Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation
Abstract
:1. Introduction
2. Results
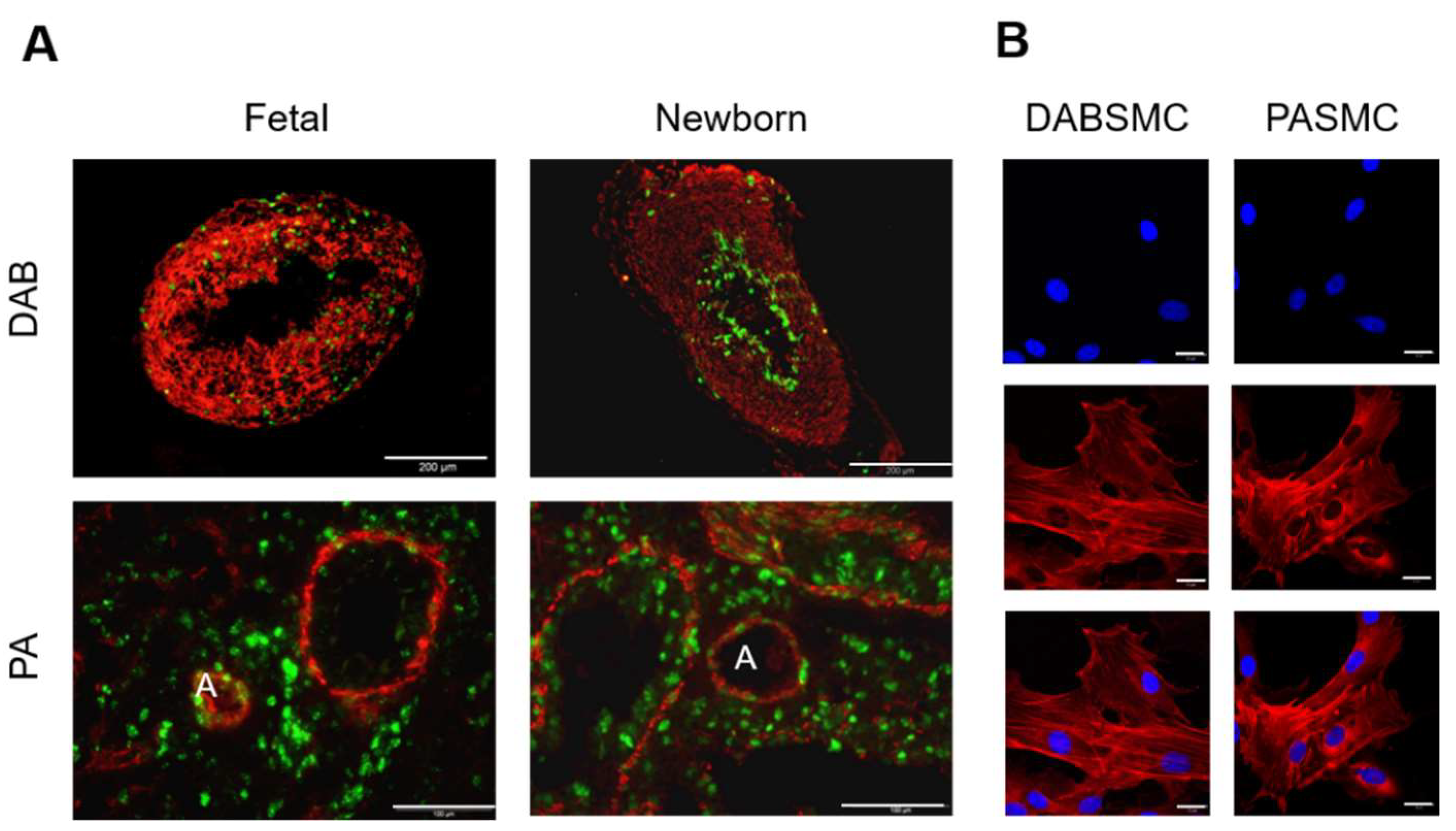
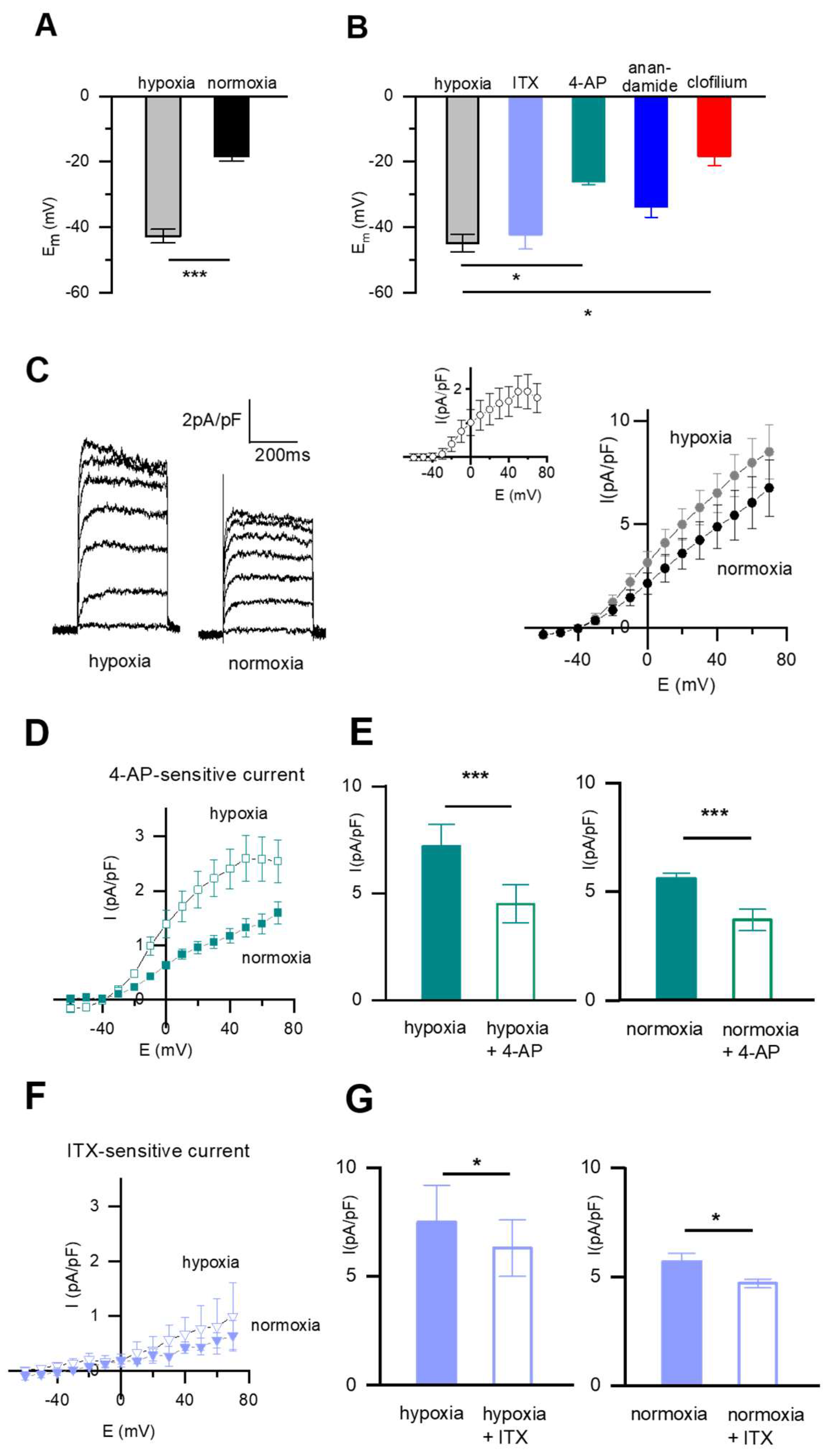
2.1. Resting Membrane Potential (Em) and Pharmacology of the Whole-Cell K+ Current (IK) in Fetal Rat DABSMC
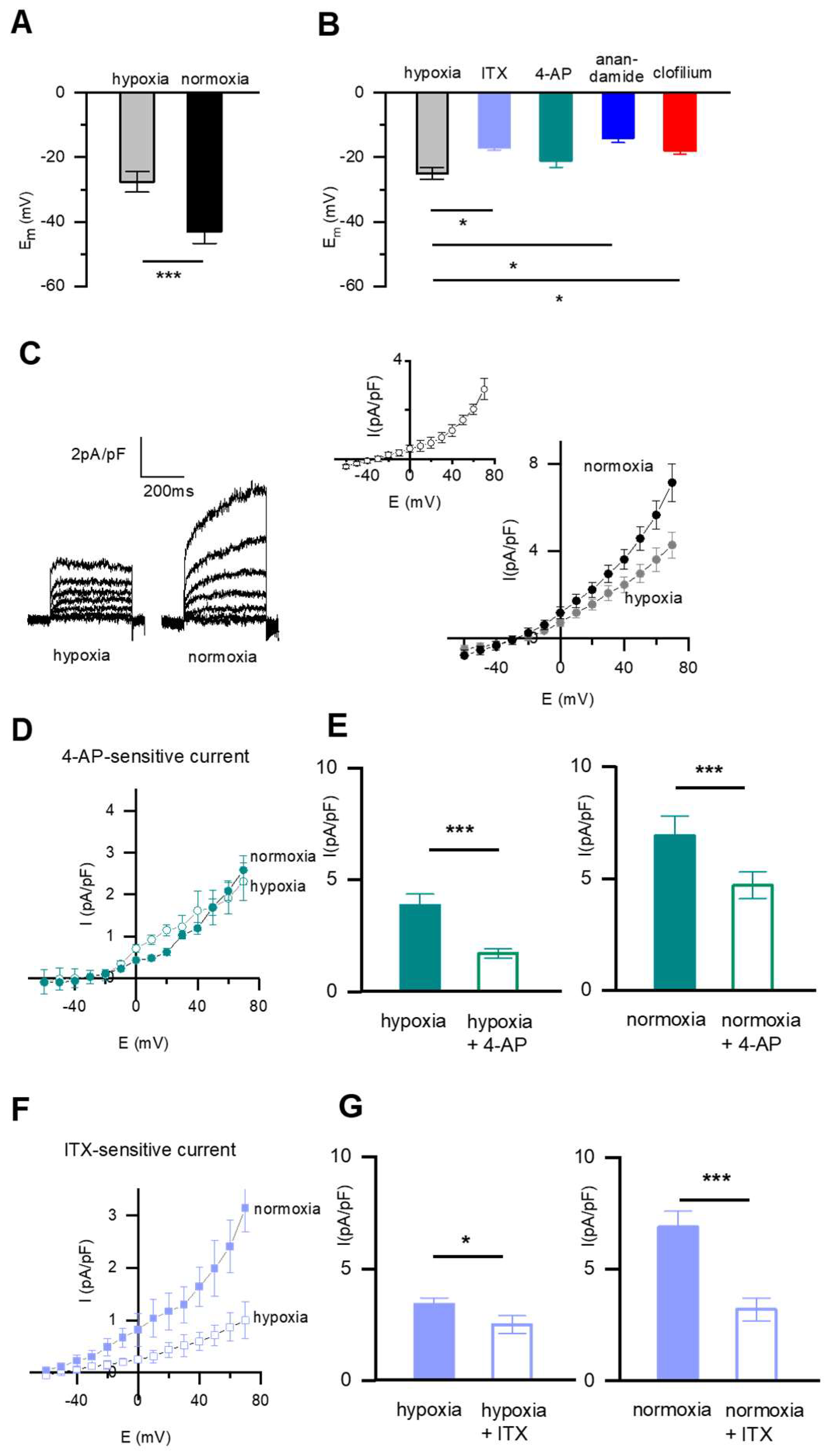
2.2. The Resting Membrane Potential (Em) and the Pharmacology of the Whole-Cell K+ Current in Fetal Rat PASMC
2.3. pH-Sensitive Em and Current Is Present in SMC from DAB and PA
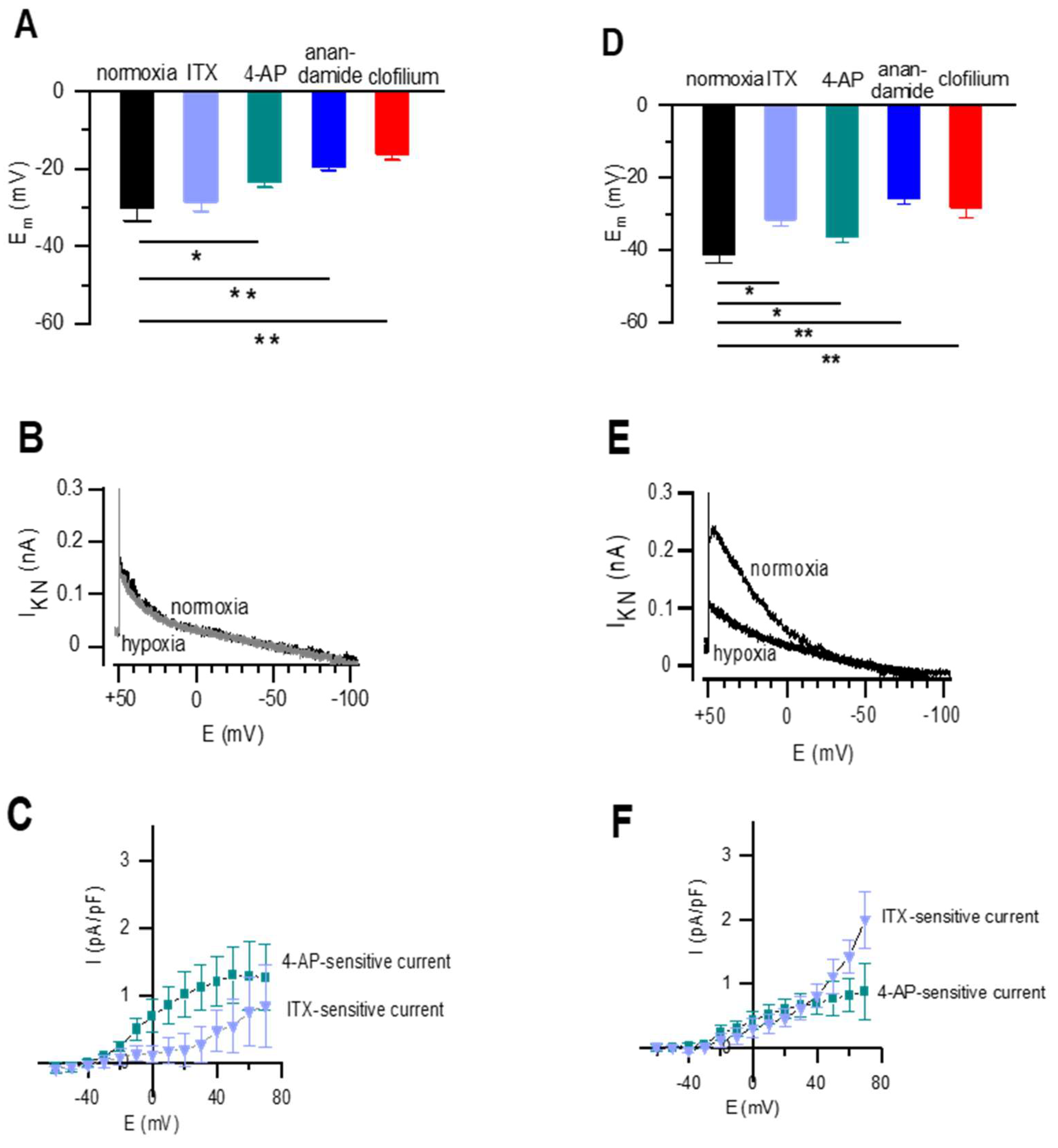
2.4. Resting Membrane Potential and Whole-Cell K+ Current in Newborn Rat DABSMC and PASMC
3. Discussion
4. Materials and Methods
4.1. Cell Isolation
4.2. Current Recording and Analysis
4.3. Solutions
4.4. Immunofluorescence STAINING
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Raj, J.U. Regulation of the pulmonary circulation in the fetus and newborn. Physiol. Rev. 2010, 90, 1291–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.C.; Yeh, J.L.; Hsu, J.H. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. Int. J. Mol. Sci. 2018, 19, 1861. [Google Scholar] [CrossRef] [Green Version]
- Mondejar-Parreno, G.; Cogolludo, A.; Perez-Vizcaino, F. Potassium (K+) channels in the pulmonary vasculature: Implications in pulmonary hypertension Physiological, pathophysiological and pharmacological regulation. Pharmacol. Ther. 2021, 225, 107835. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, A.; Papp, R.; Nagaraj, C.; Olschewski, H. Ion channels and transporters as therapeutic targets in the pulmonary circulation. Pharmacol. Ther. 2014, 144, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Burg, E.D.; Remillard, C.V.; Yuan, J.X. