A2B5 Expression in Central Nervous System and Gliomas
Abstract
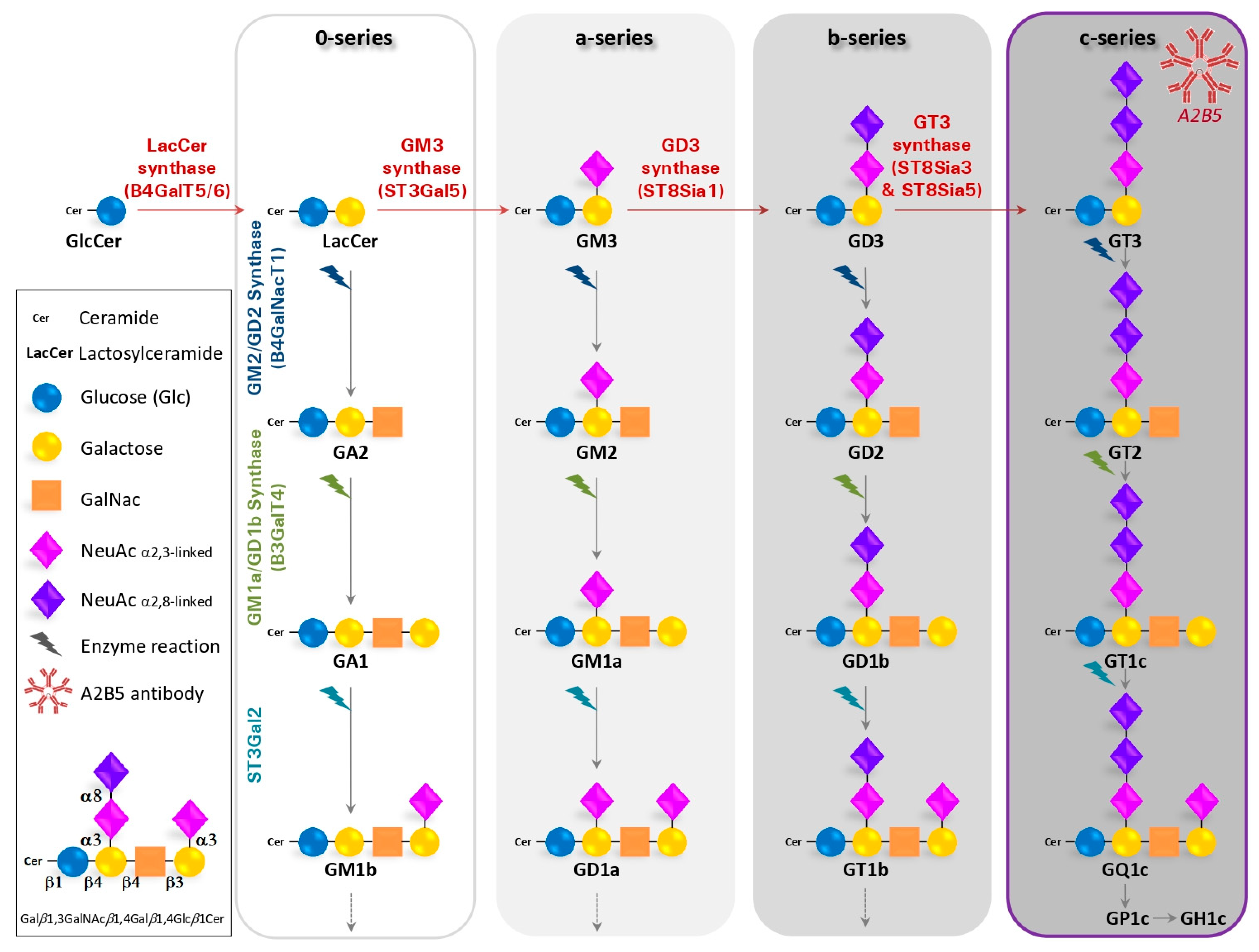
:1. Gangliosides Recognized by the A2B5 Antibody
1.1. Generation of the A2B5 Monoclonal Antibody
1.2. Structure, Biosynthesis and Expression of Gangliosides
1.3. The A2B5 Monoclonal Antibody Recognizes C-Series Gangliosides
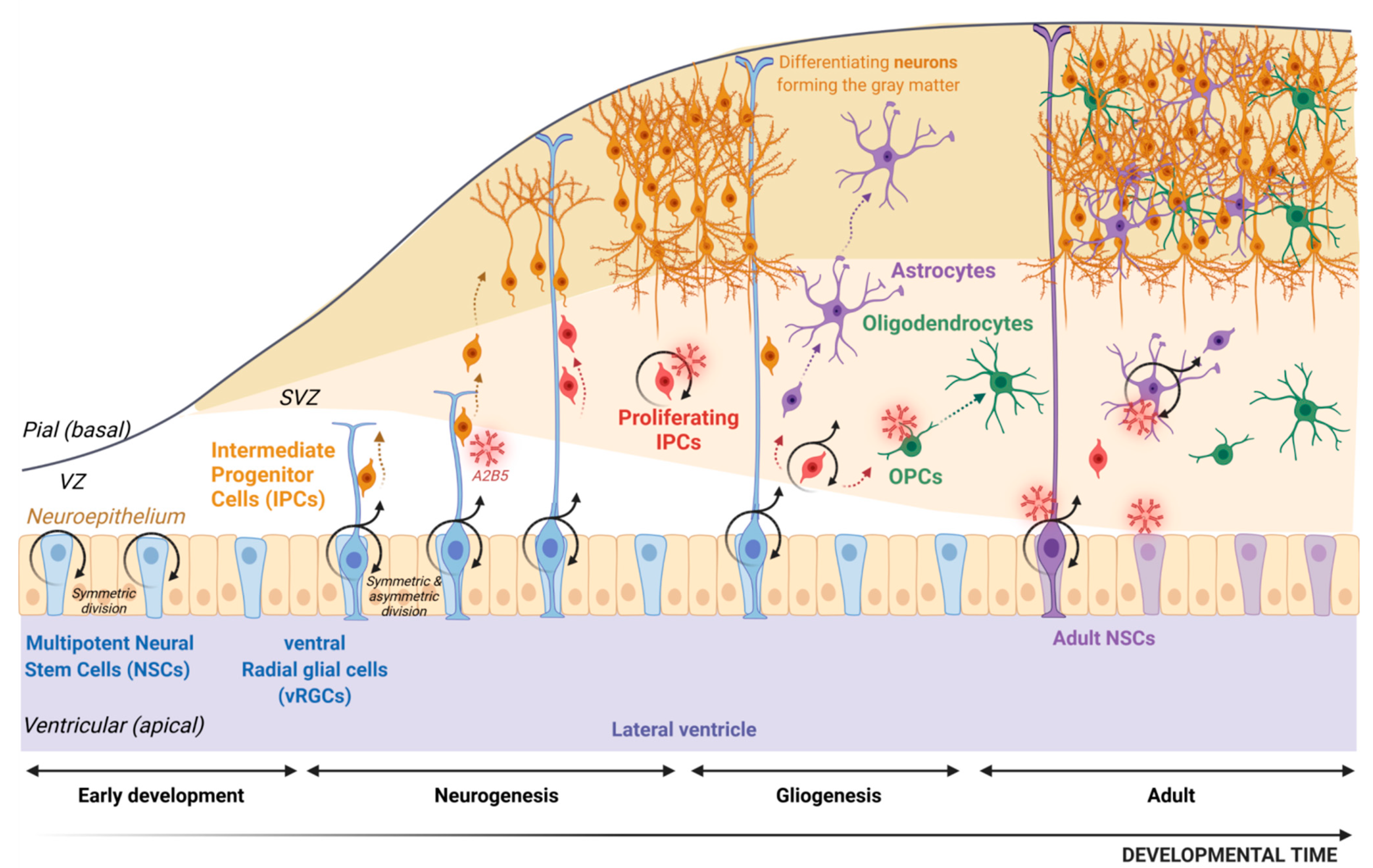
2. A2B5 Expression in the Vertebrate Central Nervous System
2.1. The Origin of Neurons and Glia
2.2. The Oligodendroglial Lineage
2.3. A2B5 Expression Characterizes the Bipotential Glial Progenitor Cells (O-2A) In Vitro
2.4. A2B5 and Other OPC Cell Surface Markers: NG2 and PDGFRα
2.5. A2B5 Expression Characterizes Glial-Restricted Precursor Cells Derived from Multipotent NSCs
2.6. A Subset of Radial Glia Cells in Rodent Expresses A2B5 during Development and in Adulthood
2.7. A2B5 Identifies OPCs and Multipotent Neural Progenitor Cells from Subcortical White Matter of the Adult Human Brain
3. A2B5 Expression and Glioma
3.1. Human Glioma: WHO Classification and Cell of Origin
3.2. A2B5 Expression Is Observed in All Glioma Types
3.3. A2B5 Expression Is a Marker of Cancer Stem Cell (CSC) in Glioblastoma
3.4. A2B5 Cells from Glioblastoma and Normal A2B5 Cells Exhibit Distinct Transcriptomic Signatures
3.5. A2B5 Cells in Glioblastoma Change Their Phenotype in Response to Environmental Clues
3.6. Generation and Functional Properties of the A2B5 Epitope in Glioblastoma Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenbarth, G.S.; Walsh, F.S.; Nirenberg, M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc. Natl. Acad. Sci. USA 1979, 76, 4913–4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klenk, E.; Hoppe Seylers, Z. Über die Natur der Phosphatide und anderer Lipoide des Gehirns und der Leber. Physiol. Chem. 1935, 235, 24–26. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Posse de Chaves, E.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svennerholm, L. Chromatographic Separation of Human Brain Gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Fischer, C.; Lee, H.; Ruch, B.; Kelm, S. Sialic Acids as Regulators of Molecular and Cellular Interactions. Lectins and Glycoconj. Oncol. 1988, 19, 5–23. [Google Scholar] [CrossRef]
- Ishii, A.; Ohta, M.; Watanabe, Y.; Matsuda, K.; Ishiyama, K.; Sakoe, K.; Nakamura, M.; Inokuchi, J.-I.; Sanai, Y.; Saito, M. Expression Cloning and Functional Characterization of Human cDNA for Ganglioside GM3 Synthase. J. Biol. Chem. 1998, 273, 31652–31655. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, M.; Yamashiro, S.; Yamamoto, A.; Furukawa, K.; Takamiya, K.; LloydK, O.; Shiku, H. Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc. Natl. Acad. Sci. USA 1994, 91, 10455–10459. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Kim, K.-S.; Do, S.-I.; Kim, C.-H.; Kim, S.-K.; Lee, Y.-C. Molecular Cloning and Expression of Human α2,8-Sialyltransferase (hST8Sia V). Biochem. Biophys. Res. Commun. 1997, 235, 327–330. [Google Scholar] [CrossRef]
