The Tbx6 Transcription Factor Dorsocross Mediates Dpp Signaling to Regulate Drosophila Thorax Closure
Abstract
1. Introduction
2. Results
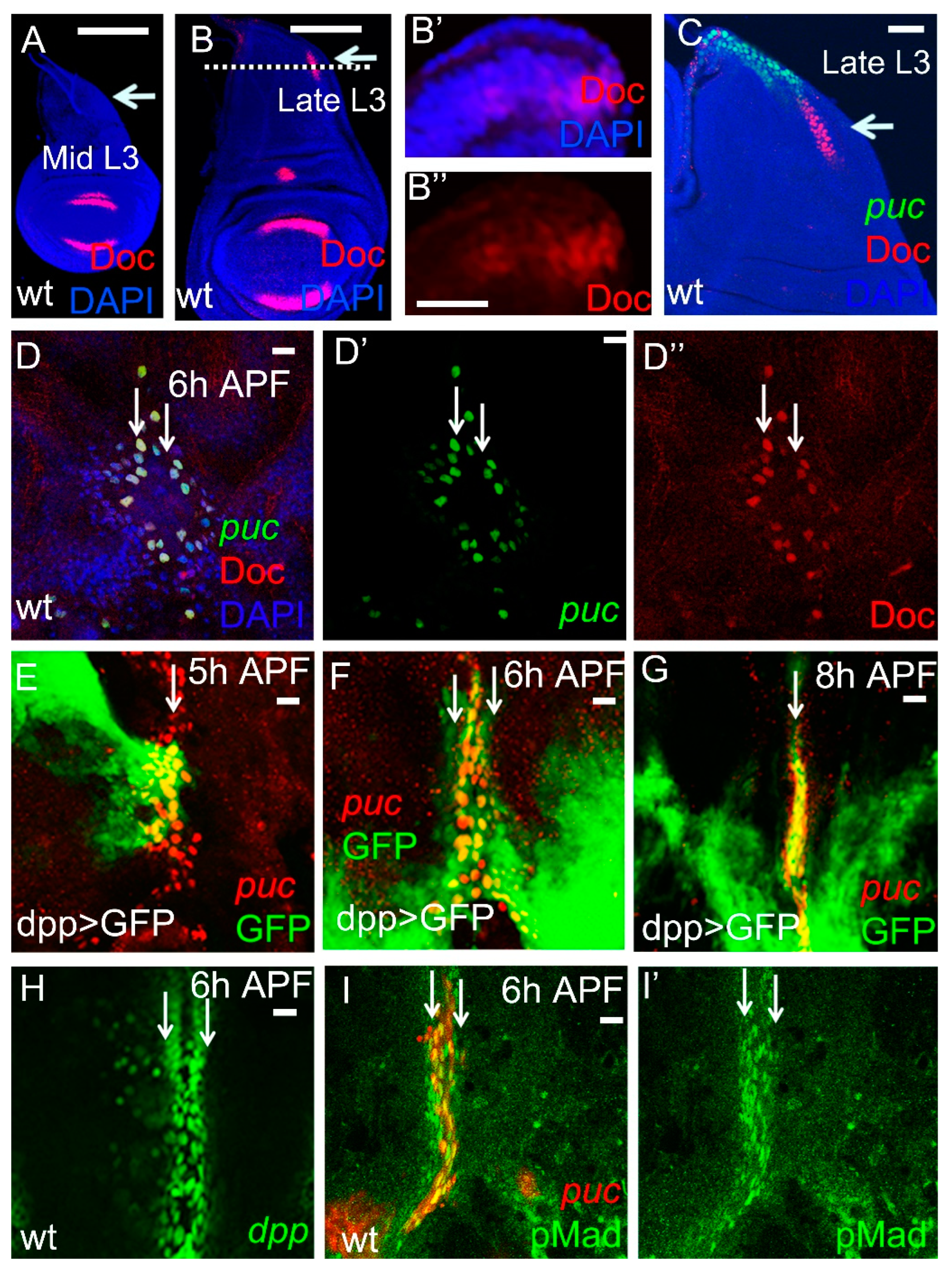
2.1. Doc Is Expressed in the Leading Edge Cells during Thorax Closure
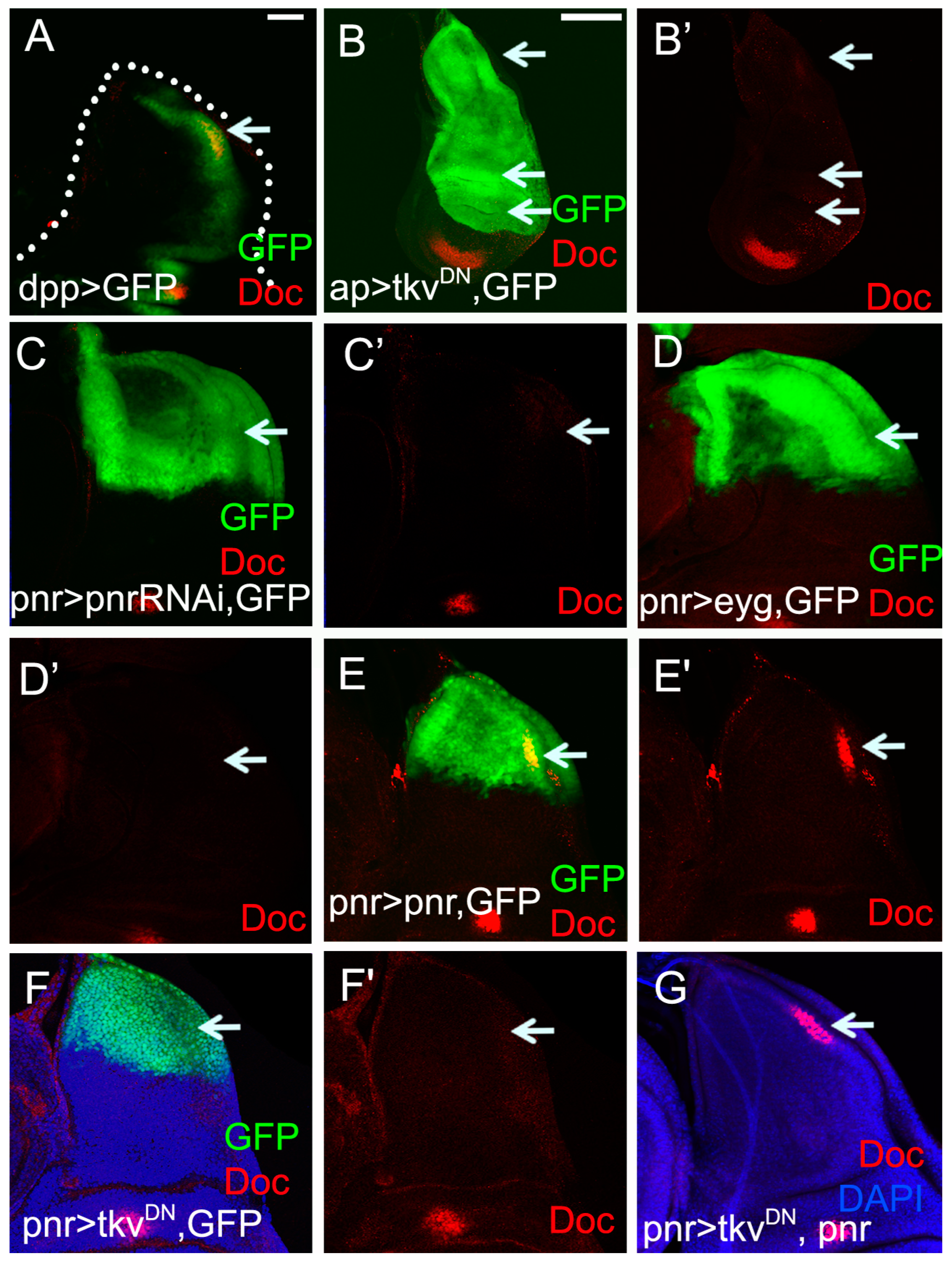
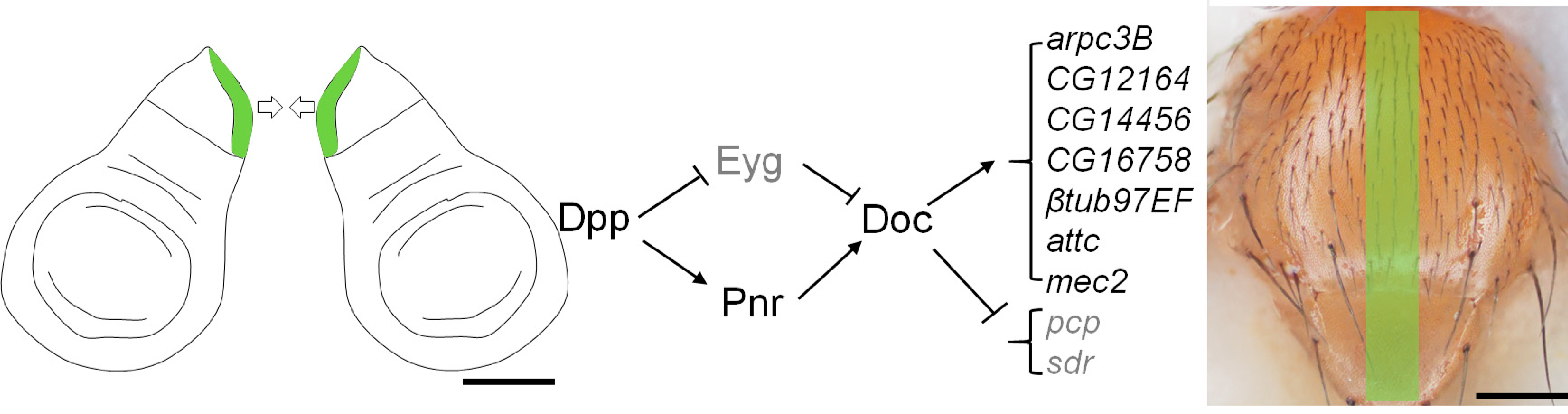
2.2. Doc Is Regulated by Dpp Signaling in the Notum Region of the Wing Disc
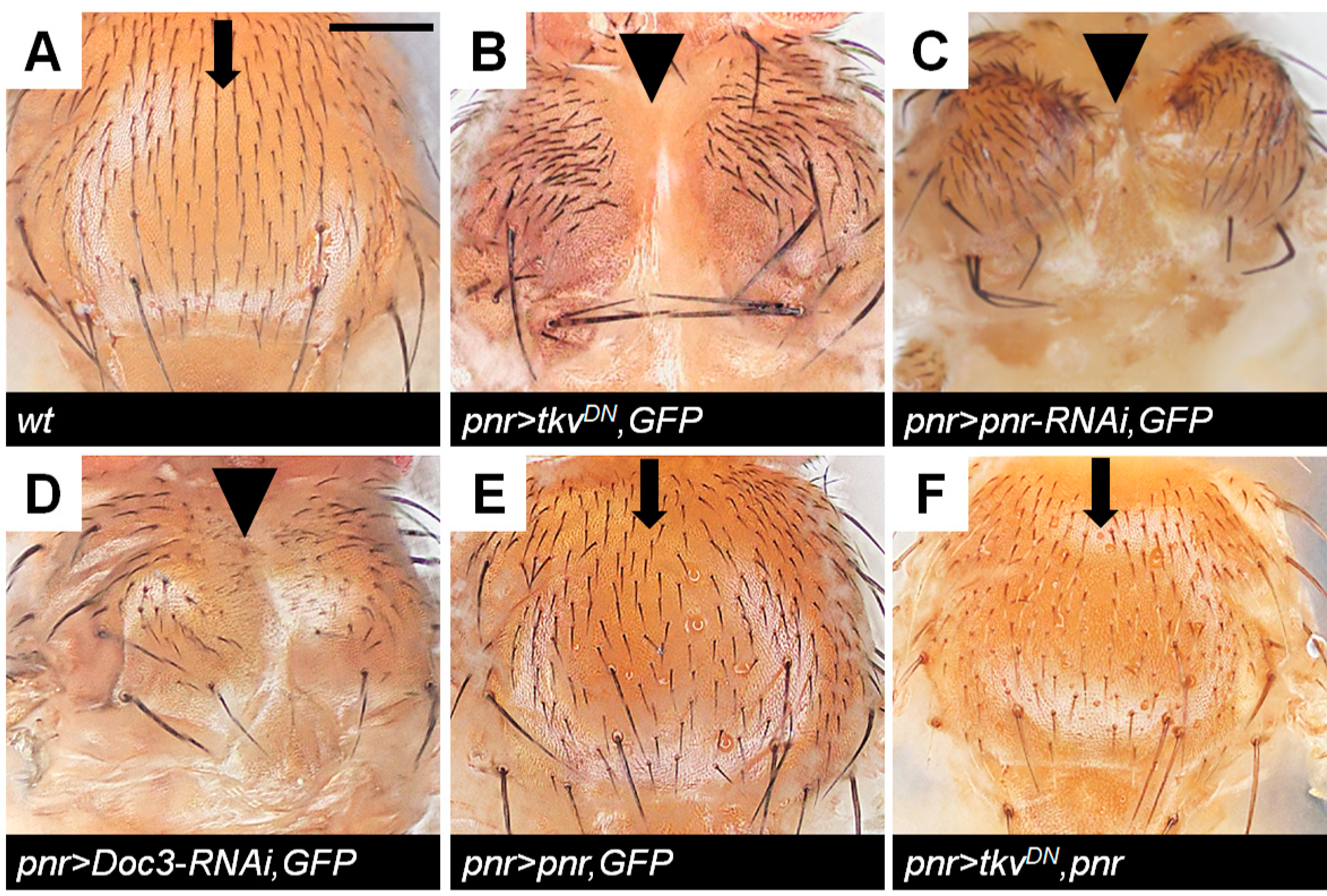
2.3. Doc Is Required for Correct Thorax Closure
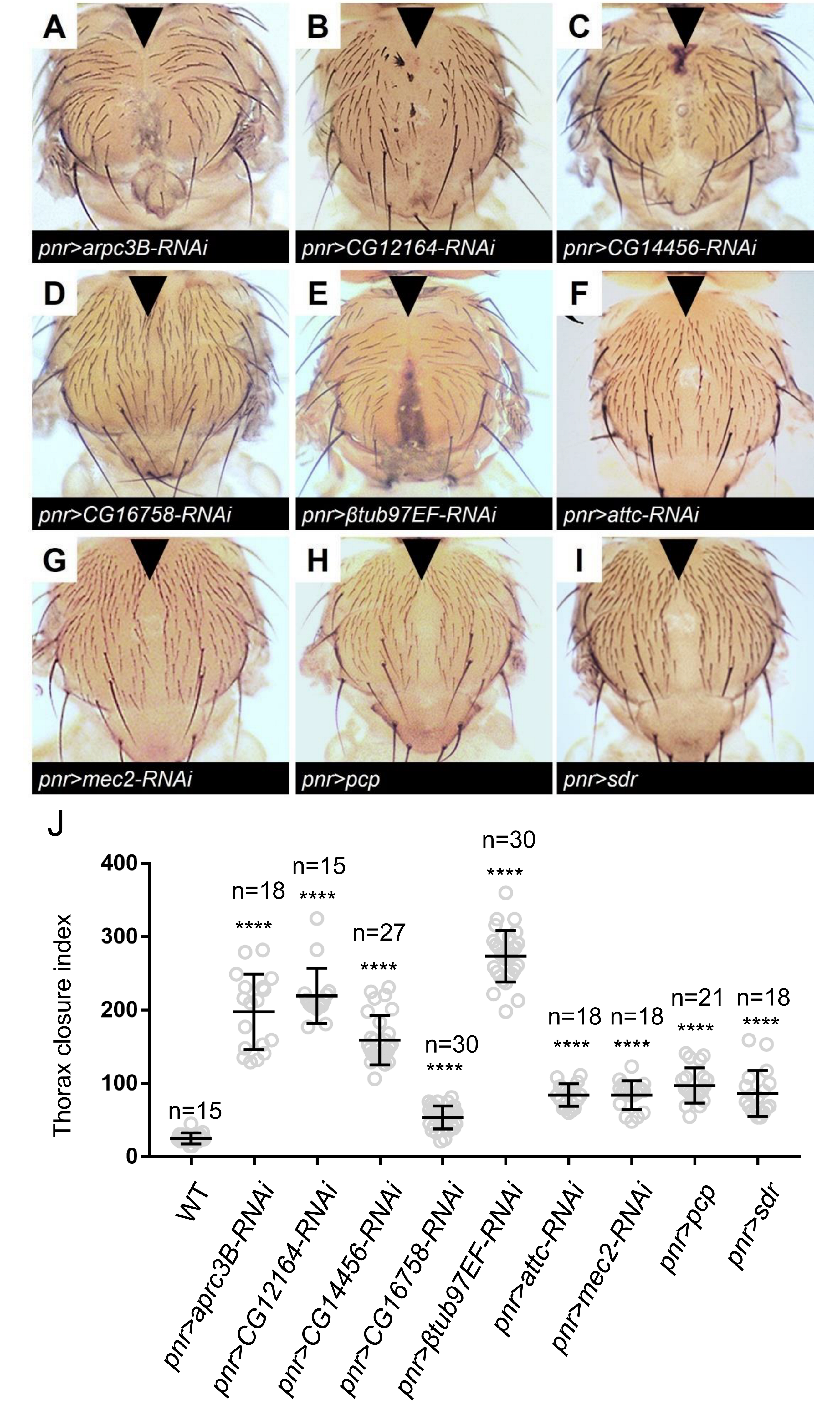
2.4. Functional Analysis of Potential Doc Target Genes
3. Discussion
3.1. Dpp Signaling Promote Thorax Closure
3.2. Doc Function in Other Epithelia
3.3. Potential Doc Target Genes in Regulating Thorax Closure
3.4. The General Character between Dorasl Cloure, Thorax Clousre and Neural Tube Closure
4. Materials and Methods
4.1. Drosophila Stocks
4.2. Dissection of Larvae
4.3. Dissection of Pupae
4.4. Immunohistochemistry
4.5. Adult Thorax Imaging
4.6. Potential Target Genes Selection
4.7. Thorax Closure Index
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallingford, J.B.; Niswander, L.A.; Shaw, G.M.; Finnell, R.H. The continuing challenge of understanding, preventing, and treating neural tube defects. Science 2013, 339, 1222002. [Google Scholar] [CrossRef] [PubMed]
- Wilde, J.J.; Petersen, J.R.; Niswander, L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 2014, 48, 583–611. [Google Scholar] [CrossRef] [PubMed]
- Pocha, S.M.; Montell, D.J. Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: Themes and variations. Annu. Rev. Genet. 2014, 48, 295–318. [Google Scholar] [CrossRef]
- Greene, N.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, A.; Woolner, S.; Martin, P. Dynamic analysis of dorsal closure in Drosophila: From genetics to cell biology. Dev. Cell 2002, 3, 9–19. [Google Scholar] [CrossRef]
- Kiehart, D.P.; Crawford, J.M.; Aristotelous, A.; Venakides, S.; Edwards, G.S. Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System. Annu. Rev. Cell Dev. Biol. 2017, 33, 169–202. [Google Scholar] [CrossRef]