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: Pharmacotherapeutic implications. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S99–S111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olschewski, A.; Weir, E.K. Redox regulation of ion channels in the pulmonary circulation. Antioxid. Redox Signal. 2015, 22, 465–485. [Google Scholar] [CrossRef] [Green Version]
- Cornfield, D.N.; Reeve, H.L.; Tolarova, S.; Weir, E.K.; Archer, S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc. Natl. Acad. Sci. USA 1996, 93, 8089–8094. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, M.T.; Porter, V.A.; Saqueton, C.B.; Herron, J.M.; Resnik, E.R.; Cornfield, D.N. Pulmonary vascular response to normoxia and K(Ca) channel activity is developmentally regulated. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1250–L1257. [Google Scholar] [CrossRef] [Green Version]
- Michelakis, E.D.; Rebeyka, I.; Wu, X.; Nsair, A.; Thebaud, B.; Hashimoto, K.; Dyck, J.R.; Haromy, A.; Harry, G.; Barr, A.; et al. O2 sensing in the human ductus arteriosus: Regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ. Res. 2002, 91, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Thebaud, B.; Michelakis, E.D.; Wu, X.C.; Moudgil, R.; Kuzyk, M.; Dyck, J.R.; Harry, G.; Hashimoto, K.; Haromy, A.; Rebeyka, I.; et al. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: Implications for infants with patent ductus arteriosus. Circulation 2004, 110, 1372–1379. [Google Scholar] [CrossRef]
- Cui, Y.; Tran, S.; Tinker, A.; Clapp, L.H. The molecular composition of K(ATP) channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am. J. Respir. Cell Mol. Biol. 2002, 26, 135–143. [Google Scholar] [CrossRef]