- Nagata, Y.; Yamashiro, S.; Yodoi, J.; Lloyd, K.O.; Shiku, H.; Furukawa, K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 1992, 267, 12082–12089. [Google Scholar] [CrossRef]
- Amado, M.; Almeida, R.; Schwientek, T.; Clausen, H. Identification and characterization of large galactosyltransferase gene families: Galactosyltransferases for all functions. Biochim. Biophys. Acta 1999, 1473, 35–53. [Google Scholar] [CrossRef]
- Monti, E.; Miyagi, T. Structure and Function of Mammalian Sialidases. Top. Curr. Chem. 2012, 366, 183–208. [Google Scholar] [CrossRef] [Green Version]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Ngamukote, S.; Yanagisawa, M.; Ariga, T.; Ando, S.; Yu, R.K. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 2007, 103, 2327–2341. [Google Scholar] [CrossRef]
- Fenderson, B.A.; Andrews, P.W.; Nudelman, E.; Clausen, H.; Hakomori, S.-I. Glycolipid core structure switching from globo- to lacto- and ganglio-series during retinoic acid-induced differentiation of TERA-2-derived human embryonal carcinoma cells. Dev. Biol. 1987, 122, 21–34. [Google Scholar] [CrossRef]
- Dubois, C.; Manuguerra, J.C.; Hauttecoeur, B.; Maze, J. Monoclonal antibody A2B5, which detects cell surface antigens, binds to ganglioside GT3 (II3 (NeuAc)3LacCer) and to its 9-O-acetylated derivative. J. Biol. Chem. 1990, 265, 2797–2803. [Google Scholar] [CrossRef]
- Saito, M.; Kitamura, H.; Sugiyama, K. The specificity of monoclonal antibody A2B5 to c-series gangliosides. J. Neurochem. 2001, 78, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Inoko, E.; Nishiura, Y.; Tanaka, H.; Takahashi, T.; Furukawa, K.; Kitajima, K.; Sato, C. Developmental stage-dependent expression of an 2,8-trisialic acid unit on glycoproteins in mouse brain. J. Glycobiol. 2010, 20, 916–928. [Google Scholar] [CrossRef] [Green Version]
- Anthony, T.; Klein, C.; Fishell, G.; Heintz, N. Radial Glia Serve as Neuronal Progenitors in All Regions of the Central Nervous System. Neuron 2004, 41, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Rakic, P. Principles of neural cell migration. J. Exp. 1990, 46, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell. Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial–cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [Green Version]
- Rakic, P.; Sidman, R.L. Supravital DNA synthesis in the developing human and mouse brain. J. Neuropathol. Exp. Neurol. 1968, 27, 246–276. [Google Scholar] [CrossRef]
- Rakic, P.; Sidman, R.L. Autoradiographic study of supravital DNA synthesis in fetal human brain. J. Neuropathol. Exp. Neurol. 1968, 27, 139–140. [Google Scholar] [CrossRef]
- Zecevic, N.; Chen, Y.; Filipovic, R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 2005, 491, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.L.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Voigt, T. Development of glial cells in the cerebral wall of ferrets: Direct tracing of their transformation from radial glia into astrocytes. J. Comp. Neurol. 1989, 289, 74–88. [Google Scholar] [CrossRef]
