The Phytotoxin Myrigalone A Triggers a Phased Detoxification Programme and Inhibits Lepidium sativum Seed Germination via Multiple Mechanisms including Interference with Auxin Homeostasis
Abstract
:1. Introduction
2. Results and Discussion
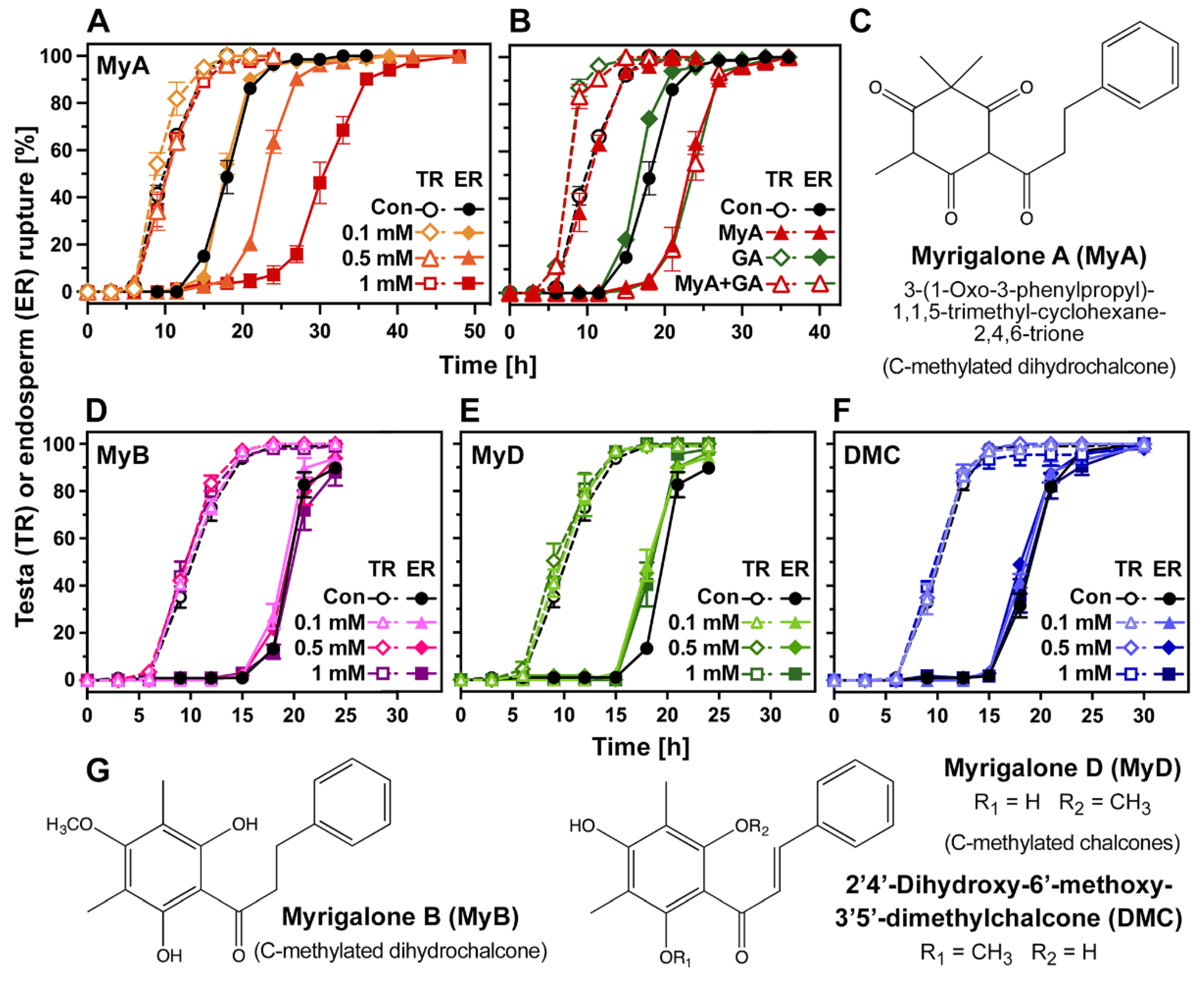
2.1. Chalcones Differ in Their Phytotoxic Bioactivity and Inhibitory Action on Seed Germination
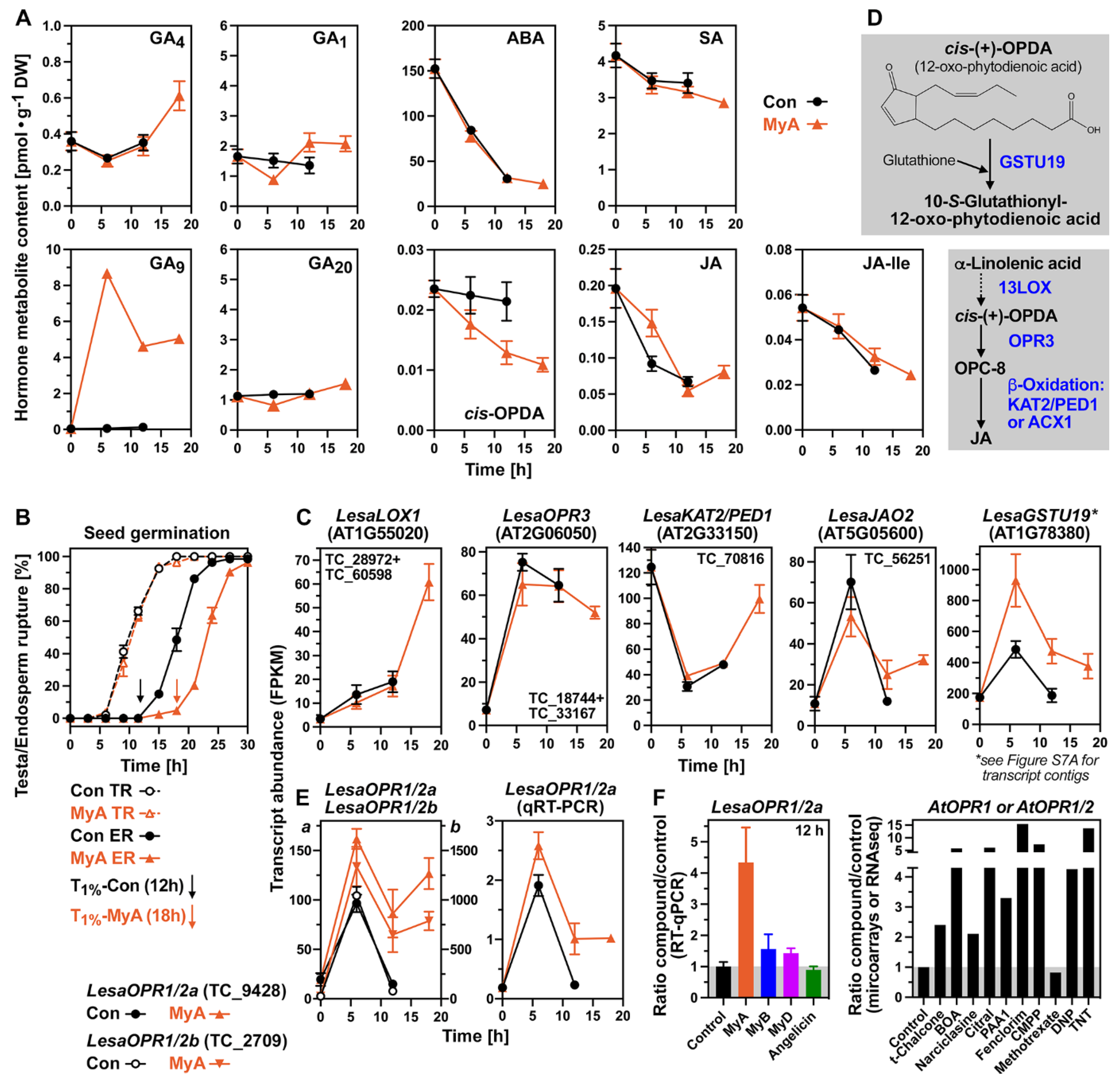
2.2. MyA-Induced Hormone and Transcriptome Changes in Germinating Seeds
2.3. MyA-Regulated OPDA Reductases, Oxylipin Pathway and Hormone Transporter Genes
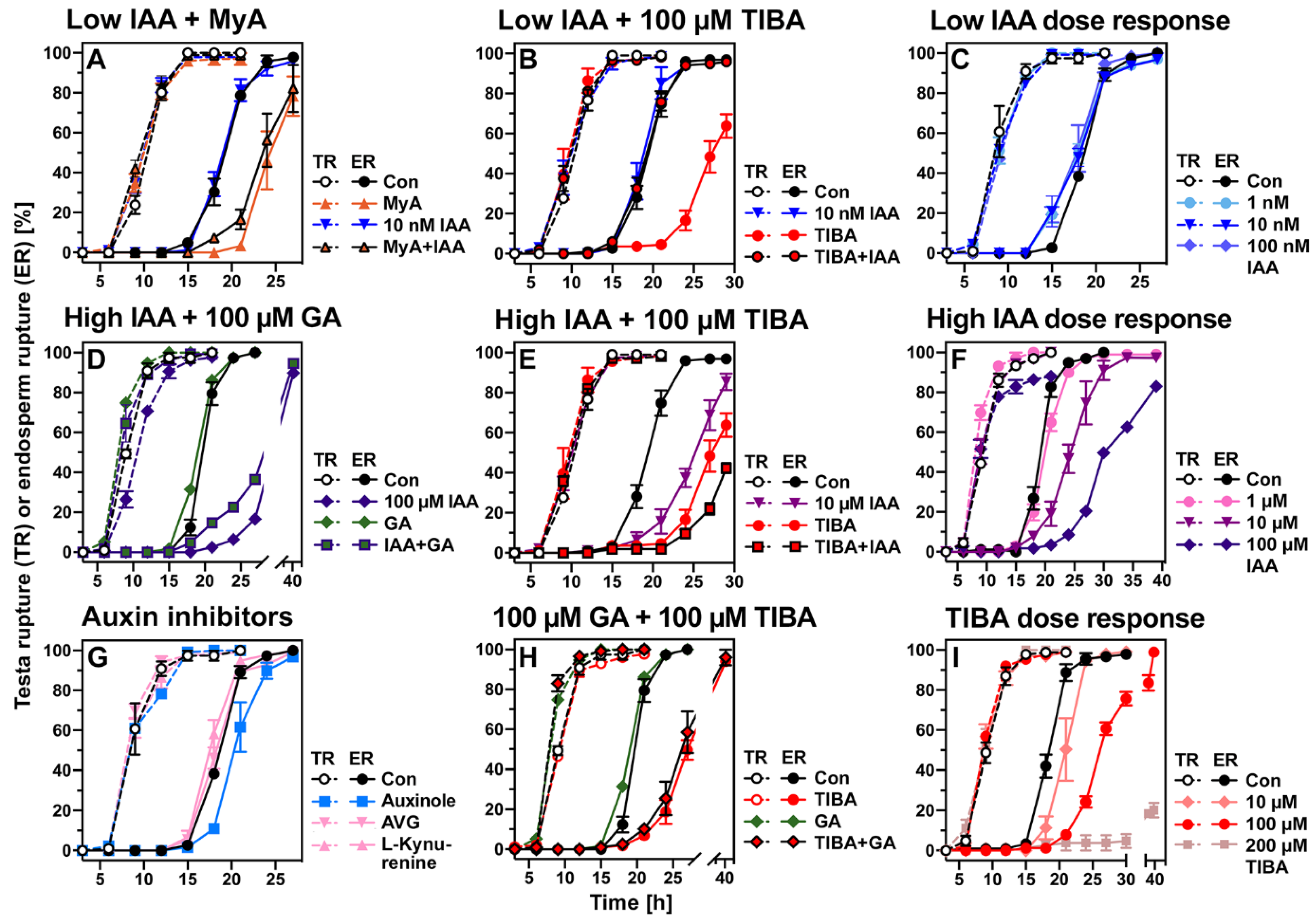
2.4. Evaluation of the Roles of Proposed Myrigalone Bioactivities during Seed Germination
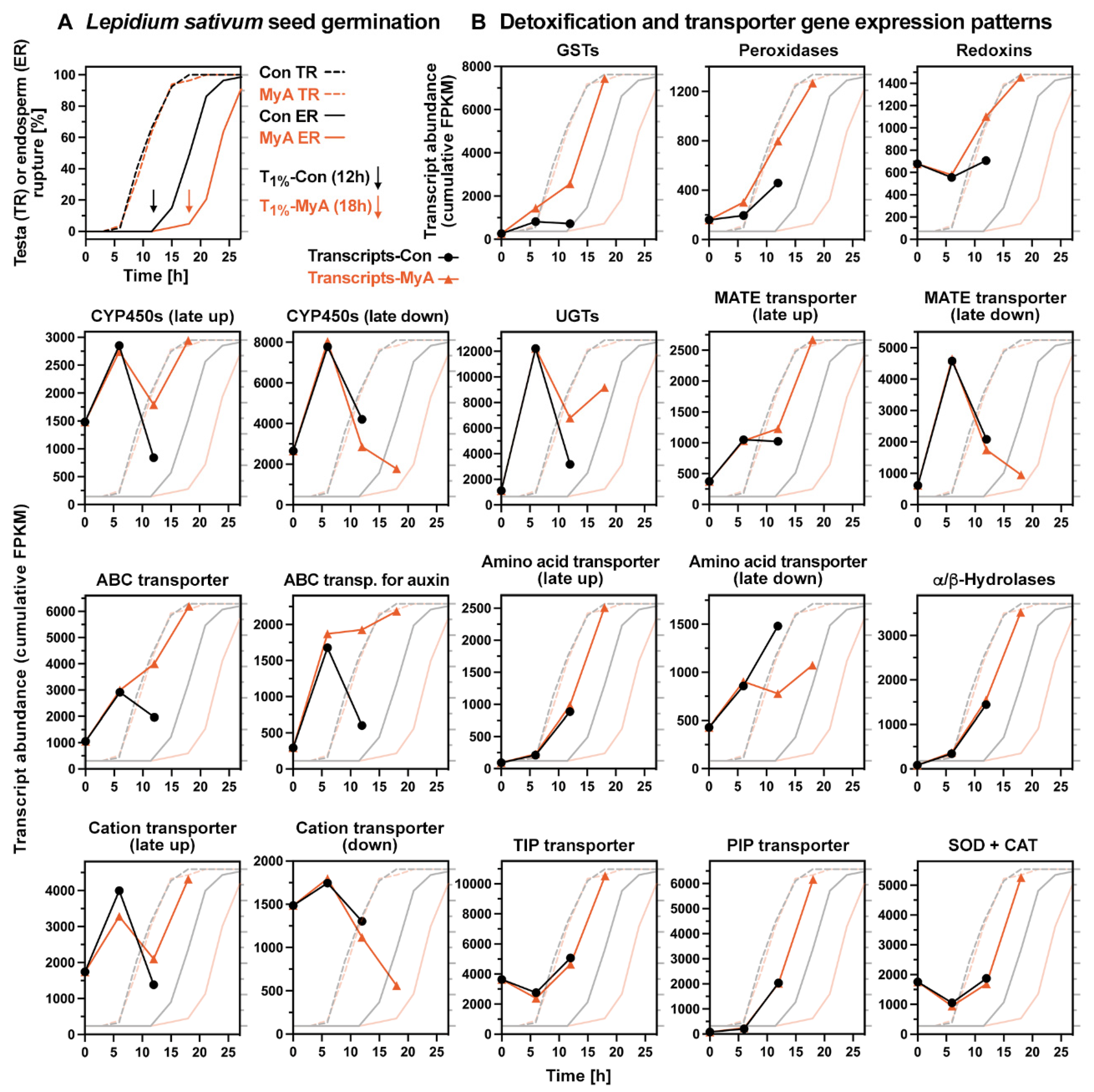
2.5. Phased Induction of the Seed’s Detoxification Programme by the Phytotoxin MyA
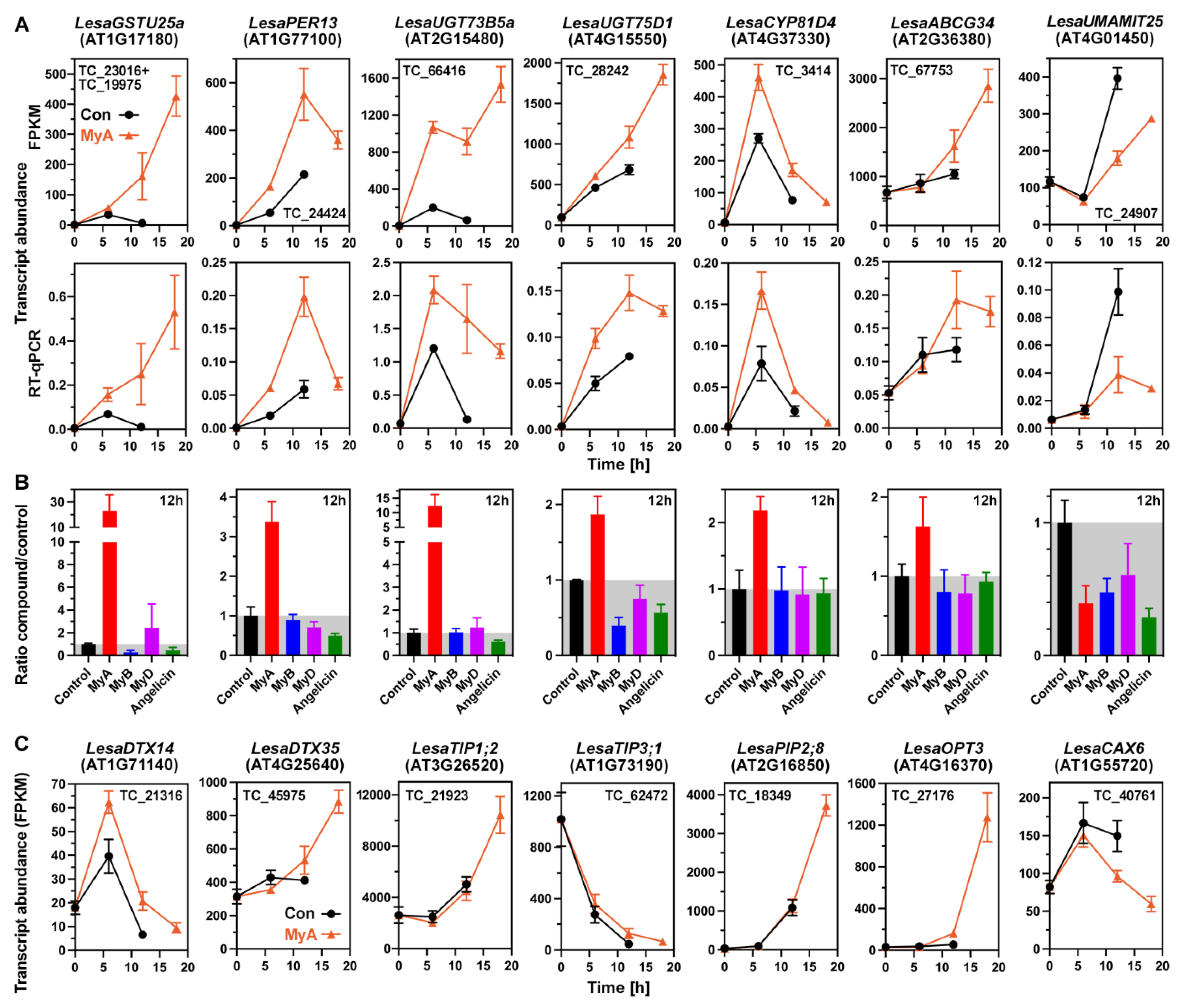
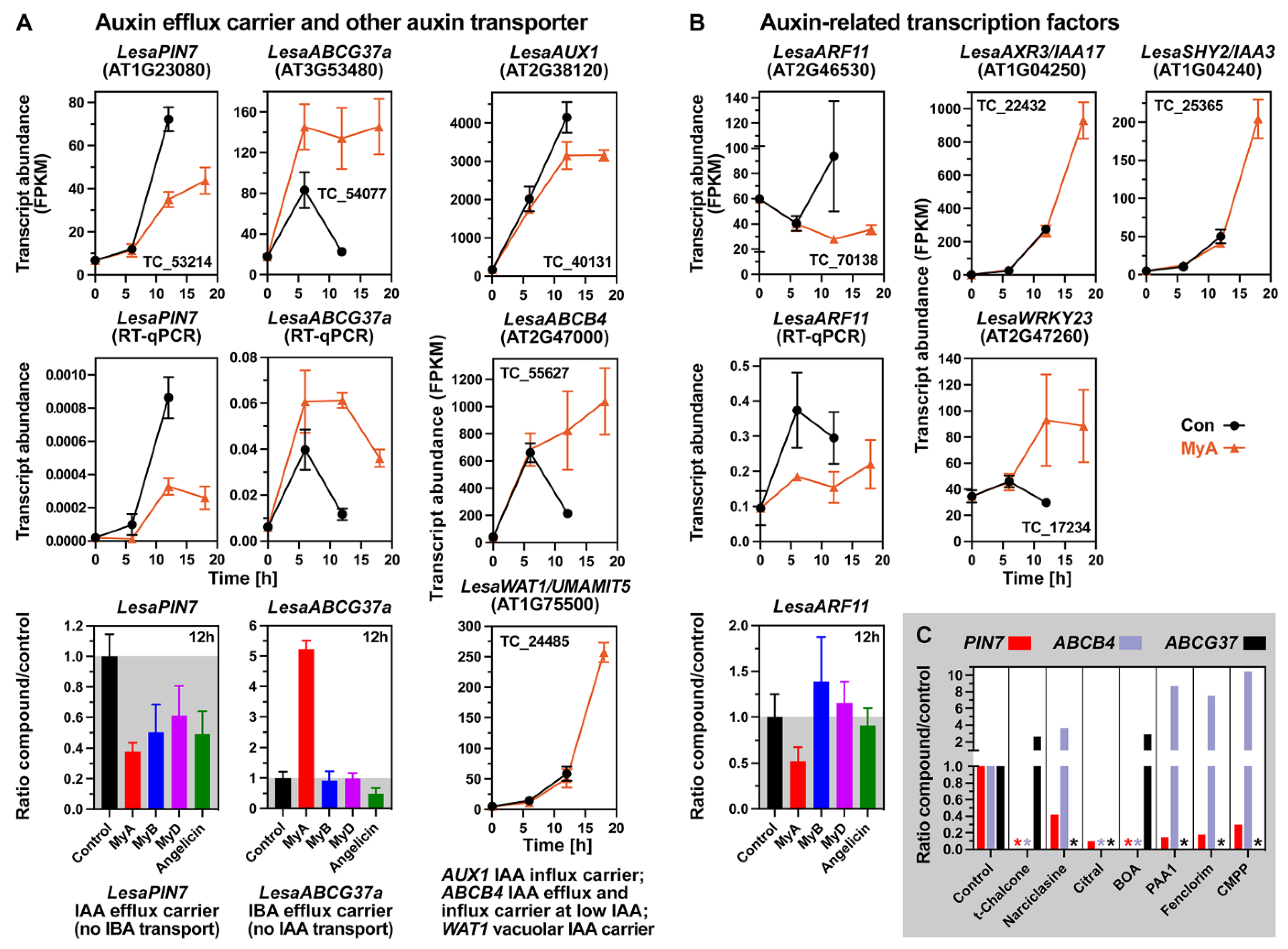
2.6. MyA Interferes with Transporter Gene Expression in Germinating Cress Seeds
2.7. MyA Interferes with the Expression of Auxin Transport and Signalling in Germinating Seeds
2.8. Conserved and Chemical-Specific Detoxification Response and Interference with Auxin Transport
3. Materials and Methods
3.1. Plant Material and Germination Assays
3.2. Chemicals
3.3. Plant Hormone Extraction and Quantification
3.4. Extraction of RNA and RT-qPCR Analysis
3.5. RNAseq Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabayashi, K.; Leubner-Metzger, G. Seed dormancy and weed emergence: From simulating environmental change to understanding trait plasticity, adaptive evolution, and population fitness. J. Exp. Bot. 2021, 72, 4181–4185. [Google Scholar] [CrossRef] [PubMed]
- Baerson, S.R.; Sanchez-Moreiras, A.; Pedrol-Bonjoch, N.; Schulz, M.; Kagan, I.A.; Agarwal, A.K.; Reigosa, M.J.; Duke, S.O. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J. Biol. Chem. 2005, 280, 21867–21881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Hu, Y.F.; Na, X.F.; Li, J.L.; Yang, L.J.; You, J.; Liang, X.L.; Wang, J.F.; Peng, L.; Bi, Y.R. Narciclasine, a potential allelochemical, affects subcellular trafficking of auxin transporter proteins and actin cytoskeleton dynamics in Arabidopsis roots. Planta 2015, 242, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Bruno, L.; Sunseri, F.; Pacenza, M.; Forgione, I.; Bitonti, M.B.; Abenavoli, M.R. The allelochemical farnesene affects Arabidopsis thaliana root meristem altering auxin distribution. Plant Physiol. Biochem. 2017, 121, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gandia-Herrero, F.; Lorenz, A.; Larson, T.; Graham, I.A.; Bowles, D.J.; Rylott, E.L.; Bruce, N.C. Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: Discovery of bifunctional O- and C-glucosyltransferases. Plant J. 2008, 56, 963–974. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Sulmon, C.; Serra, A.A.; Gouesbet, G.; Couee, I. Xenobiotic sensing and signalling in higher plants. J. Exp. Bot. 2012, 63, 3999–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, D.P.; Larsen, L.; Dayan, F.E.; Gordon, K.C.; Perry, N.B.; van Klink, J.W. Nortriketones: Antimicrobial trimethylated acylphloroglucinols from manuka (Leptospermum scoparium). J. Nat. Prod. 2016, 79, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Beaudegnies, R.; Edmunds, A.J.; Fraser, T.E.; Hall, R.G.; Hawkes, T.R.; Mitchell, G.; Schaetzer, J.; Wendeborn, S.; Wibley, J. Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors—A review of the triketone chemistry story from a Syngenta perspective. Bioorg. Med. Chem. 2009, 17, 4134–4152. [Google Scholar] [CrossRef] [PubMed]
- Skipsey, M.; Knight, K.M.; Brazier-Hicks, M.; Dixon, D.P.; Steel, P.G.; Edwards, R. Xenobiotic responsiveness of Arabidopsis thaliana to a chemical series derived from a herbicide safener. J. Biol. Chem. 2011, 286, 32268–32276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, S.O.; Pan, Z.Q.; Bajsa-Hirschel, J. Proving the mode of action of phytotoxic phytochemicals. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activity of flavokawains and related trans-chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovici, J.; Bertrand, C.; Jacquemoud, D.; Bellvert, F.; Fernandez, M.P.; Comte, G.; Piola, F. An allelochemical from Myrica gale with strong phytotoxic activity against highly invasive Fallopia x bohemica taxa. Molecules 2011, 16, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Grana, E.; Diaz-Tielas, C.; Sanchez-Moreiras, A.M.; Reigosa, M.J.; Celeiro, M.; Abagyan, R.; Teijeira, M.; Duke, M.V.; Clerk, T.; Pan, Z.Q.; et al. Transcriptome and binding data indicate that citral inhibits single strand DNA-binding proteins. Physiol. Plant. 2020, 169, 99–109. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Grana, E.; Sanchez-Moreiras, A.M.; Reigosa, M.J.; Vaughn, J.N.; Pan, Z.Q.; Bajsa-Hirschel, J.; Duke, M.V.; Duke, S.O. Transcriptome responses to the natural phytotoxin t-chalcone in Arabidopsis thaliana L. Pest Manag. Sci. 2019, 75, 2490–2504. [Google Scholar] [CrossRef]
- Cao, X.N.; Ma, F.; Xu, T.T.; Wang, J.J.; Liu, S.C.; Li, G.H.; Su, Q.; Qiao, Z.J.; Na, X. Transcriptomic analysis reveals key early events of narciclasine signaling in Arabidopsis root apex. Plant Cell Rep. 2016, 35, 2381–2401. [Google Scholar] [CrossRef]
- Vanholme, B.; El Houari, I.; Boerjan, W. Bioactivity: Phenylpropanoids’ best kept secret. Curr. Opin. Biotechnol. 2019, 56, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lohman, D.J.; McConnaughay, K.D. Patterns of defensive chemical production in wild parsnip seedlings (Apiaceae: Pastinaca sativa L.). Chemoecology 1998, 8, 195–200. [Google Scholar] [CrossRef]