- Zeitlinger, J.; Bohmann, D. Thorax closure in Drosophila: Involvement of Fos and the JNK pathway. Development 1999, 126, 3947–3956. [Google Scholar] [CrossRef]
- Martin-Blanco, E.; Pastor-Pareja, J.C.; Garcia-Bellido, A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 7888–7893. [Google Scholar] [CrossRef]
- Klein, T. Wing disc development in the fly: The early stages. Curr. Opin. Genet. Dev. 2001, 11, 470–475. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Shen, J. Asymmetric distribution of Spalt in Drosophila wing squamous and columnar epithelia ensures correct cell morphogenesis. Sci. Rep. 2016, 6, 30236. [Google Scholar] [CrossRef]
- Muñoz-Descalzo, S.; Terol, J.; Paricio, N. Cabut, a C2H2 zinc finger transcription factor, is required during Drosophila dorsal closure downstream of JNK signaling. Dev. Biol. 2005, 287, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.K.; Shashidhara, L.S. Egfr/Ras pathway mediates interactions between peripodial and disc proper cells in Drosophila wing discs. Development 2003, 130, 4931. [Google Scholar] [CrossRef] [PubMed]
- Baena-Lopez, L.A.; Pastor-Pareja, J.C.; Resino, J. Wg and Egfr signalling antagonise the development of the peripodial epithelium in Drosophila wing discs. Development 2003, 130, 6497. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, S.; Escudero, L.M.; Freeman, M. Live imaging of Drosophila imaginal disc development. Proc. Natl. Acad. Sci. USA 2010, 107, 14217–14222. [Google Scholar] [CrossRef] [PubMed]
- Manhire-Heath, R.; Golenkina, S.; Saint, R.; Murray, M.J. Netrin-dependent downregulation of Frazzled/DCC is required for the dissociation of the peripodial epithelium in Drosophila. Nat. Commun. 2013, 4, 2790. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pareja, J.C.; Grawe, F.; Martín-Blanco, E.; García-Bellido, A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell 2004, 7, 387–399. [Google Scholar] [CrossRef]
- Usui, K.; Simpson, P. Cellular Basis of the Dynamic Behavior of the Imaginal Thoracic Discs during Drosophila Metamorphosis. Dev. Biol. 2000, 225, 13–25. [Google Scholar] [CrossRef]
- Gibson, M.C.; Schubiger, G. Drosophila peripodial cells, more than meets the eye? Bioessays 2001, 23, 691–697. [Google Scholar] [CrossRef]