- Noctor, S.C.; Martínez-Cerdeño, V.; Kriegstein, A.R. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J. Comp. Neurol. 2008, 508, 28–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, G.; Yang, L.; Li, Z.; Zhang, Z.; Xu, Z.; Cai, Y.; Du, H.; Su, Z.; Wang, Z.; et al. Decoding Cortical Glial Cell Development. Neurosci. Bull. 2021, 37, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caillé, I.; Lim, D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Seri, B.; García-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.C.; Roy, N.S.; Keyoung, H.M.; Goodman, R.R.; McKhann, G., 2nd; Jiang, L.; Kang, J.; Nedergaard, M.; Goldman, S.A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003, 9, 439–447. [Google Scholar] [CrossRef]
- Goldman, S.A.; Kuypers, N.J. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.; van Bruggen, D.; Vanichkina, D.P.; Floriddia, E.M.; Munguba, H.; Väremo, L.; Giacomello, S.; Falcão, A.M.; Meijer, M.; Björklund Åsa, K.; et al. Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development. Dev. Cell 2018, 46, 504–517.e7. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A. Astrocyte Differentiation from Oligodendrocyte Precursors. In Emerging Concepts in Neuro-Oncology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 41–60. [Google Scholar]
- Tekki-Kessaris, N.; Woodruff, R.; Hall, A.C.; Gaffield, W.; Kimura, S.; Stiles, C.D.; Rowitch, D.H.; Richardson, W.D. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 2001, 128, 2545–2554. [Google Scholar] [CrossRef]
- Chapman, H.; Waclaw, R.R.; Pei, Z.; Nakafuku, M.; Campbell, K. The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 2013, 140, 2289–2298. [Google Scholar] [CrossRef] [Green Version]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2005, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gensert, J.M.; Goldman, J.E. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia 1996, 17, 39–51. [Google Scholar] [CrossRef]
- Scolding, N.; Rayner, P.; Compston, D. Identification of A2B5-positive putative oligodendrocyte progenitor cells and A2B5-positive astrocytes in adult human white matter. Neurosci. 1999, 89, 1–4. [Google Scholar] [CrossRef]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Bhaduri, A.; Velmeshev, D.; Wang, S.; Wang, L.; Rottkamp, C.A.; Alvarez-Buylla, A.; Rowitch, D.H.; Kriegstein, A.R. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020, 182, 594–608.e11. [Google Scholar] [CrossRef] [PubMed]
- Menn, B.; García-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of Oligodendrocytes in the Subventricular Zone of the Adult Brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Mirsky, R.; Fields, K.L.; Lisak, R.P.; Dorfman, S.H.; Silberberg, D.H.; Gregson, N.A.; Leibowitz, S.; Kennedy, M.C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature 1978, 274, 813–816. [Google Scholar] [CrossRef]