- Nebo, L.; Varela, R.M.; Molinillo, J.M.G.; Sampaio, O.M.; Severino, V.G.P.; Cazal, C.M.; da Silva, M.F.G.F.; Fernandes, J.B.; Macias, F.A. Phytotoxicity of alkaloids, coumarins and flavonoids isolated from 11 species belonging to the Rutaceae and Meliaceae families. Phytochem. Lett. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Macias, F.A.; Molinillo, J.M.G.; Torres, A.; Varela, R.M.; Castellano, D. Bioactive flavonoids from Helianthus annuus cultivars. Phytochemistry 1997, 45, 683–687. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Sotelo, T.; Grana, E.; Reigosa, M.J.; Sanchez-Moreiras, A.M. Phytotoxic potential of trans-chalcone on crop plants and model species. J. Plant Growth Regul. 2014, 33, 181–194. [Google Scholar] [CrossRef]
- Li, P.; Ding, L.; Zhang, L.; He, J.; Huan, Z.W. Weisiensin B inhibits primary and lateral root development by interfering with polar auxin transport in Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 139, 738–745. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mancini, E.; de Almeida, L.F.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Gonzalez, D.; Costas-Gil, A.; Reigosa, M.J.; Araniti, F.; Sanchez-Moreiras, A.M. A natural indole alkaloid, norharmane, affects PIN expression patterns and compromises root growth in Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 151, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Yang, L.J.; Na, X.F.; You, J.; Hu, W.; Liang, X.L.; Liu, J.; Mao, L.N.; Wang, X.M.; Wang, H.H.; et al. Narciclasine inhibits the responses of Arabidopsis roots to auxin. Planta 2012, 236, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Na, X.F.; Hu, Y.F.; Yue, K.; Lu, H.X.; Jia, P.F.; Wang, H.H.; Wang, X.M.; Bi, Y.R. Concentration-dependent effects of narciclasine on cell cycle progression in Arabidopsis root tips. BMC Plant Biol. 2011, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassel, G.W.; Fung, P.; Chow, T.-F.F.; Foong, J.A.; Provart, N.J.; Cutler, S.R. Elucidating the germination transcriptional program using small molecules. Plant Physiol. 2008, 147, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, C.L.; Zhang, W.; Sheng, C.Q.; Zhang, W.N.; Xing, C.G.; Miao, Z.Y. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Ndikuryayo, F.; Moosavi, B.; Yang, W.C.; Yang, G.F. 4-Hydroxyphenylpyruvate dioxygenase inhibitors: From chemical biology to agrochemicals. J. Agric. Food Chem. 2017, 65, 8523–8537. [Google Scholar] [CrossRef]
- Mathiesen, L.; Malterud, K.E.; Sund, R.B. Uncoupling of respiration and inhibition of ATP synthesis in mitochondria by C-methylated flavonoids from Myrica gale L. Eur. J. Pharm. Sci. 1996, 4, 373–379. [Google Scholar] [CrossRef]
- Mathiesen, L.; Malterud, K.E.; Sund, R.B. Hydrogen bond formation as basis for radical scavenging activity: A structure-activity study of C-methylated dihydrochalcones from Myrica gale and structurally related acetophenones. Free Radic. Biol. Med. 1997, 22, 307–311. [Google Scholar] [CrossRef]
- Malterud, K.E.; Diep, O.H.; Sund, R.B. C-methylated dihydrochalcones from Myrica gale L: Effects as antioxidants and as scavengers of 1,1-diphenyl-2-picrylhydrazyl. Pharmacol. Toxicol. 1996, 78, 111–116. [Google Scholar] [CrossRef]
- Oracz, K.; Voegele, A.; Tarkowska, D.; Jacquemoud, D.; Tureckova, V.; Urbanova, T.; Strnad, M.; Sliwinska, E.; Leubner-Metzger, G. Myrigalone A inhibits Lepidium sativum seed germination by interference with gibberellin metabolism and apoplastic superoxide production required for embryo extension growth and endosperm rupture. Plant Cell Physiol. 2012, 53, 81–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voegele, A.; Graeber, K.; Oracz, K.; Tarkowska, D.; Jacquemoud, D.; Tureckova, V.; Urbanova, T.; Strnad, M.; Leubner-Metzger, G. Embryo growth, testa permeability, and endosperm weakening are major targets for the environmentally regulated inhibition of Lepidium sativum seed germination by myrigalone A. J. Exp. Bot. 2012, 63, 5337–5350. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Scheler, C.; Weitbrecht, K.; Pearce, S.P.; Hampstead, A.; Buettner-Mainik, A.; Lee, K.; Voegele, A.; Oracz, K.; Dekkers, B.; Wang, X.; et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015, 167, 200–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; de Jong, H.; et al. DELAY OF GERMINATION 1 mediates a conserved coat dormancy mechanism for the temperature- and gibberellin-dependent control of seed germination. Proc. Natl. Acad. Sci. USA 2014, 111, E3571–E3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beynon, E.R.; Symons, Z.C.; Jackson, R.G.; Lorenz, A.; Rylott, E.L.; Bruce, N.C. The role of oxophytodienoate reductases in the detoxification of the explosive 2,4,6-trinitrotoluene by Arabidopsis. Plant Physiol. 2009, 151, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, D.P.; Edwards, R. Selective binding of glutathione conjugates of fatty acid derivatives by plant glutathione transferases. J. Biol. Chem. 2009, 284, 21249–21256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walden, N.; German, D.A.; Wolf, E.M.; Kiefer, M.; Rigault, P.; Huang, X.C.; Kiefer, C.; Schmickl, R.; Franzke, A.; Neuffer, B.; et al. Nested whole-genome duplications coincide with diversification and high morphological disparity in Brassicaceae. Nat. Commun. 2020, 11, 3795. [Google Scholar] [CrossRef]