- Hudson, J.B.; Podos, S.D.; Keith, K.; Simpson, S.L.; Ferguson, E.L. The Drosophila Medea gene is required downstream of dpp and encodes a functional homolog of human Smad4. Development 1998, 125, 1407. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.J.; Ramain, P.; Simpson, P.; Modolell, J. Different contributions of pannier and wingless to the patterning of the dorsal mesothorax of Drosophila. Development 1999, 126, 3523–3532. [Google Scholar] [CrossRef]
- Calleja, M.; Herranz, H.; Estella, C.; Casal, J.; Morata, G. Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development 2000, 127, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Navascues, J.D.; Modolell, J. tailup, a LIM-HD gene, and Iro-C cooperate in Drosophila dorsal mesothorax specification. Development 2007, 134, 1779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aldaz, S.; Morata, G.; Azpiazu, N. The Pax-homeobox gene eyegone is involved in the subdivision of the thorax of Drosophila. Development 2003, 130, 4473–4482. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Saigo, K. Involvement of pannier and u-shaped in regulation of Decapentaplegic-dependent wingless expression in developing Drosophila notum. Mech. Dev. 2000, 93, 127–138. [Google Scholar] [CrossRef]
- Harden, N. Signaling pathways directing the movement and fusion of epithelial sheets: Lessons from dorsal closure in Drosophila. Differentiation 2002, 4–5, 181–203. [Google Scholar] [CrossRef]
- Fernández, B.G.; Arias, A.M.; Jacinto, A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech. Dev. 2007, 124, 884–897. [Google Scholar] [CrossRef]
- Stathopoulos, A.; Drenth, M.V.; Erives, A.; Markstein, M.; Levine, M. Whole-Genome Analysis of Dorsal-Ventral Patterning in the Drosophila Embryo. Cell 2002, 111, 687–701. [Google Scholar] [CrossRef]
- Tomancak, P.; Beaton, A.; Weiszmann, R.; Kwan, E.; Shu, S.; Lewis, S.E.; Richards, S.; Ashburner, M.; Hartenstein, V.; Celniker, S.E.; et al. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2002, 3, research0088.1. [Google Scholar] [CrossRef]