- Raff, M.C.; Abney, E.R.; Cohen, J.; Lindsay, R.; Noble, M. Two types of astrocytes in cultures of developing rat white matter: Differences in morphology, surface gangliosides, and growth characteristics. J. Neurosci. 1983, 3, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Raff, M.C.; Abney, E.R.; Miller, R.H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev. Biol. 1984, 106, 53–60. [Google Scholar] [CrossRef]
- Monteros, A.E.D.L.; Zhang, M.; De Vellis, J. O2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Groves, A.K.; Barnett, S.C.; Franklin, R.J.; Crang, A.J.; Mayer, M.; Blakemore, W.F.; Noble, M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature 1993, 362, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B. The NG2 antigen, a putative lineage marker: Immunofluorescent localization in primary cultures of rat brain. Dev. Biol. 1981, 83, 154–165. [Google Scholar] [CrossRef]
- Hart, I.K.; Richardson, W.D.; Heldin, C.H.; Westermark, B.; Raff, M.C. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development 1989, 105, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, C.; Raff, M.C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 1986, 319, 499–502. [Google Scholar] [CrossRef]
- Wren, D.; Wolswijk, G.; Noble, M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J. Cell Biol. 1992, 116, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.H.; Raff, M.C. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J. Neurosci. 1984, 4, 585–592. [Google Scholar] [CrossRef]
- Richardson, W.D.; Pringle, N.; Mosley, M.J.; Westermark, B.; Dubois-Dalcg, M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 1988, 53, 309–319. [Google Scholar] [CrossRef]
- Raff, M.C.; Lillien, L.E.; Richardson, W.D.; Burne, J.F.; Noble, M.D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 1988, 333, 562–565. [Google Scholar] [CrossRef]
- Pringle, N.P.; Richardson, W.D. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development 1993, 117, 525–533. [Google Scholar] [CrossRef]
- Stallcup, W.; Beasley, L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J. Neurosci. 1987, 7, 2737–2744. [Google Scholar] [CrossRef]
- Nishiyama, A.; Lin, X.H.; Giese, N.; Heldin, C.H.; Stallcup, W.B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci. Res. 1996, 43, 315–330. [Google Scholar] [CrossRef]
- Nishiyama, A.; Lin, X.-H.; Giese, N.; Heldin, C.-H.; Stallcup, W. Co-localization of NG2 proteoglycan and PDGF?—Receptor on O2A progenitor cells in the developing rat brain. J. Neurosci. Res. 1996, 43, 299–314. [Google Scholar] [CrossRef]