- Mueller, S.; Hilbert, B.; Dueckershoff, K.; Roitsch, T.; Krischke, M.; Mueller, M.J.; Berger, S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 2008, 20, 768–785. [Google Scholar] [CrossRef] [Green Version]
- Wulff, N.; Ernst, H.A.; Jorgensen, M.E.; Lambertz, S.; Maierhofer, T.; Belew, Z.M.; Crocoll, C.; Motawia, M.S.; Geiger, D.; Jorgensen, F.S.; et al. An optimized screen reduces the number of GA transporters and provides insights into nitrate transporter 1/peptide transporter family sSubstrate determinants. Front. Plant Sci. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gräfe, K.; Schmitt, L. The ABC transporter G subfamily in Arabidopsis thaliana. J. Exp. Bot. 2021, 72, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Eom, S.; Shin, K.; Lee, R.A.; Choi, S.; Lee, J.H.; Lee, S.; Soh, M.S. Identification of lysine histidine transporter 2 as an 1-aminocyclopropane carboxylic acid transporter in Arabidopsis thaliana by transgenic complementation approach. Front. Plant Sci. 2019, 10, 1092. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Wu, F.; Lin, W.-C.; Luo, J. The utilization of photophosphorylation uncoupler to improve lipid production of Chlorella, a case study using transcriptome and functional gene expression analysis to reveal its mechanism. Biochem. Eng. J. 2022, 178, 108275. [Google Scholar] [CrossRef]
- Jayasinghe, U.L.; Ratnayake, R.M.; Medawala, M.M.; Fujimoto, Y. Dihydrochalcones with radical scavenging properties from the leaves of Syzygium jambos. Nat. Prod. Res. 2007, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering hydrogen peroxide-induced signalling towards stress tolerance in plants. 3 Biotech 2019, 9, 395. [Google Scholar] [CrossRef]
- Podgorska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.R.; Li, Z.H.; Huang, P.X.; Li, B.S.; Fang, S.; Chu, J.F.; Guo, H.W. A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell 2017, 29, 2854–2870. [Google Scholar] [CrossRef]
- Zhang, L.P.; Chen, L.G.; Yu, D.Q. Transcription factor WRKY75 interacts with DELLA proteins to affect flowering. Plant Physiol. 2018, 176, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, L.; Ji, Y.; Jing, Y.; Li, L.; Chen, Y.; Wang, R.; Zhang, H.; Yu, D.; Chen, L. Arabidopsis SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2 inhibit WRKY75 function in abscisic acid-mediated leaf senescence and seed germination. J. Exp. Bot. 2022, 73, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.A.; Wilson, I.W.; Llewellyn, D.J.; Dennis, E.S. The low-oxygen induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis thaliana seeds following low-oxygen treatment. Plant Physiol. 2009, 149, 1724–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, S.; Ksas, B.; Havaux, M. Decoding beta-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell 2018, 30, 2495–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, T.; Hajny, J.; Grunewald, W.; Vasileva, M.; Molnar, G.; Tejos, R.; Schmid, M.; Sauer, M.; Friml, J. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLoS Genet. 2018, 14, e1007177. [Google Scholar] [CrossRef]
- Grunewald, W.; De Smet, I.; Lewis, D.R.; Lofke, C.; Jansen, L.; Goeminne, G.; Bossche, R.V.; Karimi, M.; De Rybel, B.; Vanholme, B.; et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef] [Green Version]
- Tzafestas, K.; Ahmad, L.; Dani, M.P.; Grogan, G.; Rylott, E.L.; Bruce, N.C. Structure-guided mechanisms behind the metabolism of 2,4,6-trinitrotoluene by glutathione transferases U25 and U24 that lead to alternate product distribution. Front. Plant Sci. 2018, 9, 1846. [Google Scholar] [CrossRef] [Green Version]
- Jemmat, A.M.; Ranocha, P.; Le Ru, A.; Neel, M.; Jauneau, A.; Raggi, S.; Ferrari, S.; Burlat, V.; Dunand, C. Coordination of five class III peroxidase-encoding genes for early germination events of Arabidopsis thaliana. Plant Sci. 2020, 298, 110565. [Google Scholar] [CrossRef]
- Linkies, A.; Schuster-Sherpa, U.; Tintelnot, S.; Leubner-Metzger, G.; Müller, K. Peroxidases identified in a substractive cDNA library approach show tissue-specific transcript abundance and enzyme activity during seed germination of Lepidium sativum. J. Exp. Bot. 2010, 61, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Jiang, A.L.; Guo, Z.L.; Pan, J.W.; Yang, Y.Z.; Zhuang, Y.; Zuo, D.Q.; Hao, C.; Gao, Z.X.; Xin, P.Y.; Chu, J.F.; et al. The PIF1-miR408-PLANTACYANIN repression cascade regulates light-dependent seed germination. Plant Cell 2021, 33, 1506–1529. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Jin, S.H.; Jiang, X.Y.; Dong, R.R.; Li, P.; Li, Y.J.; Hou, B.K. Ectopic expression of UGT75D1, a glycosyltransferase preferring indole-3-butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana. Plant Mol. Biol. 2016, 90, 77–93. [Google Scholar] [CrossRef]
- Simon, C.; Langlois-Meurinne, M.; Didierlaurent, L.; Chaouch, S.; Bellvert, F.; Massoud, K.; Garmier, M.; Thareau, V.; Comte, G.; Noctor, G.; et al. The secondary metabolism glycosyltransferases UGT73B3 and UGT73B5 are components of redox status in resistance of Arabidopsis to Pseudomonas syringae pv. tomato. Plant Cell Environ. 2014, 37, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; de Cotte, B.V.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [Green Version]