- Gurney, A.M.; Osipenko, O.N.; MacMillan, D.; McFarlane, K.M.; Tate, R.J.; Kempsill, F.E. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ. Res. 2003, 93, 957–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennant, B.P.; Cui, Y.; Tinker, A.; Clapp, L.H. Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: Evidence for a major role of Kir2.4 subunits. J. Membr. Biol. 2006, 213, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, D.A.; Barhanin, J.; Gestreau, C.; Guyenet, P.G. The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch. 2015, 467, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Ghanayem, N.S.; Gordon, J.B. Modulation of pulmonary vasomotor tone in the fetus and neonate. Respir. Res. 2001, 2, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H.; Chatfield, B.A.; Hall, S.L.; McMurtry, I.F. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am. J. Physiol. 1990, 259, H1921–H1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accurso, F.J.; Alpert, B.; Wilkening, R.B.; Petersen, R.G.; Meschia, G. Time-dependent response of fetal pulmonary blood flow to an increase in fetal oxygen tension. Respir. Physiol. 1986, 63, 43–52. [Google Scholar] [CrossRef]
- Cornfield, D.N.; Chatfield, B.A.; McQueston, J.A.; McMurtry, I.F.; Abman, S.H. Effects of birth-related stimuli on L-arginine-dependent pulmonary vasodilation in ovine fetus. Am. J. Physiol. 1992, 262, H1474–H1481. [Google Scholar] [CrossRef] [PubMed]
