M.M.; Lj Stojanovic, D.; Jancic, S.A. Involvement of leptin receptors expression in proliferation and neoangiogenesis in colorectal carcinoma. J. Off. J. Balk. Union Oncol. 2015, 20, 100–108. [Google Scholar]
- Shahraki, N.; Mehrabian, A.; Amiri-Darban, S.; Moosavian, S.A.; Jaafari, M.R. Preparation and characterization of PEGylated liposomal Doxorubicin targeted with leptin-derived peptide and evaluation of their anti-tumor effects, in vitro and in vivo in mice bearing C26 colon carcinoma. Colloids Surf. B Biointerfaces 2021, 200, 111589. [Google Scholar] [CrossRef] [PubMed]
- Darban, S.A.; Nikoofal, S.; Amiri, N.; Kiamanesh, N.; Mehrabian, A.; Zendehbad, B.; Gholizadeh, Z.; Jaafari, M.R. Targeting the leptin receptor: To evaluate therapeutic efficacy and anti-tumor effects of Doxil, in vitro and in vivo in mice bearing C26 colon carcinoma tumor. Colloids Surf. B Biointerfaces 2018, 164, 107–115. [Google Scholar] [CrossRef] [PubMed]
- ElSaeed, G.; Mousa, N.; El-Mougy, F.; Hafez, M.; Khodeera, S.; Alhelbawy, M.; Fouda, E.; Elsheikh, S.; ElKaffas, R.; Eldeeb, S.; et al. Monogenic leptin deficiency in early childhood obesity. Pediatr. Obes. 2020, 15, e12574. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Alzoubi, K.H.; Kubas, M.A.; Banihani, M.N.; Hamdan, N.; Al-Jaberi, T.M. High levels of leptin and non-high molecular weight-adiponectin in patients with colorectal cancer: Association with chemotherapy and common genetic polymorphisms. Biomed. Rep. 2021, 14, 13. [Google Scholar] [CrossRef]
- IST Database. Available online: https://ist.medisapiens.com/ (accessed on 24 June 2021).
- Lawrence, J.E.; Cook, N.J.; Rovin, R.A.; Winn, R.J. Leptin Promotes Glioblastoma. Neurol. Res. Int. 2012, 2012, 870807. [Google Scholar] [CrossRef]
- Surmacz, E. Leptin and Adiponectin: Emerging Therapeutic Targets in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 321–332. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Beales, I.L.P. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int. J. Color. Dis. 2007, 22, 401–409. [Google Scholar] [CrossRef]
- Hardwick, J.C.; Van Den Brink, G.R.; Offerhaus, G.; Van Deventer, S.J.; Peppelenbosch, M.P. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 2001, 121, 79–90. [Google Scholar] [CrossRef]
- Stattin, P.; Palmqvist, R.; Söderberg, S.; Biessy, C.; Ardnor, B.; Hallmans, G.; Kaaks, R.; Olsson, T. Plasma leptin and colorectal cancer risk: A prospective study in Northern Sweden. Oncol. Rep. 2003, 10, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Wolff, R.K.; Herrick, J.; Caan, B.J.; Potter, J.D. Leptin and leptin receptor genotypes and colon cancer: Gene-gene and gene-lifestyle interactions. Int. J. Cancer 2008, 122, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhong, R.; Wei, S.; Xiang, H.; Chen, J.; Xie, D.; Yin, J.; Zou, L.; Sun, J.; Chen, W.; et al. The Leptin Gene Family and Colorectal Cancer: Interaction with Smoking Behavior and Family History of Cancer. PLoS ONE 2013, 8, e60777. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Ahnen, D.J.; Baron, J.A.; Batts, K.P.; Burke, C.A.; Burt, R.W.; Goldblum, J.R.; Guillem, J.G.; Kahi, C.J.; Kalady, M.F.; et al. Serrated Lesions of the Colorectum: Review and Recommendations from an Expert Panel. Am. J. Gastroenterol. 2012, 107, 1315–1329. [Google Scholar] [CrossRef]