- Gardin, J.A.C.; Gouzy, J.; Carrere, S.; Delye, C. ALOMYbase, a resource to investigate non-target-site-based resistance to herbicides inhibiting acetolactate-synthase (ALS) in the major grass weed Alopecurus myosuroides (black-grass). BMC Genom. 2015, 16, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, R.; Boachon, B.; Renault, H.; Gavira, C.; Miesch, L.; Iglesias, J.; Ginglinger, J.F.; Allouche, L.; Miesch, M.; Grec, S.; et al. Dual function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol. 2014, 166, 1149. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.Q.; Zhao, S.; Wang, L.; Dong, W.; Liu, L.; He, Y.K. Structure of the Arabidopsis thaliana NADPH-cytochrome P450 reductase 2 (ATR2) provides insight into its function. FEBS J. 2017, 284, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, N.; Kar, D.; Mahajan, B.D.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, H.; Moriyama, S.; Kusakizako, T.; Kumazaki, K.; Nakane, T.; Yamashita, K.; Hirata, K.; Dohmae, N.; Nishizawa, T.; Ito, K.; et al. Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef]
- Martinoia, E. Vacuolar transporters—Companions on a longtime journey. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef]
- Thompson, E.P.; Wilkins, C.; Demidchik, V.; Davies, J.M.; Glover, B.J. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J. Exp. Bot. 2010, 61, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Kovinich, N.; Wang, Y.Q.; Adegboye, J.; Chanoca, A.A.; Otegui, M.S.; Durkin, P.; Grotewold, E. Arabidopsis MATE45 antagonizes local abscisic acid signaling to mediate development and abiotic stress responses. Plant Direct 2018, 2, e00087. [Google Scholar] [CrossRef] [Green Version]
- Do, T.H.T.; Martinoia, E.; Lee, Y.; Hwang, J.U. 2021 update on ATP-binding cassette (ABC) transporters: How they meet the needs of plants. Plant Physiol. 2021, 187, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.W.; Novak, O.R.; Wei, Z.Y.; Gou, M.Y.; Zhang, X.B.; Yu, Y.; Yang, H.J.; Cai, Y.H.; Strnad, M.; Liu, C.J. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 2014, 5, 3274. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Aryal, B.; di Donato, M.; Hao, P.C. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.C.; Xia, J.; Liu, J.; Di Donato, M.; Pakula, K.; Bailly, A.; Jasinski, M.; Geisler, M. Auxin-transporting ABC transporters are defined by a conserved D/E-P motif regulated by a prolylisomerase. J. Biol. Chem. 2020, 295, 13094–13105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pratelli, R.; Yu, S.; Shelley, B.; Collakova, E.; Pilot, G. Detailed characterization of the UMAMIT proteins provides insight into their evolution, amino acid transport properties, and role in the plant. J. Exp. Bot. 2021, 72, 6400–6417. [Google Scholar] [CrossRef]
- Yao, X.H.; Nie, J.; Bai, R.X.; Sui, X.L. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Besnard, J.; Zhao, C.S.; Avice, J.C.; Vitha, S.; Hyodo, A.; Pilot, G.; Okumoto, S. Arabidopsis UMAMIT24 and 25 are amino acid exporters involved in seed loading. J. Exp. Bot. 2018, 69, 5221–5232. [Google Scholar] [CrossRef]
- Tan, P.P.; Du, X.H.; Shang, Y.J.; Zhu, K.K.; Joshi, S.; Kaur, K.; Khare, T.; Kumar, V. Ion transporters and their exploration for conferring abiotic stress tolerance in plants. Plant Growth Regul. 2022, 96, 1–23. [Google Scholar] [CrossRef]
- Zhang, M.L.; Huang, P.P.; Ji, Y.; Wang, S.W.; Wang, S.S.; Li, Z.; Guo, Y.; Ding, Z.J.; Wu, W.H.; Wang, Y. KUP9 maintains root meristem activity by regulating K+ and auxin homeostasis in response to low K. Embo Rep. 2020, 21, e50164. [Google Scholar] [CrossRef]
- Hoai, P.T.T.; Tyerman, S.D.; Schnell, N.; Tucker, M.; McGaughey, S.A.; Qiu, J.E.; Groszmann, M.; Byrt, C.S. Deciphering aquaporin regulation and roles in seed biology. J. Exp. Bot. 2020, 71, 1763–1773. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Thakral, V.; Padalkar, G.; Rajora, N.; Dhiman, P.; Raturi, G.; Sharma, Y.; Tripathi, D.K.; Deshmukh, R.; Sharma, T.R.; et al. Significance of solute specificity, expression, and gating mechanism of tonoplast intrinsic protein during development and stress response in plants. Physiol. Plant. 2021, 172, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Footitt, S.; Clewes, R.; Feeney, M.; Finch-Savage, W.E.; Frigerio, L. Aquaporins influence seed dormancy and germination in response to stress. Plant Cell Environ. 2019, 42, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Perez, M.; Steinbrecher, T.; Gawthrop, F.; Pavlovic, I.; Novak, O.; Tarkowska, D.; Strnad, M.; Marone, F.; Nakabayashi, K.; et al. Molecular mechanisms and hormonal regulation underpinning morphological dormancy: A case study using Apium graveolens (Apiaceae). Plant J. 2021, 108, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Belin, C.; Megies, C.; Hauserova, E.; Lopez-Molina, L. Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling. Plant Cell 2009, 21, 2253–2268. [Google Scholar] [CrossRef] [Green Version]
- Marhava, P. Recent developments in the understanding of PIN polarity. New Phytol. 2022, 233, 624–630. [Google Scholar] [CrossRef]
- Feraru, E.; Feraru, M.I.; Barbez, E.; Waidmann, S.; Sun, L.; Gaidora, A.; Kleine-Vehn, J. PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2019, 116, 3893–3898. [Google Scholar] [CrossRef] [Green Version]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratge-Faillie, C.; Novak, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef]
- Teale, W.; Palme, K. Naphthylphthalamic acid and the mechanism of polar auxin transport. J. Exp. Bot. 2018, 69, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Neve, J.; Hirose, M.; Kuboki, A.; Shimada, Y.; Kepinski, S.; Nozaki, H. Rational design of an auxin antagonist of the SCF(TIR1) auxin receptor complex. ACS Chem. Biol. 2012, 7, 590–598. [Google Scholar] [CrossRef]