- Butler, M.J.; Jacobsen, T.L.; Cain, D.M.; Jarman, M.G.; Michael, H.; Whittle, J.; Roger, P.; Amanda, S. Discovery of genes with highly restricted expression patterns in the Drosophila wing disc using DNA oligonucleotide microarrays. Development 2003, 130, 659. [Google Scholar] [CrossRef]
- Reim, I.; Lee, H.H.; Frasch, M. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development 2003, 130, 3187–3204. [Google Scholar] [CrossRef]
- Sebé-Pedrós, A.; Ruiz-Trillo, I. Evolution and Classification of the T-Box Transcription Factor Family. Curr. Top Dev. Biol. 2017, 122, 1–26. [Google Scholar] [PubMed]
- Reim, I.; Frasch, M.; Schaub, C. T-Box Genes in Drosophila Mesoderm Development. Curr. Top. Dev. Biol. 2017, 122, 161–193. [Google Scholar] [CrossRef]
- Reim, I. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 2005, 132, 4911. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Yabe, S.; Uchiyama, H.; Murakami, R. Drosophila Tbx6-related gene, Dorsocross, mediates high levels of Dpp and Scw signal required for the development of amnioserosa and wing disc primordium. Dev. Biol. 2004, 265, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Pflugfelder, G.O.; Shen, J. The Dorsocross T-box transcription factors promote tissue morphogenesis in the Drosophila wing imaginal disc. Development 2012, 139, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.H.; Wilk, R.; Lipshitz, H.D. Downregulation of Jun kinase signaling in the amnioserosa is essential for dorsal closure of the Drosophila embryo. Curr. Biol. 2001, 11, 1098–1108. [Google Scholar] [CrossRef]
- Mcewen, D.G.; Cox, R.T.; Peifer, M. The canonical Wg and JNK signaling cascades collaborate to promote both dorsal closure and ventral patterning. Development 2000, 127, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Agnès, F.; Suzanne, M.; Noselli, S. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development 1999, 126, 5453. [Google Scholar] [CrossRef]
- Hashimoto, H.; Robin, F.B.; Sherrard, K.M.; Munro, E.M. Sequential Contraction and Exchange of Apical Junctions Drives Zippering and Neural Tube Closure in a Simple Chordate. Dev. Cell 2015, 32, 241–255. [Google Scholar] [CrossRef]