- Bouchard, C. The causes of obesity: Advances in molecular biology but stagnation on the genetic front. Diabetologia 1996, 39, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Arslan, M.; Froguel, P. Genetics of Obesity in Consanguineous Populations: Toward Precision Medicine and the Discovery of Novel Obesity Genes. Obesity 2018, 26, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-Y.; Lee, Y.-H.; Na, H.-K.; Baek, J.-H.; Surh, Y.-J. Leptin induces SIRT1 expression through activation of NF-E2-related factor 2: Implications for obesity-associated colon carcinogenesis. Biochem. Pharmacol. 2018, 153, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Yehuda, H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer 2016, 16, 882. [Google Scholar] [CrossRef]
- Montague, C.; Farooqi, S.; Whitehead, J.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Funcke, J.-B.; Von Schnurbein, J.; Denzer, F.; Lahr, G.; Mazen, I.; El Gammal, M.; Denzer, C.; Moss, A.; Debatin, K.-M.; et al. Severe Early-Onset Obesity Due to Bioinactive Leptin Caused by a p.N103K Mutation in the Leptin Gene. J. Clin. Endocrinol. Metab. 2015, 100, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Bonnefond, A.; Manzoor, J.; Shabir, F.; Ayesha, H.; Philippe, J.; Durand, E.; Crouch, H.; Sand, O.; Ali, M.; et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity 2015, 23, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Echwald, S.; Rasmussen, S.; Sørensen, T.; Andersen, T.; Tybjærg-Hansen, A.; Clausen, J.; Hansen, L.; Hansen, T.; Pedersen, O. Identification of two novel missense mutations in the human OB gene. Int. J. Obes. 1997, 21, 321–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strobel, A.; Issad, T.; Camoin, L.; Ozata, M.; Strosberg, A.D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 1998, 18, 213–215. [Google Scholar] [CrossRef]
- Chekhranova, M.K.; Karpova, S.K.; Yatsyshina, S.; Pankov, J.A. A new mutation c.422C > G (p.S141C) in homoand heterozygous forms of the human leptin gene. Russ. J. Bioorganic Chem. 2008, 34, 768–770. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Mazen, I.; El-Gammal, M.; Abdel-Hamid, M.; Amr, K. A novel homozygous missense mutation of the leptin gene (N103K) in an obese Egyptian patient. Mol. Genet. Metab. 2009, 97, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; von Schnurbein, J.; Moepps, B.; Lahr, G.; Strauss, G.; Barth, T.F.; Kassubek, J.; Muhleder, H.; Moller, P.; Debatin, K.M.; et al. A New Missense Mutation in the Leptin Gene Causes Mild Obesity and Hypogonadism without Affecting Tcell Responsiveness. J. Clin. Endocrinol. Metab. 2010, 95, 2836–2840. [Google Scholar] [CrossRef]
- Fatima, W.; Shahid, A.; Imran, M.; Manzoor, J.; Hasnain, S.; Rana, S.; Mahmood, S. Leptin deficiency andleptingene mutations in obese children from Pakistan. Int. J. Pediatr. Obes. 2011, 6, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Butt, T.A.; Anwer, M.; Arslan, M.; Froguel, P. High prevalence of leptin and melanocortin-4 receptor gene mu-tations in children with severe obesity from Pakistani consanguineous families. Mol. Genet. Metab. 2012, 106, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, A.; Dubey, S.; Saxena, R.; Peters, A.; Singhal, A. A novel mutation of the leptin gene in an Indian patient. Clin. Genet. 2014, 86, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hong, N.; Liu, X.; Wu, B.; Tang, S.; Yang, J.; Hu, C.; Jia, W. A Novel Mutation in Leptin Gene Is Associated with Severe Obesity in Chinese Individuals. BioMed Res. Int. 2014, 2014, 912052. [Google Scholar] [CrossRef] [PubMed]