- Lu, Q.; Yuk, D.-I.; Alberta, J.; Zhu, Z.; Pawlitzky, I.; Chan, J.; McMahon, A.P.; Stiles, C.D.; Rowitch, D.H. Sonic Hedgehog–Regulated Oligodendrocyte Lineage Genes Encoding bHLH Proteins in the Mammalian Central Nervous System. Neuron 2000, 25, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wang, S.; Anderson, D.J. Identification of a Novel Family of Oligodendrocyte Lineage-Specific Basic Helix–Loop–Helix Transcription Factors. Neuron 2000, 25, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Kuhlbrodt, K.; Herbarth, B.; Sock, E.; Hermans-Borgmeyer, I.; Wegner, M. Sox10, a Novel Transcriptional Modulator in Glial Cells. J. Neurosci. 1998, 18, 237–250. [Google Scholar] [CrossRef]
- Gregath, A.; Lu, Q.R. Epigenetic modifications—insight into oligodendrocyte lineage progression, regeneration, and disease. FEBS Lett. 2018, 592, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Seeker, L.A.; Williams, A. Oligodendroglia heterogeneity in the human central nervous system. Acta Neuropathol. 2021, 143, 143–157. [Google Scholar] [CrossRef]
- Pouly, S.; Becher, B.; Blain, M.; Antel, J. Expression of a homologue of rat NG2 on human microglia. Glia 1999, 27, 259–268. [Google Scholar] [CrossRef]
- Baracskay, K.L.; Kidd, G.J.; Miller, R.H.; Trapp, B.D. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 2007, 55, 1001–1010. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Wang, Z.; Wang, Q.; Hu, C.; Wang, X.; Lu, S.; Li, K.; Yang, Y.; Luan, Z. Identifying the functions of two biomarkers in human oligodendrocyte progenitor cell development. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Rao, M.; Mayer-Proschel, M. Glial-Restricted Precursors Are Derived from Multipotent Neuroepithelial Stem Cells. Dev. Biol. 1997, 188, 48–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Babiarz, J.; Woodbury, J.; Kane-Goldsmith, N.; Grumet, M. Spatiotemporal heterogeneity of CNS radial glial cells and their transition to restricted precursors. Dev. Biol. 2004, 271, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungblut, M.; Tiveron, M.C.; Barral, S.; Abrahamsen, B.; Knöbel, S.; Pennartz, S.; Schmitz, J.; Perraut, M.; Pfrieger, F.W.; Stoffel, W.; et al. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia 2012, 60, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wang, S.; Harrison-Restelli, C.; Benraiss, A.; Fraser, R.A.R.; Gravel, M.; Braun, P.E.; Goldman, S.A. Identification, Isolation, and Promoter-Defined Separation of Mitotic Oligodendrocyte Progenitor Cells from the Adult Human Subcortical White Matter. J. Neurosci. 1999, 19, 9986–9995. [Google Scholar] [CrossRef] [Green Version]
- Scherer, S.S.; Braun, P.E.; Grinspan, J.; Collarini, E.; Wang, D.-Y.; Kamholz, J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron 1994, 12, 1363–1375. [Google Scholar] [CrossRef]
- Lojewski, X.; Hermann, A.; Wegner, F.; Araúzo-Bravo, M.J.; Hallmeyer-Elgner, S.; Kirsch, M.; Schwarz, J.; Schöler, H.R.; Storch, A. Human Adult White Matter Progenitor Cells Are Multipotent Neuroprogenitors Similar to Adult Hippocampal Progenitors. Stem Cells Transl. Med. 2014, 3, 458–469. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Vescovi, A.L.; Galli, R.; Reynolds, B.A. Brain tumour stem cells. Nat. Cancer 2006, 6, 425–436. [Google Scholar] [CrossRef]