- Ray, H.J.; Niswander, L. Mechanisms of tissue fusion during development. Development 2012, 139, 1701–1711. [Google Scholar] [CrossRef]
- Staehling-Hampton, K.; Hoffmann, F.M.; Baylies, M.K.; Rushtont, E.; Bate, M. Dpp induces mesodermal gene expression in Drosophila. Nature 1994, 372, 783–786. [Google Scholar] [CrossRef]
- Herranz, H.; Morata, G. The functions of pannier during Drosophila embryogenesis. Development 2001, 128, 4837–4846. [Google Scholar] [CrossRef]
- Fromental-Ramain, C.; Vanolst, L.; Delaporte, C.; Ramain, P. pannier encodes two structurally related isoforms that are differentially expressed during Drosophila development and display distinct functions during thorax patterning. Mech. Dev. 2008, 125, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.; Navascués, J.D.; Gorfinkiel, N. α-Catenin stabilises Cadherin-Catenin complexes and modulates actomyosin dynamics to allow pulsatile apical contraction. J. Cell Sci. 2016, 129, 4496–4508. [Google Scholar] [CrossRef] [PubMed]
- Gorfinkiel, N.; Arias, A.M. Requirements for adherens junction components in the interaction between epithelial tissues during dorsal closure in Drosophila. J. Cell Sci. 2007, 120, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Rousset, R.; Carballès, F.; Parassol, N.; Schaub, S.; Cérézo, D.; Noselli, S. Signalling crosstalk at the leading edge controls tissue closure dynamics in the Drosophila embryo. PLoS Genet. 2017, 13, e1006640. [Google Scholar] [CrossRef] [PubMed]
- Rousset, R.; Bono-Lauriol, S.; Gettings, M.; Suzanne, M.; Spéder, P.; Noselli, S. The Drosophila serine protease homologue Scarface regulates JNK signalling in a negative-feedback loop during epithelial morphogenesis. Development 2010, 137, 2177–2186. [Google Scholar] [CrossRef]
- Simin, K.; Bates, E.A.; Horner, M.A.; Letsou, A. Genetic analysis of punt, a type II Dpp receptor that functions throughout the Drosophila melanogaster life cycle. Genetics 1998, 148, 801–813. [Google Scholar] [CrossRef]
- Chen, Y.J.; Riese, M.J.; Killinger, M.A.; Hoffmann, F.M. A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development 1998, 125, 1759–1768. [Google Scholar] [CrossRef]