- Dayal, D.; Seetharaman, K.; Panigrahi, I.; Muthuvel, B.; Agarwal, A. Severe Early Onset Obesity due to a Novel Missense Mutation in Exon 3 of the Leptin Gene in an Infant from Northwest India. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yupanqui-Lozno, H.; Bastarrachea, R.A.; Yupanqui-Velazco, M.E.; Alvarez-Jaramillo, M.; Medina-Méndez, E.; Giraldo-Peña, A.P.; Arias-Serrano, A.; Torres-Forero, C.; Garcia-Ordoñez, A.M.; Mastronardi, C.A.; et al. Congenital Leptin Deficiency and Leptin Gene Missense Mutation Found in Two Colombian Sisters with Severe Obesity. Genes 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Hsiao, M. Leptin and Cancer: Updated Functional Roles in Carcinogenesis, Therapeutic Niches, and Developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef] [PubMed]
- Paracchini, V.; Pedotti, P.; Taioli, E. Genetics of Leptin and Obesity: A HuGE Review. Am. J. Epidemiol. 2005, 162, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Colmenarejo, G.; Selma, M.V.; Espín, J.C. Genetic Polymorphisms, Mediterranean Diet and Microbiota-Associated Urolithin Metabotypes can Predict Obesity in Childhood-Adolescence. Sci. Rep. 2020, 10, 7850. [Google Scholar] [CrossRef]
- Hager, J.; Clement, K.; Francke, S.; Dina, C.; Raison, J.; Lahlou, N.; Rich, N.; Pelloux, V.; Basdevant, A.; Guy-Grand, B.; et al. A polymorphism in the 5′ untranslated region of the human ob gene is associated with low leptin levels. Int. J. Obes. 1998, 22, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Ding, Y.; Xu, W.; Cather, C.; Green, E.D.; Price, R.A. Extreme Obesity May Be Linked to Markers Flanking the Human OB Gene. Diabetes 1996, 45, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, V.S.; Zembrzuski, V.M.; Hutz, M.H. Association analysis of genes involved in the leptin-signaling pathway with obesity in Brazil. Int. J. Obes. 2002, 26, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wilk, J.; Borecki, I.; Williamson, S.; DeStefano, A.; Xu, G.; Liu, J.; Ellison, R.; Province, M.; Myers, R. Common Variants in the 5′ Region of the Leptin Gene Are Associated with Body Mass Index in Men from the National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Hum. Genet. 2004, 75, 220–230. [Google Scholar] [CrossRef]
- Erez, G.; Tirosh, A.; Rudich, A.; Meiner, V.; Schwarzfuchs, D.; Sharon, N.; Shpitzen, S.; Blüher, M.; Stumvoll, M.; Thiery, J.; et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int. J. Obes. 2011, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.M.; Northrup, H.; King, T.M.; Fletcher, J.M.; Townsend, I.; Tyerman, G.H.; Au, K.S. Genes in Glucose Metabolism and Association With Spina Bifida. Reprod. Sci. 2008, 15, 51–58. [Google Scholar] [CrossRef][Green Version]
- Dasgupta, S.; Salman, M.; Siddalingaiah, L.B.; Lakshmi, G.L.; Xaviour, D.; Sreenath, J. Genetic variants in leptin: Determinants of obesity and leptin levels in South Indian population. Adipocyte 2014, 4, 135–140. [Google Scholar] [CrossRef]
- Oksanen, L.; Kainulainen, K.; Heiman, M.; Mustajoki, P.; Kauppinen-Mäkelin, R.; Kontula, K. Novel polymorphism of the human ob gene promoter in lean and morbidly obese subjects. Int. J. Obes. 1997, 21, 489–494. [Google Scholar] [CrossRef]
