TRPA1 Role in Inflammatory Disorders: What Is Known So Far?
Abstract
:1. Introduction
2. Transient Receptor Potential Ankyrin 1 (TRPA1)
3. TRPA1 and Asthma and Chronic Obstructive Pulmonary Disease (COPD)
4. TRPA1 and Rheumatoid Arthritis
5. TRPA1 and Endometriosis
6. TRPA1 and Inflammatory Bowel Disease
7. TRPA1 and Atherosclerosis
8. TRPA1 and Inflammatory Skin Diseases
9. TRPA1 and Neurodegenerative Inflammatory Diseases
9.1. TRPA1 and Alzheimer’s Disease
9.2. TRPA1 and Parkinson’s Disease
9.3. TRPA1 and Multiple Sclerosis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Yang, Y.; Xu, H. Organellar TRP channels. Nat. Struct. Mol. Biol. 2018, 25, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Logu, F.; Geppetti, P. Ion channel pharmacology for pain modulation. Concepts Princ. Pharmacol. 2019, 260, 161–186. [Google Scholar] [CrossRef]
- De Logu, F.; Li Puma, S.; Landini, L.; Tuccinardi, T.; Poli, G.; Preti, D.; De Siena, G.; Patacchini, R.; Tsagareli, M.G.; Geppetti, P.; et al. The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol. Res. 2019, 142, 127–139. [Google Scholar] [CrossRef]
- Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Oliveira, S.M.; Silva, M.A.; Silva, C.R.; Fusi, C.; Tonello, R.; Minocci, D.; Guerra, G.P.; et al. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic. Biol. Med. 2014, 72, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Souza Monteiro de Araújo, D.; De Logu, F.; Adembri, C.; Rizzo, S.; Janal, M.N.; Landini, L.; Magi, A.; Mattei, G.; Cini, N.; Pandolfo, P.; et al. TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death Dis. 2020, 11, 633. [Google Scholar] [CrossRef]
- Robledo-Avila, F.H.; Ruiz-Rosado, J.D.; Brockman, K.L.; Partida-Sánchez, S. The TRPM2 ion channel regulates inflammatory functions of neutrophils during listeria monocytogenes infection. Front. Immunol. 2020, 11, 97. [Google Scholar] [CrossRef]
- Najder, K.; Rugi, M.; Lebel, M.; Schroder, J.; Oster, L.; Schimmelpfennig, S.; Sargin, S.; Petho, Z.; Bulk, E.; Schwab, A. Role of the intracellular sodium homeostasis in chemotaxis of activated murine neutrophils. Front. Immunol. 2020, 11, 2124. [Google Scholar] [CrossRef]
- Nadolni, W.; Immler, R.; Hoelting, K.; Fraticelli, M.; Ripphahn, M.; Rothmiller, S.; Matsushita, M.; Boekhoff, I.; Gudermann, T.; Sperandio, M.; et al. TRPM7 kinase is essential for neutrophil recruitment and function via regulation of Akt/mTOR signaling. Front. Immunol. 2020, 11, 606893. [Google Scholar] [CrossRef] [PubMed]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.P.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Abraham, S.; Niese, K.A.; Southern, B.D.; Grove, L.M.; Hite, R.D.; McDonald, C.; Hamilton, T.A.; Olman, M.A. TRPV4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J. Immunol. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Michalick, L.; Tang, C.; Tabuchi, A.; Goldenberg, N.; Dan, Q.; Awwad, K.; Wang, L.; Erfinanda, L.; Nouailles, G.; et al. Role of transient receptor potential vanilloid 4 in neutrophil activation and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2016, 54, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Kung, C. A possible unifying principle for mechanosensation. Nature 2005, 436, 647–654. [Google Scholar] [CrossRef]
- Christensen, A.P.; Corey, D.P. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521. [Google Scholar] [CrossRef]
- Hamanaka, K.; Jian, M.Y.; Townsley, M.I.; King, J.A.; Liedtke, W.; Weber, D.S.; Eyal, F.G.; Clapp, M.M.; Parker, J.C. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L353–L362. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Liang, L.; Smillie, S.J.; Kaiser, F.; Purcell, R.; Rivett, D.W.; Alam, S.; Howat, S.; Collins, H.; Thompson, S.J.; et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 2012, 188, 5741–5751. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.F.; Ching, L.C.; Kou, Y.R.; Lin, S.J.; Wei, J.; Shyue, S.K.; Lee, T.S. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-alpha-induced inflammation in macrophages: Role of liver X receptor α. Mediat. Inflamm. 2013, 2013, 925171. [Google Scholar] [CrossRef] [Green Version]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Honore, E. Sensing pressure with ion channels. Trends Neurosci. 2012, 35, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Chang, R.B.; Waters, H.N.; McKemy, D.D.; Liman, E.R. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J. Biol. Chem. 2008, 283, 32691–32703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, G.; Benemei, S.; Materazzi, S.; De Logu, F.; De Siena, G.; Fusi, C.; Fortes Rossato, M.; Coppi, E.; Marone, I.M.; Ferreira, J.; et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016, 139, 1361–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [Green Version]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Li Puma, S.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [Green Version]
- Doerner, J.F.; Gisselmann, G.; Hatt, H.; Wetzel, C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007, 282, 13180–13189. [Google Scholar] [CrossRef] [Green Version]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 2007, 10, 277–279. [Google Scholar] [CrossRef]