- Shao, F.; Liu, C. Revisit the Candidacy of Brain Cell Types as the Cell(s) of Origin for Human High-Grade Glioma. Front. Mol. Neurosci. 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin, C.; Baeza, N.; Tong, S.; Bouvier, C.; Quilichini, B.; Durbec, P.; Figarella-Branger, D. In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol. Appl. Neurobiol. 2006, 32, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Kleihues, P.; Louis, D.N.; Scheithauer, B.W.; Rorke, L.B.; Reifenberger, G.; Burger, P.C.; Cavenee, W.K. The WHO Classification of Tumors of the Nervous System. J. Neuropathol. Exp. Neurol. 2002, 61, 215–225. [Google Scholar] [CrossRef]
- Shoshan, Y.; Nishiyama, A.; Chang, A.; Mörk, S.; Barnett, G.H.; Cowell, J.K.; Trapp, B.D.; Staugaitis, S.M. Expression of oligodendrocyte progenitor cell antigens by gliomas: Implications for the histogenesis of brain tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 10361–10366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebetz, J.; Tian, D.; Persson, A.; Widegren, B.; Salford, L.G.; Englund, E.; Gisselsson, D.; Fan, X. Glial Progenitor-Like Phenotype in Low-Grade Glioma and Enhanced CD133-Expression and Neuronal Lineage Differentiation Potential in High-Grade Glioma. PLoS ONE 2008, 3, e1936. [Google Scholar] [CrossRef] [Green Version]
- Auvergne, R.M.; Sim, F.J.; Wang, S.; Chandler-Militello, D.; Burch, J.; Al Fanek, Y.; Davis, D.; Benraiss, A.; Walter, K.; Achanta, P.; et al. Transcriptional Differences between Normal and Glioma-Derived Glial Progenitor Cells Identify a Core Set of Dysregulated Genes. Cell Rep. 2013, 3, 2127–2141. [Google Scholar] [CrossRef] [Green Version]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef]
- Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef] [Green Version]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; DiMeco, F.; Vescovi, A. Isolation and Characterization of Tumorigenic, Stem-like Neural Precursors from Human Glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Ogden, A.T.; Waziri, A.E.; Lochhead, R.A.; Fusco, D.; Lopez, K.; Ellis, J.A.; Kang, J.; Assanah, M.; McKhann, G.M.; Sisti, M.B.; et al. Identification of A2b5+Cd133—Tumor-Initiating Cells in Adult Human Gliomas. Neurosurgery 2008, 62, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchoghandjian, A.; Baeza, N.; Colin, C.; Cayre, M.; Metellus, P.; Beclin, C.; Ouafik, L.; Figarella-Branger, D. A2B5 Cells from Human Glioblastoma have Cancer Stem Cell Properties. Brain Pathol. 2010, 20, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, G.; Li, Y.; Xie, X.; Zhou, Y.; Du, Z. Aggressive invasion is observed in CD133(-)/A2B5(+) glioma-initiating cells. Oncol. Lett. 2015, 10, 3399–3406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchoghandjian, A.; Baeza-Kallee, N.; Beclin, C.; Metellus, P.; Colin, C.; Ducray, F.; Adélaïde, J.; Rougon, G.; Figarella-Branger, D. Cortical and Subventricular Zone Glioblastoma-Derived Stem-Like Cells Display Different Molecular Profiles and Differential In Vitro and In Vivo Properties. Ann. Surg. Oncol. 