- Lin, J.; Xie, Z.; Lan, B.; Guo, Z.; Tang, W.F.; Liu, C.; Zhang, S.; Chen, G.; Guo, F.; Chen, Y. Investigation of Leptin and its receptor (LEPR) for single nucleotide polymorphisms in colorectal cancer: A case-control study involving 2306 subjects. Am. J. Transl. Res. 2020, 12, 3613. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Farahani, H.; Nobakht, H.; Dabiri, R.; Zali, M.R. Genetic Variations in Leptin and Leptin Receptor and Susceptibility to Colorectal Cancer and Obesity. Iran. J. Cancer Prev. 2016, 9, e7013. [Google Scholar] [CrossRef]
- Mammès, O.; Betoulle, D.; Aubert, R.; Giraud, V.; Tuzet, S.; Petiet, A.; Colas-Linhart, N.; Fumeron, F. Novel polymorphisms in the 5′ region of the LEP gene: Association with leptin levels and response to low-calorie diet in human obesity. Diabetes 1998, 47, 487–489. [Google Scholar] [CrossRef]
- Lucantoni, R.; Ponti, E.; Berselli, M.E.; Savia, G.; Minocci, A.; Calò, G.; De Medici, C.; Liuzzi, A.; Di Blasio, A.M. The A19G Polymorphism in the 5′ Untranslated Region of the Human Obese Gene Does Not Affect Leptin Levels in Severely Obese Patients. J. Clin. Endocrinol. Metab. 2000, 85, 3589–3591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, S.-H.; Say, Y.-H. Leptin and leptin receptor gene polymorphisms and their association with plasma leptin levels and obesity in a multi-ethnic Malaysian suburban population. J. Physiol. Anthropol. 2014, 33, 15. [Google Scholar] [CrossRef]
- Hinuy, H.M.; Hirata, M.H.; Forti, N.; Diament, J.; Sampaio, M.F.; Armaganijan, D.; Salazar, L.A.; Hirata, R.D.C. Leptin G-2548A promoter polymorphism is associated with increased plasma leptin and BMI in Brazilian women. Arq. Bras. Endocrinol. Metabol. 2008, 52, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.F.; Chuah, H.S.; Lau, C.L.; Lee, C.H.; Say, Y.H. Prevalence of the leptin and leptin receptor gene variants and obesity risk factors among Malaysian university students of Setapak, Kuala Lumpur. Asian J. Epidemiol. 2009, 2, 49–58. [Google Scholar] [CrossRef]
- Kazunari, Y.; Maciukiewicz, M.; Zai, C.C.; Gonçalves, V.F.; Brandl, E.J.; Lieberman, J.A.; Meltzer, H.Y.; Tiwari, A.K.; Kennedy, J.L.; Müller, D.J. Association between the -2548G/A polymorphism of the leptin gene and antipsychotic-induced weight gain: Analysis of the CATIE sample and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109952. [Google Scholar]
- Ben Ali, S.; Kallel, A.; Ftouhi, B.; Sediri, Y.; Feki, M.; Slimane, H.; Jemaa, R.; Kaabachi, N. Association of G-2548A LEP polymorphism with plasma leptin levels in Tunisian obese patients. Clin. Biochem. 2009, 42, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Naka, I.; Yamauchi, T.; Natsuhara, K.; Kimura, R.; Nakazawa, M.; Ishida, T.; Inaoka, T.; Matsumura, Y.; Ataka, Y.; et al. The Q223R polymorphism in LEPR is associated with obesity in Pacific Islanders. Hum. Genet. 2009, 127, 287–294. [Google Scholar] [CrossRef]
- Constantin, A.; Costache, G.; Sima, A.V.; Glavce, C.S.; Vladica, M.; Popov, D.L. Leptin G-2548A and leptin receptor Q223R gene polymorphisms are not associated with obesity in Romanian subjects. Biochem. Biophys. Res. Commun. 2010, 391, 282–286. [Google Scholar] [CrossRef]
- Okpechi, I.G.; Rayner, B.L.; Van Der Merwe, L.; Mayosi, B.M.; Adeyemo, A.; Tiffin, N.; Ramesar, R. Genetic Variation at Selected SNPs in the Leptin Gene and Association of Alleles with Markers of Kidney Disease in a Xhosa Population of South Africa. PLoS ONE 2010, 5, e9086. [Google Scholar] [CrossRef]