- Matilla, A.J. Auxin: Hormonal signal required for seed development and dormancy. Plants 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.T.; Kang, X.K.; Lei, W.; Yao, X.H.; Zou, L.J.; Zhang, D.W.; Lin, H.H. SHY2 as a node in the regulation of root meristem development by auxin, brassinosteroids, and cytokinin. J. Integr. Plant Biol. 2020, 62, 1500–1517. [Google Scholar] [CrossRef]
- Kamimoto, Y.; Terasaka, K.; Hamamoto, M.; Takanashi, K.; Fukuda, S.; Shitan, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Wang, B.J.; et al. Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant Cell Physiol. 2012, 53, 2090–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.; Lee, Z.W.; Cho, H.T. ATP-binding cassette B4, an auxin-efflux transporter, stably associates with the plasma membrane and shows distinctive intracellular trafficking from that of PIN-FORMED proteins. Plant Physiol. 2012, 159, 642. [Google Scholar] [CrossRef] [Green Version]
- Kubes, M.; Yang, H.B.; Richter, G.L.; Cheng, Y.; Mlodzinska, E.; Wang, X.; Blakeslee, J.J.; Carraro, N.; Petrasek, J.; Zazimalova, E.; et al. The Arabidopsis concentration-dependent influx/efflux transporter ABCB4 regulates cellular auxin levels in the root epidermis. Plant J. 2012, 69, 640–654. [Google Scholar] [CrossRef]
- Aryal, B.; Huynh, J.; Schneuwly, J.; Siffert, A.; Liu, J.; Alejandro, S.; Ludwig-Muller, J.; Martinoia, E.; Geisler, M. ABCG36/PEN3/PDR8 is an exporter of the auxin precursor, indole-3-butyric acid, and involved in auxin-controlled development. Front. Plant Sci. 2019, 10, 889. [Google Scholar] [CrossRef]
- Ruzicka, K.; Strader, L.C.; Bailly, A.; Yang, H.B.; Blakeslee, J.; Langowski, L.; Nejedla, E.; Fujita, H.; Itoh, H.; Syono, K.; et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl. Acad. Sci. USA 2010, 107, 10749–10753. [Google Scholar] [CrossRef] [Green Version]
- Grana, E.; Diaz-Tielas, C.; Lopez-Gonzalez, D.; Martinez-Penalver, A.; Reigosa, M.J.; Sanchez-Moreiras, A.M. The plant secondary metabolite citral alters water status and prevents seed formation in Arabidopsis thaliana. Plant Biol. 2016, 18, 423–432. [Google Scholar] [CrossRef]
- Taguchi, G.; Ubukata, T.; Nozue, H.; Kobayashi, Y.; Takahi, M.; Yamamoto, H.; Hayashida, N. Malonylation is a key reaction in the metabolism of xenobiotic phenolic glucosides in Arabidopsis and tobacco. Plant J. 2010, 63, 1031–1041. [Google Scholar] [CrossRef]
- Park, S.W.; Li, W.; Viehhauser, A.; He, B.; Kim, S.; Nilsson, A.K.; Andersson, M.X.; Kittle, J.D.; Ambavaram, M.M.R.; Luan, S.; et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 9559–9564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.K.; Chauhan, N.; Rajakumari, S.; Daum, G.; Rajasekharan, R. At4g24160, a soluble acyl-coenzyme A-dependent lysophosphatidic acid acyltransferase. Plant Physiol. 2009, 151, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mene-Saffrane, L.; Lopez-Molina, L. An endosperm-associated cuticle is required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015, 11, e1005708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.; Kim, J.Y.; Choi, H.; Kim, H.; Lee, I.; Soh, M.S.; Nam, H.G.; Chang, Y.T.; Lim, P.O.; Woo, H.R. Rootin, a compound that inhibits root development through modulating PIN-mediated auxin distribution. Plant Sci. 2015, 233, 116–126. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.Y.; Hu, L.Y.; Cao, W.; Sun, K.; Sun, Q.B.; Siddikee, A.; Shi, R.H.; Dai, C.C. Evidence for the involvement of auxin, ethylene and ROS signaling during primary root inhibition of Arabidopsis by the allelochemical benzoic acid. Plant Cell Physiol. 2018, 59, 1889–1904. [Google Scholar] [CrossRef]
- Urbanova, T.; Tarkowska, D.; Novak, O.; Hedden, P.; Strnad, M. Analysis of gibberellins as free acids by ultra performance liquid chromatography-tandem mass spectrometry. Talanta 2013, 112, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Flokova, K.; Tarkowska, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novak, O. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8’-hydroxylases: Key enzymes in ABA catabolism. Eur. Mol. Biol. Organ. J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef] [Green Version]
- Graeber, K.; Linkies, A.; Wood, A.T.; Leubner-Metzger, G. A guideline to family-wide comparative state-of-the-art quantitative RT-PCR analysis exemplified with a Brassicaceae cross-species seed germination case study. Plant Cell 2011, 23, 2045–2063. [Google Scholar] [CrossRef] [Green Version]
- Holloway, T.; Steinbrecher, T.; Perez, M.; Seville, A.; Stock, D.; Nakabayashi, K.; Leubner-Metzger, G. Coleorhiza-enforced seed dormancy: A novel mechanism to control germination in grasses. New Phytol. 2021, 229, 2179–2191. [Google Scholar] [CrossRef]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.M.; Birol, I. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009, 19, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Cherveux, B.; Wetter, T.; Suhai, S. Genome sequence assembly using trace signals and additional sequence information. Comput. Sci. Biol. Proc. Ger. Conf. Bioinf. 1999, 99, 45–56. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Phyton framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [Green Version]

| Comparison | Up-Regulated Contigs in MyA Treatment | Down-Regulated Contigs in MyA Treatment |
|---|---|---|
| 6 h MyA/6 h control | 11 a (12) b | 3 (4) |
| 12 h MyA/12 h control | 180 (4723) | 24 (27) |