2011, 19, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, H.; Mimura, Y.; Zahra, M.H.; Katayama, S.; Hassan, G.; Afify, S.M.; Seno, M. Isolation and characterization of cancer stem cells derived from human glioblastoma. Am. J. Cancer Res. 2021, 11, 441–457. [Google Scholar] [PubMed]
- Auvergne, R.; Wu, C.; Connell, A.; Au, S.; Cornwell, A.; Osipovitch, M.; Benraiss, A.; Dangelmajer, S.; Guerrero-Cazares, H.; Quinoneshinojosa, A.; et al. PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene 2016, 35, 3817–3828. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, Q.; Li, Z.; Sathornsumetee, S.; Wang, H.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Targeting Cancer Stem Cells through L1CAM Suppresses Glioma Growth. Cancer Res. 2008, 68, 6043–6048. [Google Scholar] [CrossRef] [Green Version]
- Ehtesham, M.; Mapara, K.Y.; Stevenson, C.B.; Thompson, R.C. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009, 274, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Son, M.J.; Woolard, K.; Nam, D.-H.; Lee, J.; Fine, H.A. SSEA-1 Is an Enrichment Marker for Tumor-Initiating Cells in Human Glioblastoma. Cell Stem Cell 2009, 4, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lathia, J.D.; Wu, Q.; Wang, J.; Li, Z.; Heddleston, J.M.; Eyler, C.E.; Elderbroom, J.; Gallagher, J.; Schuschu, J.; et al. Targeting Interleukin 6 Signaling Suppresses Glioma Stem Cell Survival and Tumor Growth. Stem Cells 2009, 27, 2393–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietras, E.J.; Katz, A.M.; Ekström, E.J.; Wee, B.; Halliday, J.J.; Pitter, K.; Werbeck, J.L.; Amankulor, N.M.; Huse, J.T.; Holland, E.C. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell Stem Cell 2014, 14, 357–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lathia, J.D.; Gallagher, J.; Heddleston, J.M.; Wang, J.; Eyler, C.E.; MacSwords, J.; Wu, Q.; Vasanji, A.; McLendon, R.E.; Hjelmeland, A.B.; et al. Integrin Alpha 6 Regulates Glioblastoma Stem Cells. Cell Stem Cell 2010, 6, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer Stem Cell-Specific Scavenger Receptor CD36 Drives Glioblastoma Progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef] [Green Version]
- Erhart, F.; Blauensteiner, B.; Zirkovits, G.; Printz, D.; Soukup, K.; Klingenbrunner, S.; Fischhuber, K.; Reitermaier, R.; Halfmann, A.; Lötsch, D.; et al. Gliomasphere marker combinatorics: Multidimensional flow cytometry detects CD44+/CD133+/ITGA6+/CD36+ signature. J. Cell. Mol. Med. 2018, 23, 281–292. [Google Scholar] [CrossRef]