- Primo, D.; Izaola, O.; De Luis, D. Leptin gene polymorphism (rs 7799039; G2548A) is associated with changes in lipid profile during a partial meal-replacement hypocaloric diet. J. Hum. Nutr. Diet. 2020, 34, 456–463. [Google Scholar] [CrossRef]
- Mao, F.; Niu, X.; Gu, S.; Ji, L.; Wei, B.; Wang, H. Investigation of Leptin G19A polymorphism with bladder cancer risk: A case-control study. J. Clin. Lab. Anal. 2020, 34, e23351. [Google Scholar] [CrossRef] [PubMed]
- Partida-Pérez, M.; de la Luz Ayala-Madrigal, M.; Peregrina-Sandoval, J.; Macías-Gómez, N.; Moreno-Ortiz, J.; Leal-Ugarte, E.; Cárdenas-Meza, M.; Centeno-Flores, M.; Maciel-Gutiérrez, V.; Cabrales, E.; et al. Association of LEP and ADIPOQ common variants with colorectal cancer in Mexican patients. Cancer Biomark. 2010, 7, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Dubern, B.; Clément, K.; Poitou, C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes. Facts 2016, 9, 158–173. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.H.; Trzaskowski, M.; Plomin, R.; Wardle, J. Finding the missing heritability in pediatric obesity: The contribution of genome-wide complex trait analysis. Int. J. Obes. 2013, 37, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manolio, T.A.; Pasquale, L.R.; Boerwinkle, E.; Caporaso, N.; Cunningham, J.M.; de Andrade, M.; Feenstra, B.; Feingold, E.; Hayes, M.G.; et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 2011, 43, 519–525. [Google Scholar] [CrossRef]
- Coleman, D.L. Obesity Genes: Beneficial Effects in Heterozygous Mice. Science 1979, 203, 663–665. [Google Scholar] [CrossRef]
- Ma, R.; He, Q. A Variant of Leptin Gene Decreases the Risk of Gastric Cancer in Chinese Individuals: Evidence from a Case-Control Study. Pharm. Pers. Med. 2020, 13, 397–404. [Google Scholar] [CrossRef]
- Hebebrand, J.; Sommerlad, C.; Geller, F.; Görg, T.; Hinney, A. The genetics of obesity: Practical implications. Int. J. Obes. 2001, 25, S10–S18. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Saffarian, A.; Mulet, C.; Regnault, B.; Amiot, A.; Tran-Van-Nhieu, J.; Ravel, J.; Sobhani, I.; Sansonetti, P.J.; Pédron, T. Crypt- and Mucosa-Associated Core Microbiotas in Humans and Their Alteration in Colon Cancer Patients. mBio 2019, 10, e01315–e01319. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Rawls, J.F.; Randall, T.A.; Burcall, L.; Mpande, C.; Jenkins, N.; Jovov, B.; Abdo, Z.; Sandler, R.S.; Keku, T.O. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 2010, 1, 138–147. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Drewes, J.L.; Housseau, F.; Sears, C.L. Sporadic colorectal cancer: Microbial contributors to disease prevention, development and therapy. Br. J. Cancer 2016, 115, 273–280. [Google Scholar] [CrossRef]
- Miao, Z.H.; Zhou, W.X.; Cheng, R.Y.; Liang, H.J.; Jiang, F.L.; Shen, X.; Lu, J.H.; Li, M.; He, F. Dysbiosis of intestinal microbiota in early life aggravates high-fat diet induced dysmetabolism in adult mice. BMC Microbiol. 2021, 21, 209. [Google Scholar] [CrossRef]