| 18 h MyA/12 h control | 889 (1341) | 419 (491) |
| Gene Name | Control 12 h/Con 12 h | MyA b 12 h/Con 12 h | MyA b 18 h/Con 12 h | MyB b 12 h/Con 12 h | MyD b 12 h/Con 12 h | Angelicin c 12 h/Con 12 h | Angelicin c 18 h/Con 12 h |
|---|---|---|---|---|---|---|---|
| LesaGSTU25 | 1.0 ± 0.1 | 23.1 ± 12.7 | 49.0 ± 15.4 | 0.3 ± 0.2 | 2.5 ± 2.1 | 0.5 ± 0.3 | 0.2 ± 0.1 |
| LesaNAC102 | 1.0 ± 0.1 | 14.6 ± 4.9 | 4.1 ± 0.7 | 0.3 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.1 |
| LesaUGT73B5 | 1.0 ± 0.2 | 12.4 ± 3.9 | 8.7 ± 0.8 | 1.0 ± 0.2 | 1.2 ± 0.4 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| LesaERF2 | 1.0 ± 0.2 | 6.6 ± 2.8 | 0.5 ± 0.1 | 0.3 ± 0.2 | 2.0 ± 0.9 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| LesaABCG37 | 1.0 ± 0.2 | 5.2 ± 0.3 | 3.1 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.2 | 0.5 ± 0.2 | 1.2 ± 0.4 |
| LesaOXI1 | 1.0 ± 0.4 | 4.8 ± 2.3 | 0.8 ± 0.1 | 0.7 ± 0.5 | 0.8 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| LesaCYP81D8 | 1.0 ± 0.3 | 4.4 ± 2.2 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.6 ± 0.3 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| LesaOPR1/2 | 1.0 ± 0.1 | 4.3 ± 1.1 | 4.4 ± 0.1 | 1.6 ± 0.5 | 1.4 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| LesaWRKY75 | 1.0 ± 0.1 | 3.6 ± 1.1 | 2.9 ± 0.3 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.2 |
| LesaPER13 | 1.0 ± 0.2 | 3.4 ± 0.5 | 1.1 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.2 |
| LesaWRKY23 | 1.0 ± 0.1 | 2.9 ± 1.5 | 0.9 ± 0.1 | 1.0 ± 0.5 | 1.7 ± 0.9 | 0.6 ± 0.0 | 0.6 ± 0.1 |
| LesaNAC005 | 1.0 ± 0.5 | 2.8 ± 1.5 | 6.5 ± 1.8 | 1.8 ± 0.6 | 1.7 ± 0.3 | 1.0 ± 0.3 | 3.6 ± 0.7 |
| LesaUGT74E2 | 1.0 ± 0.3 | 2.7 ± 0.7 | 0.7 ± 0.1 | 0.4 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| LesaABCB4 | 1.0 ± 0.2 | 2.3 ± 1.2 | 1.5 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.3 |
| LesaAOX1A | 1.0 ± 0.1 | 2.2 ± 1.3 | 0.5 ± 0.1 | 0.2 ± 0.0 | 0.6 ± 0.4 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| LesaCYP81D4 | 1.0 ± 0.3 | 2.2 ± 0.2 | 0.4 ± 0.1 | 1.0 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.2 | 0.2 ± 0.1 |
| LesaUGT75B1 | 1.0 ± 0.3 | 2.1 ± 1 | 0.5 ± 0.1 | 1.5 ± 1.0 | 0.9 ± 0.2 | 0.4 ± 0.0 | 0.4 ± 0.2 |
| LesaUGT75D1 | 1.0 ± 0.0 | 1.9 ± 0.2 | 1.6 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.1 | 1.1 ± 0.5 |
| Monooxygenase (AT5G64250) a | 1.0 ± 0.1 | 1.7 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.4 ± 0.1 |
| LesaABCG34 | 1.0 ± 0.2 | 1.6 ± 0.4 | 1.5 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.1 | 1.5 ± 0.8 |
| LesaGSTU19 | 1.0 ± 0.1 | 1.5 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.1 | 0.3 ± 0.1 |
| Oxidoreductase (AT3G44190) a | 1.0 ± 0.1 | 1.5 ± 0.2 | 4.3 ± 0.7 | 1.1 ± 0.0 | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.7 ± 0.1 |
| LesaFSD1 | 1.0 ± 0.0 | 1.5 ± 0.1 | 5.5 ± 1.6 | 0.6 ± 0.1 | 0.7 ± 0.3 | 0.5 ± 0.1 | 4.1 ± 1.0 |
| LesaPER70 | 1.0 ± 0.4 | 0.6 ± 0.2 | 0.4 ± 0.1 | 1.1 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.7 ± 0.2 |
| LesaTAT2 | 1.0 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.9 ± 0.3 | 0.7 ± 0.1 | 0.5 ± 0.2 |
| LesaPER45 | 1.0 ± 0.5 | 0.6 ± 0.3 | 1.6 ± 0.6 | 0.3 ± 0.2 | 0.9 ± 0.3 | 0.3 ± 0.1 | 3.2 ± 1.0 |
| LesaARF11 | 1.0 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.2 | 1.4 ± 0.5 | 1.2 ± 0.2 | 0.9 ± 0.2 | 1.1 ± 0.4 |
| LesaCYP78A7 | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.3 ± 0.1 |
| LesaUMAMIT25 | 1.0 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.2 |
| LesaPIN7 | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.3 |
| LesaLHT1 | 1.0 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.2 | 0.3 ± 0.1 | 1.1 ± 0.2 |
| LesaSKS15 | 1.0 ± 0.0 | 0.3 ± 0.2 | 2.2 ± 0.7 | 0.6 ± 0.1 | 0.9 ± 0.3 | 0.6 ± 0.2 | 3.5 ± 0.4 |
| Ratio MyA/Con | Ratio Compound/Control: ≥2 (UP), ≤2 (DN; Down) or Not 2-Fold Regulated (“-”) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myrigalone A | MyA | tCHC | tCHC | NCS | Citral | Citral | BOA | PAA1 | FEN | CMPP | MTX | DNP | |||
| AGI | Gene Name | 12/12 h | 18/12 h | Seeds | Roots | Shoots | Roots | Roots | Shoots | Seedlings | Seedlings | Root | Root | Seeds | Seeds |
| Cult. | Cult. | ||||||||||||||
| Selected hormone transporter genes (known hormone specificity) | |||||||||||||||
| AT1G15520 | ABCG40 (ABA) | 4.3 | 4.6 | UP | UP | UP | - | UP | UP | UP | UP | - | UP | - | - |
| AT1G71960 | ABCG25 (ABA) | 0.9 | 0.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| AT1G31770 | ABCG14 (CK) | 0.9 | 3.6 | UP | - | - | - | DN | - | - | - | - | - | - | DN |
| AT4G39850 | ABCD1/CTS (OPDA) | 0.9 | 0.9 | - | - | - | - | DN | - | - | - | - | - | - | - |
| AT3G55090 | ABCG16 (JA) | n.a. | n.a. | n.a. | UP | - | - | UP | - | - | UP | - | - | UP | - |
| AT2G26690 | NPF6.2 (GA) | 1.0 | 4.9 | UP | - | - | DN | - | DN | - | - | - | - | DN | DN |
| AT1G12110 | NPF6.3 (GA) | 1.0 | 3.2 | UP | DN | - | - | DN | - | - | - | - | DN | - | - |
| AT5G40780 | LHT1 (ACC) | 0.5 | 0.8 | DN | DN | - | - | DN | - | - | - | - | - | DN | DN |
| Auxin transporter: IAA influx (AUX1) and efflux (PIN, PILS) carrier | |||||||||||||||
| AT2G38120 | AUX1 | 0.8 | 0.8 | - | - | - | UP * | - | - | - | DN | - | DN | - | - |
| AT1G73590 | PIN1 | 0.8 | 0.7 | - | - | - | DN * | DN | - | - | DN | - | - | - | - |
| AT5g57090 | PIN2 | 0.8 | 0.7 | - | - | - | UP * | DN | - | - | - | - | - | - | - |
| AT1G70940 | PIN3 | 0.8 | 1.3 | - | - | - | DN * | - | DN | - | - | DN | - | - | - |