- Riesgoescovar, J.R.; Hafen, E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev. 1997, 11, 1717–1727. [Google Scholar] [CrossRef]
- Martin-Blanco, E. Regulation of cell differentiation by the Drosophila Jun kinase cascade. Curr. Opin. Genet. Dev. 1997, 7, 666–671. [Google Scholar] [CrossRef]
- Hou, X.S.; Goldstein, E.S.; Perrimon, N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes Dev. 1997, 11, 1728–1737. [Google Scholar] [CrossRef]
- Solon, J.; Kaya-Copur, A.; Colombelli, J.; Brunner, D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 2009, 137, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Gates, J.; Mahaffey, J.P.; Rogers, S.L.; Emerson, M.; Rogers, E.M.; Sottile, S.L.; Van Vactor, D.; Gertler, F.B.; Peifer, M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development 2007, 134, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Zeitlinger, J.; Kockel, L.; Peverali, F.A.; Jackson, D.B.; Mlodzik, M.; Bohmann, D. Defective dorsal closure and loss of epidermal decapentaplegic expression in Drosophila fos mutants. Embo J. 1997, 16, 7393–7401. [Google Scholar] [CrossRef]
- Ricos, M.G.; Harden, N.; Sem, K.P.; Lim, L.; Chia, W. Dcdc42 acts in TGF-beta signaling during Drosophila morphogenesis: Distinct roles for the Drac1/JNK and Dcdc42/TGF-beta cascades in cytoskeletal regulation. J. Cell Sci. 1999, 112 Pt 8, 1225–1235. [Google Scholar] [CrossRef]
- Woolner, S.; Jacinto, A.; Martin, P. The small GTPase Rac plays multiple roles in epithelial sheet fusion--dynamic studies of Drosophila dorsal closure. Dev. Biol. 2005, 282, 163–173. [Google Scholar] [CrossRef]
- Milán, M.; Cohen, S.M. Regulation of LIM homeodomain activity in vivo: A tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Mol. Cell 1999, 4, 267–273. [Google Scholar] [CrossRef]
- Horn, T.; Panfilio, K.A. Novel functions for Dorsocross in epithelial morphogenesis in the beetle Tribolium castaneum. Development 2016, 143, 3002–3011. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Terhzaz, S.; Wang, J.; Al Bratty, M.; Watson, D.G.; Herzyk, P.; Davies, S.A.; Dow, J.A. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS ONE 2012, 7, e32577. [Google Scholar] [CrossRef]