- Shen, X.; Wang, M.; Zhang, X.; He, M.; Li, M.; Cheng, G.; Wan, C.; He, F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019, 19, 123. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Pham, D.-V.; Park, P.-H. Recent insights on modulation of inflammasomes by adipokines: A critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch. Pharmacal Res. 2020, 43, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, V. The Impact of Obesity on Microglial Function: Immune, Metabolic and Endocrine Perspectives. Cells 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.-J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.-R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 Tcell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Font-Burgada, J.; Sun, B.; Karin, M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016, 23, 48–62. [Google Scholar] [CrossRef]
- Geurts, L.; Lazarevic, V.; Derrien, M.; Everard, A.; Van Roye, M.; Knauf, C.; Valet, P.; Girard, M.; Muccioli, G.G.; François, P.; et al. Altered Gut Microbiota and Endocannabinoid System Tone in Obese and Diabetic Leptin-Resistant Mice: Impact on Apelin Regulation in Adipose Tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.W.; Patterson, C.M.; Opp, J.S.; Foltin, S.; Young, V.; Myers, M.G. Leptin Acts Independently of Food Intake to Modulate Gut Microbial Composition in Male Mice. Endocrinology 2014, 155, 748–757. [Google Scholar] [CrossRef]
- Duggal, P.; Guo, X.; Haque, R.; Peterson, K.M.; Ricklefs, S.; Mondal, D.; Alam, F.; Noor, Z.; Verkerke, H.P.; Marie, C.; et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J. Clin. Investig. 2011, 121, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Roberts, M.R.; Becker, S.M.; Podd, B.; Zhang, Y.; Chua, S.C.; Myers, M.G.; Duggal, P.; Houpt, E.R.; Petri, W.A. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011, 4, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.-O. The Gut Microbiota Reduces Leptin Sensitivity and the Expression of the Obesity-Suppressing Neuropeptides Proglucagon (Gcg) and Brain-Derived Neurotrophic Factor (Bdnf) in the Central Nervous System. Endocrinology 2013, 154, 3643–3651. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in ob/ob Mice by Partially Restoring Leptin-related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Portune, K.; Campillo, I.; López-Almela, I.; la Paz, S.M.-D.; Romaní-Pérez, M.; Benítez-Páez, A.; Sanz, Y. Bacteroides uniformis CECT 7771 alleviates inflammation within the gut-adipose tissue axis involving TLR5 signaling in obese mice. Sci. Rep. 2021, 11, 11788. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4713. https://doi.org/10.3390/ijms23094713
Socol CT, Chira A, Martinez-Sanchez MA, Nuñez-Sanchez MA, Maerescu CM, Mierlita D, Rusu AV, Ruiz-Alcaraz AJ, Trif M, Ramos-Molina B. Leptin Signaling in Obesity and Colorectal Cancer. International Journal of Molecular Sciences. 2022; 23(9):4713. https://doi.org/10.3390/ijms23094713
Chicago/Turabian StyleSocol, Claudia Terezia, Alexandra Chira, Maria Antonia Martinez-Sanchez, Maria Angeles Nuñez-Sanchez, Cristina Maria Maerescu, Daniel Mierlita, Alexandru Vasile Rusu, Antonio Jose Ruiz-Alcaraz, Monica Trif, and Bruno Ramos-Molina. 2022. "Leptin Signaling in Obesity and Colorectal Cancer" International Journal of Molecular Sciences 23, no. 9: 4713. https://doi.org/10.3390/ijms23094713
APA StyleSocol, C. T., Chira, A., Martinez-Sanchez, M. A., Nuñez-Sanchez, M. A., Maerescu, C. M., Mierlita, D., Rusu, A. V., Ruiz-Alcaraz, A. J., Trif, M., & Ramos-Molina, B. (2022). Leptin Signaling in Obesity and Colorectal Cancer. International Journal of Molecular Sciences, 23(9), 4713. https://doi.org/10.3390/ijms23094713