- Fineman, J.R.; Wong, J.; Morin, F.C., 3rd; Wild, L.M.; Soifer, S.J. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J. Clin. Investig. 1994, 93, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Reeve, H.L.; Hampl, V.; Tolarova, S.; Michelakis, E.; Weir, E.K. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ. Res. 1996, 78, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Franco-Obregon, A.; Urena, J.; Lopez-Barneo, J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc. Natl. Acad. Sci. USA 1995, 92, 4715–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Barneo, J.; Lopez-Lopez, J.R.; Urena, J.; Gonzalez, C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 1988, 241, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, A.; Hong, Z.; Nelson, D.P.; Weir, E.K. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1143–L1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ. Res. 2006, 98, 1072–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.S.; Han, J.; Kim, N.; Ko, J.H.; Kim, S.J.; Earm, Y.E. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2461–H2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.S.; Son, Y.K.; Kim, N.; Ko, J.H.; Kang, S.H.; Warda, M.; Earm, Y.E.; Jung, I.D.; Park, Y.M.; Han, J. Acute hypoxia induces vasodilation and increases coronary blood flow by activating inward rectifier K(+) channels. Pflugers Arch. 2007, 454, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A.; Polak, J. Hypoxia. 4. Hypoxia and ion channel function. Am. J. Physiol. Cell Physiol. 2011, 300, C951–C967. [Google Scholar] [CrossRef]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am. J. Physiol. 1993, 264, L116–L123. [Google Scholar] [CrossRef]