- Balasubramanian, M.K.; Feoktistova, A.; Mccollum, D.; Gould, K.L. Fission yeast Sop2p: A novel and evolutionarily conserved protein that interacts with Arp3p and modulates profilin function. Embo J. 1996, 15, 6426–6437. [Google Scholar] [CrossRef] [PubMed]
- Mccollum, D.; Feoktistova, A.; Morphew, M.; Balasubramanian, M.; Gould, K.L. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. Embo J. 1996, 15, 6438–6446. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.L.; Morphew, M.; Gould, K.L. A Mutant of Arp2p Causes Partial Disassembly of the Arp2/3 Complex and Loss of Cortical Actin Function in Fission Yeast. Mol. Biol. Cell 1999, 10, 4201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hudson, A.M.; Cooley, L. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu. Rev. Genet. 2002, 36, 455–488. [Google Scholar] [CrossRef] [PubMed]
- Rohn, J.L.; Sims, D.; Liu, T.; Fedorova, M.; Schöck, F.; Dopie, J.; Vartiainen, M.K.; Kiger, A.A.; Perrimon, N.; Baum, B. Comparative RNAi screening identifies a conserved core metazoan actinome by phenotype. J. Cell Biol. 2011, 194, 789–805. [Google Scholar] [CrossRef]
- Myachina, F.; Bosshard, F.; Bischof, J.; Kirschmann, M.; Lehne, C.F. Drosophila β-Tubulin 97EF is upregulated at low temperature and stabilizes microtubules. Development 2017, 144, 4573–4587. [Google Scholar] [CrossRef]
- Ring, J.M.; Martinez Arias, A. puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Development 1993, 119, 251–259. [Google Scholar] [CrossRef]
- Young, P.E.; Richman, A.M.; Ketchum, A.S.; Kiehart, D.P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993, 7, 29–41. [Google Scholar] [CrossRef]
- Jankovics, F.; Brunner, D. Transiently reorganized microtubules are essential for zippering during dorsal closure in Drosophila melanogaster. Dev. Cell 2006, 11, 375–385. [Google Scholar] [CrossRef]
- Millard, T.H.; Martin, P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 2008, 135, 621–626. [Google Scholar] [CrossRef]