| AT2G01420 | PIN4 | 0.9 | 1.2 | - | - | - | DN | - | DN | - | - | - | - | - | DN |
| AT5G16530 | PIN5 | n.a. | n.a. | n.a. | - | - | - | - | - | - | - | - | - | - | - |
| AT1G77110 | PIN6 | 0.9 | 0.8 | - | - | - | - | - | DN | - | - | - | - | DN | - |
| AT1G23080 | PIN7 | 0.5 | 0.6 | DN | - | - | DN | - | DN | - | DN | DN | DN | - | - |
| AT5G15100 | PIN8 | n.a. | n.a. | n.a. | - | - | - | - | - | - | - | - | - | - | DN |
| AT1G20925 | PILS1 | n.a. | n.a. | n.a. | - | - | - | - | - | - | - | - | - | - | - |
| AT1G71090 | PILS2 | 0.9 | 1.1 | - | - | - | - | DN | - | - | - | - | - | - | - |
| AT1G76520 | PILS3 | 1.3 | 1.3 | - | UP | - | UP | - | - | UP | - | UP | UP | - | - |
| AT1G76530 | PILS4 | n.a. | n.a. | n.a. | - | UP | - | - | - | - | - | - | - | UP | UP |
| AT2G17500 | PILS5 | 1.2 | 1.4 | - | UP | UP | - | UP | - | - | - | UP | - | - | - |
| AT5G01990 | PILS6 | 0.9 | 0.8 | - | - | - | - | DN | - | - | - | - | - | - | - |
| AT5G65980 | PILS7 | 0.9 | 1.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| Auxin transporter: ABC transporter and UMAMIT15 (WAT1) auxin carrier (known auxin specificity) | |||||||||||||||
| AT1G75500 | WAT1 (IAA) | 0.9 | 4.4 | UP | - | - | DN | - | - | - | DN | - | DN | - | - |
| AT2G36910 | ABCB1 (IAA) | 0.7 | 0.9 | - | - | - | - | - | - | - | - | - | - | - | - |
| AT2G47000 | ABCB4 (IAA) | 3.8 | 4.8 | UP | - | - | UP | - | - | - | UP | UP | UP | - | - |
| AT1G02520 | ABCB11 | 2.1 | 1.9 | UP | - | UP | UP | DN | - | - | - | - | - | - | - |
| AT1G28010 | ABCB14 (cytokinin) | 1.7 | 7.1 | UP | - | - | - | - | DN | - | - | - | - | DN | DN |
| AT3G28345 | ABCB15 | 1.1 | 0.8 | - | - | - | - | DN | - | - | - | - | - | UP | - |
| AT3G28860 | ABCB19 (IAA) | 0.8 | 0.8 | - | - | - | DN | - | DN | - | - | - | - | - | - |
| AT3G62150 | ABCB21 (IAA) | 1.0 | 0.9 | - | - | - | UP | - | DN | - | - | - | - | - | - |
| AT3G53480 | ABCG37 (IBA) | 4.6 | 5.1 | UP | - | UP | - | - | - | UP | - | - | - | - | - |
| AT1G59870 | ABCG36 (IBA) | 1.1 | 0.9 | - | - | - | UP | DN | - | - | - | - | - | - | - |
| AT2G39350 | ABCG1 | 1.2 | 1.8 | - | - | - | UP | - | - | - | - | UP | UP | - | - |
| Selected auxin signalling genes | |||||||||||||||
| AT3G62980 | TIR1 | 0.9 | 0.8 | - | - | - | - | - | DN | - | - | - | - | - | - |
| AT1G04240 | IAA3/SHY2 | 0.8 | 4.1 | UP | - | - | - | - | DN | - | DN | DN | DN | UP | - |
| AT1G04250 | IAA17/AXR3 | 1.0 | 3.4 | UP | - | DN | DN | DN | - | - | - | - | - | DN | - |
| AT2G22670 | IAA8 | 0.9 | 1.2 | - | - | - | DN | - | DN | - | - | - | - | - | - |
| AT5G57420 | IAA33 | 1.2 | 0.4 | DN | - | - | - | DN | - | - | - | - | - | - | - |
| AT1G59750 | ARF1 | 0.8 | 0.5 | DN | - | - | - | - | DN | - | - | - | - | - | - |
| AT2G46530 | ARF11 | 0.3 | 0.4 | DN | - | - | - | - | DN | - | - | - | - | - | - |
| AT3G61830 | ARF18 | 0.8 | 0.5 | DN | - | - | - | - | - | - | - | - | - | - | - |
| AT5G13080 | WRKY75 | 4.5 | 5.7 | UP | - | - | - | UP | - | - | UP | UP | UP | DN | UP |
| AT3G56710 | SIB1 | 0.6 | 0.4 | DN | - | - | - | - | - | UP | - | UP | UP | - | - |
| AT2G41180 | SIB2 | 0.8 | 0.4 | DN | - | UP | - | - | DN | - | - | - | - | - | - |
| AT2G47260 | WRKY23 | 3.1 | 3.0 | UP | - | - | - | DN | - | - | - | - | - | - | - |
| AT1G62300 | WRKY6 | 2.1 | 2.4 | UP | - | - | UP | DN | - | UP | - | UP | UP | - | - |
| Ratio MyA/Con | Ratio Compound/Control: ≥2 (UP), ≤2 (DN; Down) or Not 2-Fold Regulated (“-”) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myrigalone A | MyA | tCHC | tCHC | NCS | Citral | Citral | BOA | PAA1 | FEN | CMPP | MTX | DNP | |||
| AGI | Gene Name | 12/12 h | 18/12 h | Seeds | Roots | Shoots | Roots | Roots | Shoots | Seedlings | Seedlings | Root | Root | Seeds | Seeds |
| Cult. | Cult. | ||||||||||||||
| GST, peroxidase, glutathione and ascorbate system genes (substrate) | |||||||||||||||
| AT1G17180 | GSTU25 | 7.5 | 35.3 | UP | UP | UP | UP | - | - | UP | UP | UP | - | - | - |
| AT1G78380 | GSTU19 (OPDA) | 2.5 | 2.0 | UP | UP | UP | - | - | UP | - | UP | UP | UP | - | UP |
| AT1G78340 | GSTU22 | 3.6 | 8.8 | UP | UP | UP | UP | DN | - | UP | UP | - | UP | - | - |
| AT2G29490 | GSTU1 | 5.2 | 7.1 | UP | UP | UP | UP | DN | UP | UP | - | UP | UP | - | UP |
| AT3G09270 | GSTU8 | 17.2 | 30.9 | UP | - | UP | - | DN | - | UP | - | UP | UP | DN | DN |
| AT2G31570 | GPX2 | 1.0 | 2.6 | UP | - | - | - | DN | - | - | - | - | - | - | - |
| AT1G75270 | DHAR2 | 4.9 | 8.0 | UP | UP | UP | - | - | UP | - | UP | UP | UP | - | UP |
| AT1G07890 | APX1 | 1.0 | 2.4 | UP | - | - | - | DN | - | - | - | - | - | - | DN |
| AT4G35970 | APX5 | 1.1 | 4.8 | UP | - | - | - | DN | - | - | - | - | - | DN | DN |
| AT1G77100 | PER13 | 2.6 | 1.7 | UP | - | - | - | - | - | - | - | - | - | - | - |
| AT4G30170 | PER45 | 0.7 | 5.0 | UP | DN | - | DN | DN | - | DN | - | - | DN | DN | DN |
| AT4G25100 | FSD1 | 1.0 | 6.9 | UP | - | - | - | DN | DN | - | - | - | - | - | - |
| AT3G22370 | AOX1A | 2.3 | 3.3 | UP | - | - | UP | - | - | - | - | UP | UP | UP | UP |
| CYP750 and UGT protein genes (substrate) | |||||||||||||||
| AT4G37330 | CYP81D4 | 2.2 | 0.9 | UP | - | - | - | - | - | - | - | - | - | - | - |
| AT4G37370 | CYP81D8 | 5.3 | 5.6 | UP | UP | - | UP | UP | UP | UP | - | UP | UP | UP | UP |
| AT3G26290 | CYP71B26 | 0.9 | 3.1 | UP | - | DN | - | - | - | - | - | - | - | - | - |
| AT3G20950 | CYP75A32 | 1.2 | 5.3 | UP | - | - | - | DN | - | - | - | - | - | UP | DN |
| AT2G45570 | CYP76C2 | 2.5 | 3.7 | UP | - | - | - | - | UP | - | - | - | - | DN | DN |
| AT4G15550 | UGT75D1 (IBA) | 1.6 | 2.7 | UP | - | UP | - | - | UP | - | UP | UP | - | - | UP |