- Cogolludo, A.L.; Moral-Sanz, J.; van der Sterren, S.; Frazziano, G.; van Cleef, A.N.; Menendez, C.; Zoer, B.; Moreno, E.; Roman, A.; Perez-Vizcaino, F.; et al. Maturation of O2 sensing and signaling in the chicken ductus arteriosus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L619–L630. [Google Scholar] [CrossRef] [Green Version]
- Gurney, A.; Manoury, B. Two-pore potassium channels in the cardiovascular system. Eur. Biophys. J. 2009, 38, 305–318. [Google Scholar] [CrossRef]
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, T.M.; Boyd, R.D.; Platt, H.S.; Strang, L.B. Composition of alveolar liquid in the foetal lamb. J. Physiol. 1969, 204, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraj, C.; Tang, B.; Balint, Z.; Wygrecka, M.; Hrzenjak, A.; Kwapiszewska, G.; Stacher, E.; Lindenmann, J.; Weir, E.K.; Olschewski, H.; et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur. Respir. J. 2013, 41, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.; Hong, F.; Olschewski, A.; Cabrera, J.A.; Varghese, A.; Nelson, D.P.; Weir, E.K. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation 2006, 114, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Connolly, M.; Nagaraj, C.; Tang, B.; Balint, Z.; Popper, H.; Smolle-Juettner, F.M.; Lindenmann, J.; Kwapiszewska, G.; Aaronson, P.I.; et al. Peroxisome proliferator-activated receptor-beta/delta, the acute signaling factor in prostacyclin-induced pulmonary vasodilation. Am. J. Respir. Cell Mol. Biol. 2012, 46, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Akaike, N.; Harata, N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn. J. Physiol. 1994, 44, 433–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippiat, J.D. Whole-cell recording using the perforated patch clamp technique. Methods Mol. Biol. 2008, 491, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Linley, J.E. Perforated whole-cell patch-clamp recording. Methods Mol. Biol. 2013, 998, 149–157. [Google Scholar] [CrossRef]