- Liu, R.; Woolner, S.; Johndrow, J.E.; Metzger, D.; Flores, A.; Parkhurst, S.M. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development 2008, 135, 53–63. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.L. Ultrastructural studies of amphibian neural fold fusion. Dev. Biol. 1978, 65, 435–446. [Google Scholar] [CrossRef]
- Rolo, A.; Savery, D.; Escuin, S.; de Castro, S.C.; Armer, H.E.; Munro, P.M.; Molè, M.A.; Greene, N.D.; Copp, A.J. Regulation of cell protrusions by small GTPases during fusion of the neural folds. Elife 2016, 5, e13273. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.E. Topographical changes along the neural fold associated with neurulation in the hamster and mouse. Am. J. Anat. 1976, 146, 151–171. [Google Scholar] [CrossRef]
- Pyrgaki, C.; Liu, A.; Niswander, L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev. Biol. 2011, 353, 38–49. [Google Scholar] [CrossRef]
- Werth, M.; Walentin, K.; Aue, A.; Schönheit, J.; Wuebken, A.; Pode-Shakked, N.; Vilianovitch, L.; Erdmann, B.; Dekel, B.; Bader, M.; et al. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development 2010, 137, 3835–3845. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Wang, Y.; Wang, X.; Wang, D.; Pflugfelder, G.O.; Shen, J. The Tbx6 Transcription Factor Dorsocross Mediates Dpp Signaling to Regulate Drosophila Thorax Closure. Int. J. Mol. Sci. 2022, 23, 4543. https://doi.org/10.3390/ijms23094543
Lu J, Wang Y, Wang X, Wang D, Pflugfelder GO, Shen J. The Tbx6 Transcription Factor Dorsocross Mediates Dpp Signaling to Regulate Drosophila Thorax Closure. International Journal of Molecular Sciences. 2022; 23(9):4543. https://doi.org/10.3390/ijms23094543
Chicago/Turabian StyleLu, Juan, Yingjie Wang, Xiao Wang, Dan Wang, Gert O. Pflugfelder, and Jie Shen. 2022. "The Tbx6 Transcription Factor Dorsocross Mediates Dpp Signaling to Regulate Drosophila Thorax Closure" International Journal of Molecular Sciences 23, no. 9: 4543. https://doi.org/10.3390/ijms23094543
APA StyleLu, J., Wang, Y., Wang, X., Wang, D., Pflugfelder, G. O., & Shen, J. (2022). The Tbx6 Transcription Factor Dorsocross Mediates Dpp Signaling to Regulate Drosophila Thorax Closure. International Journal of Molecular Sciences, 23(9), 4543. https://doi.org/10.3390/ijms23094543