| AT1G05680 | UGT74E2 (IBA) | 2.4 | 3.1 | UP | - | - | UP | UP | UP | UP | - | UP | UP | UP | UP |
| AT1G05560 | UGT75B1 (IBA) | 2.6 | 2.2 | UP | - | UP | UP | UP | UP | UP | UP | UP | - | - | UP |
| AT2G15480 | UGT73B5 | 10.3 | 13.1 | UP | - | UP | UP | UP | UP | UP | - | UP | UP | - | UP |
| AT4G34138 | UGT73B1 | 1.7 | 2.3 | UP | - | UP | - | - | UP | UP | - | UP | UP | DN | UP |
| MATE, ABC, UMAMIT and aquaporin transporter (* see Table 3 for hormone-specific ABC transporter) | |||||||||||||||
| AT1G71140 | DTX14 | 3.2 | 1.4 | UP | UP | - | UP | UP | - | - | UP | - | UP | - | UP |
| AT4G25640 | DTX35 | 1.3 | 2.1 | UP | - | UP | - | - | - | - | - | UP | UP | - | - |
| AT1G66760 | DTX9 | 1.1 | 2.9 | UP | UP | - | - | - | DN | - | - | - | - | - | - |
| AT1G33100 | DTX2 | 1.8 | 2.8 | UP | - | - | - | DN | - | - | - | - | - | - | - |
| AT5G52450 | DTX16 | 1.1 | 3.0 | UP | - | - | UP | DN | - | - | - | - | - | - | - |
| AT2G36380 | ABCG34 * | 1.5 | 2.7 | UP | - | UP | - | DN | - | - | - | - | - | - | UP |
| AT4G01450 | UMAMIT30 | 1.4 | 2.7 | UP | - | - | DN | DN | - | - | - | - | - | - | - |
| AT4G28040 | UMAMIT33 | 1.1 | 3.3 | UP | UP | UP | DN | DN | DN | - | - | - | - | DN | - |
| AT4G30420 | UMAMIT34 | 2.2 | 8.8 | UP | - | - | - | DN | - | - | - | - | - | - | - |
| AT3G56620 | UMAMIT10 | 1.6 | 5.1 | UP | - | - | - | DN | - | - | - | - | - | UP | - |
| AT3G26520 | TIP1;2 | 0.9 | 2.1 | UP | - | - | DN | DN | DN | - | - | - | - | DN | DN |
| AT1G73190 | TIP3;1 | 2.7 | 1.4 | UP | - | - | - | - | - | - | - | - | - | UP | UP |
| AT1G01620 | PIP1;3 | 0.9 | 2.1 | UP | - | - | DN | DN | - | - | - | - | - | - | - |
| AT2G16850 | PIP2;8 | 1.0 | 3.4 | UP | - | - | - | - | DN | - | - | - | - | - | - |
| AT2G37170 | PIP2;2 | 0.8 | 2.2 | UP | DN | - | DN | DN | - | - | - | - | - | - | - |
| AT2G39010 | PIP2;6 | 1.7 | 4.2 | UP | - | - | DN | - | DN | - | - | - | - | DN | - |
| Transcription factors | |||||||||||||||
| AT1G02250 | NAC005 | 3.9 | 9.5 | UP | - | - | - | DN | - | - | - | - | - | - | - |
| AT1G77450 | NAC032 | 3.1 | 2.8 | UP | UP | UP | - | DN | UP | - | - | UP | UP | - | UP |
| AT5G08790 | NAC081/ATAF2 | 2.1 | 1.5 | UP | UP | - | UP | UP | - | - | - | - | - | - | - |
| AT5G63790 | NAC102 | 3.8 | 3.9 | UP | UP | UP | UP | - | UP | UP | UP | - | UP | - | UP |
| AT1G71520 | ERF20 | 12.4 | 8.3 | UP | UP | - | UP | UP | UP | - | - | - | - | - | - |
| AT1G74930 | ERF18/ORA47 | 7.0 | 4.0 | UP | - | - | DN | - | DN | - | - | - | - | - | - |
| AT3G23230 | ERF98/TDR1 | 5.1 | 8.3 | UP | - | - | - | - | - | - | - | - | - | - | - |
| AT5G47220 | ERF2 | 2.9 | 1.8 | UP | - | - | - | - | DN | - | - | - | - | - | UP |
| Other genes | |||||||||||||||
| AT1G76680 | OPR1/2 | 8.0 | 9.9 | UP | UP | - | UP | UP | UP | UP | UP | UP | UP | - | UP |
| AT5G22140 | Oxidoreductase | 27.9 | 49.4 | UP | UP | - | UP | UP | UP | - | - | - | - | - | - |
| AT3G44190 | Oxidoreductase | 91 | 349 | UP | UP | - | - | - | UP | - | - | UP | - | - | - |
| AT4G13180 | Oxidoreductase | 6.4 | 43.5 | UP | UP | UP | - | - | - | UP | UP | UP | UP | DN | UP |
| AT1G55920 | SAT1 | 4.7 | 5.6 | UP | UP | - | UP | - | UP | UP | UP | UP | UP | - | UP |
| AT5G39050 | PMAT1 | 2.0 | 2.6 | UP | UP | UP | UP | UP | UP | UP | - | UP | UP | - | UP |
| AT4G01870 | tolB | 6.7 | 5.3 | UP | UP | - | UP | UP | UP | UP | UP | UP | UP | - | UP |
| AT4G24160 | α/β-hydrolase | 2.0 | 1.5 | UP | UP | - | UP | - | UP | UP | - | UP | UP | - | UP |
| AT1G64670 | BDG1 (α/β-hyd.) | 1.0 | 3.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| AT1G06570 | HPPD | 1.1 | 0.9 | - | UP | - | - | - | DN | - | - | - | - | - | UP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakabayashi, K.; Walker, M.; Irwin, D.; Cohn, J.; Guida-English, S.M.; Garcia, L.; Pavlović, I.; Novák, O.; Tarkowská, D.; Strnad, M.; et al. The Phytotoxin Myrigalone A Triggers a Phased Detoxification Programme and Inhibits Lepidium sativum Seed Germination via Multiple Mechanisms including Interference with Auxin Homeostasis. Int. J. Mol. Sci. 2022, 23, 4618. https://doi.org/10.3390/ijms23094618
Nakabayashi K, Walker M, Irwin D, Cohn J, Guida-English SM, Garcia L, Pavlović I, Novák O, Tarkowská D, Strnad M, et al. The Phytotoxin Myrigalone A Triggers a Phased Detoxification Programme and Inhibits Lepidium sativum Seed Germination via Multiple Mechanisms including Interference with Auxin Homeostasis. International Journal of Molecular Sciences. 2022; 23(9):4618. https://doi.org/10.3390/ijms23094618
Chicago/Turabian StyleNakabayashi, Kazumi, Matthew Walker, Dianne Irwin, Jonathan Cohn, Stephanie M. Guida-English, Lucio Garcia, Iva Pavlović, Ondřej Novák, Danuše Tarkowská, Miroslav Strnad, and et al. 2022. "The Phytotoxin Myrigalone A Triggers a Phased Detoxification Programme and Inhibits Lepidium sativum Seed Germination via Multiple Mechanisms including Interference with Auxin Homeostasis" International Journal of Molecular Sciences 23, no. 9: 4618. https://doi.org/10.3390/ijms23094618
APA StyleNakabayashi, K., Walker, M., Irwin, D., Cohn, J., Guida-English, S. M., Garcia, L., Pavlović, I., Novák, O., Tarkowská, D., Strnad, M., Pérez, M., Seville, A., Stock, D., & Leubner-Metzger, G. (2022). The Phytotoxin Myrigalone A Triggers a Phased Detoxification Programme and Inhibits Lepidium sativum Seed Germination via Multiple Mechanisms including Interference with Auxin Homeostasis. International Journal of Molecular Sciences, 23(9), 4618. https://doi.org/10.3390/ijms23094618