- Stieber, D.; Golebiewska, A.; Evers, L.; Lenkiewicz, E.; Brons, N.H.C.; Nicot, N.; Oudin, A.; Bougnaud, S.; Hertel, F.; Bjerkvig, R.; et al. Glioblastomas are composed of genetically divergent clones with distinct tumourigenic potential and variable stem cell-associated phenotypes. Acta Neuropathol. 2013, 127, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Innes, J.A.; Lowe, A.S.; Fonseca, R.; Aley, N.; El-Hassan, T.; Constantinou, M.; Lau, J.; Eddaoudi, A.; Marino, S.; Brandner, S. Phenotyping clonal populations of glioma stem cell reveals a high degree of plasticity in response to changes of microenvironment. Lab. Investig. 2021, 102, 172–184. [Google Scholar] [CrossRef]
- Baeza-Kallee, N.; Bergès, R.; Soubéran, A.; Colin, C.; Denicolaï, E.; Appay, R.; Tchoghandjian, A.; Figarella-Branger, D. Glycolipids Recognized by A2B5 Antibody Promote Proliferation, Migration, and Clonogenicity in Glioblastoma Cells. Cancers 2019, 11, 1267. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.; Furukawa, K.; Takahashi, T.; Urano, T.; Sanai, Y.; Nagino, M.; Nimura, Y. Fundamental study of small interfering RNAs for ganglioside GD3 synthase gene as a therapeutic target of lung cancers. Oncogene 2006, 25, 6924–6935. [Google Scholar] [CrossRef] [Green Version]
- Mountney, A.; Zahner, M.R.; Lorenzini, I.; Oudega, M.; Schramm, L.P.; Schnaar, R.L. Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 11561–11566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joksimovic, S.L.; Evans, J.G.; McIntire, W.E.; Orestes, P.; Barrett, P.Q.; Jevtovic-Todorovic, V.; Todorovic, S.M. Glycosylation of CaV3.2 Channels Contributes to the Hyperalgesia in Peripheral Neuropathy of Type 1 Diabetes. Front. Cell. Neurosci. 2020, 14, 605312. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yao, Y.; Hua, W.; Wu, Z.B.; Zhong, P.; Mao, Y.; Zhou, L.; Luo, F.; Chu, Y. Mouse glioma immunotherapy mediated by A2B5+ GL261 cell lysate-pulsed dendritic cells. J. Neuro Oncol. 2014, 116, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F.; et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 2016, 7, 41172–41185. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figarella-Branger, D.; Colin, C.; Baeza-Kallee, N.; Tchoghandjian, A. A2B5 Expression in Central Nervous System and Gliomas. Int. J. Mol. Sci. 2022, 23, 4670. https://doi.org/10.3390/ijms23094670
Figarella-Branger D, Colin C, Baeza-Kallee N, Tchoghandjian A. A2B5 Expression in Central Nervous System and Gliomas. International Journal of Molecular Sciences. 2022; 23(9):4670. https://doi.org/10.3390/ijms23094670
Chicago/Turabian StyleFigarella-Branger, Dominique, Carole Colin, Nathalie Baeza-Kallee, and Aurélie Tchoghandjian. 2022. "A2B5 Expression in Central Nervous System and Gliomas" International Journal of Molecular Sciences 23, no. 9: 4670. https://doi.org/10.3390/ijms23094670
APA StyleFigarella-Branger, D., Colin, C., Baeza-Kallee, N., & Tchoghandjian, A. (2022). A2B5 Expression in Central Nervous System and Gliomas. International Journal of Molecular Sciences, 23(9), 4670. https://doi.org/10.3390/ijms23094670