- Olschewski, A.; Hong, Z.; Peterson, D.A.; Nelson, D.P.; Porter, V.A.; Weir, E.K. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L15–L22. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, A.; Brau, M.E.; Olschewski, H.; Hempelmann, G.; Vogel, W. ATP-dependent potassium channel in rat cardiomyocytes is blocked by lidocaine. Possible impact on the antiarrhythmic action of lidocaine. Circulation 1996, 93, 656–659. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, C.; Li, Y.; Tang, B.; Bordag, N.; Guntur, D.; Enyedi, P.; Olschewski, H.; Olschewski, A. Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation. Int. J. Mol. Sci. 2022, 23, 4681. https://doi.org/10.3390/ijms23094681
Nagaraj C, Li Y, Tang B, Bordag N, Guntur D, Enyedi P, Olschewski H, Olschewski A. Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation. International Journal of Molecular Sciences. 2022; 23(9):4681. https://doi.org/10.3390/ijms23094681
Chicago/Turabian StyleNagaraj, Chandran, Yingji Li, Bi Tang, Natalie Bordag, Divya Guntur, Péter Enyedi, Horst Olschewski, and Andrea Olschewski. 2022. "Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation" International Journal of Molecular Sciences 23, no. 9: 4681. https://doi.org/10.3390/ijms23094681
APA StyleNagaraj, C., Li, Y., Tang, B., Bordag, N., Guntur, D., Enyedi, P., Olschewski, H., & Olschewski, A. (2022). Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation. International Journal of Molecular Sciences, 23(9), 4681. https://doi.org/10.3390/ijms23094681