- Samad, A.; Sura, L.; Benedikt, J.; Ettrich, R.; Minofar, B.; Teisinger, J.; Vlachova, V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem. J. 2011, 433, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Anoveros, J.; Duggan, A. TRPA1 in Auditory and Nociceptive Organs. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Earley, S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J. Cardiovasc. Pharmacol. 2011, 57, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Kobayashi, K.; Hao, Y.; Kanda, H.; Kondo, T.; Kogure, Y.; Yamanaka, H.; Yamamoto, S.; Li, J.; et al. TRPA1-expressing lamina propria mesenchymal cells regulate colonic motility. JCI Insight 2019, 4, e122402. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Gomes, P.; Aranake, S.; Shetty, M.; Karnik, P.; Damle, M.; Kuruganti, S.; Thorat, S.; Khairatkar-Joshi, N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J. Recept. Signal Transduct. 2011, 31, 350–358. [Google Scholar] [CrossRef]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [Green Version]
- Luostarinen, S.; Hämäläinen, M.; Hatano, N.; Muraki, K.; Moilanen, E. The inflammatory regulation of TRPA1 expression in human A549 lung epithelial cells. Pulm. Pharmacol. Ther. 2021, 70, 102059. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012, 133, 189–204. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Preti, D.; Materazzi, S.; Geppetti, P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J. Med. Chem. 2010, 53, 5085–5107. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2383–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buch, T.R.; Schafer, E.A.; Demmel, M.T.; Boekhoff, I.; Thiermann, H.; Gudermann, T.; Steinritz, D.; Schmidt, A. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem. Biol. Interact. 2013, 206, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Earley, S. TRPA1 channels in the vasculature. Br. J. Pharmacol. 2012, 167, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Yanagihara, A.A.; Small-Howard, A.L.; Turner, H.; Stokes, A.J. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J. Immunol. 2008, 181, 5024–5034. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, M.; Harada, K.; Nakamura, K.; Tsuboi, T. Transient receptor potential ankyrin 1 channels are involved in spontaneous peptide hormone release from astrocytes. Biochem. Biophys. Res. Commun. 2018, 501, 988–995. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Kolodziejczyk, K.; Kougioumtzidou, E.; Attwell, D. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 2016, 529, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Li, S.; He, L.; Han, X.; Tang, F.; Huang, R.; Lin, Z.; Deng, S.; Xu, J.; Huang, H.; et al. Transient receptor potential ankyrin 1 contributes to the lysophosphatidylcholine-induced oxidative stress and cytotoxicity in OLN-93 oligodendrocyte. Cell Stress Chaperones 2020, 25, 955–968. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Goncalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef] [Green Version]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; De Pra, S.D.; de David Antoniazzi, C.T.; Kudsi, S.Q.; Ferro, P.R.; Landini, L.; Rigo, F.K.; de Bem Silveira, G.; Silveira, P.C.L.; Oliveira, S.M.; et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain Behav. Immun. 2020, 88, 535–546. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Marini, M.; Landini, L.; Souza Monteiro de Araujo, D.; Bartalucci, N.; Trevisan, G.; Bruno, G.; Marangoni, M.; Schmidt, B.L.; Bunnett, N.W.; et al. Peripheral nerve resident macrophages and schwann cells mediate cancer-induced pain. Cancer Res. 2021, 81, 3387–3401. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E.; Ghatta, S.; Bettner, W.; Undem, B.J. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol. Pharmacol. 2009, 75, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Orengo, L.; Dhaka, A.; Heuermann, R.J.; Young, T.J.; Montana, M.C.; Cavanaugh, E.J.; Kim, D.; Story, G.M. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol. Pain 2008, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Clark, T.E.; Undem, B.J.; Macglashan, D.W., Jr.; Ghatta, S.; Carr, M.J.; McAlexander, M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol. Pharmacol. 2008, 73, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materazzi, S.; Nassini, R.; Andre, E.; Campi, B.; Amadesi, S.; Trevisani, M.; Bunnett, N.W.; Patacchini, R.; Geppetti, P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2008, 105, 12045–12050. [Google Scholar] [CrossRef] [Green Version]
- Geppetti, P.; Holzer, P. Neurogenic Inflammation; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; Souza Monteiro de Araujo, D.; Ramírez-Garcia, P.; et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Pratter, M.R. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 72S–74S. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.S.; Curley, F.J.; French, C.L. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am. Rev. Respir. Dis. 1990, 141, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Morrison, D.; Donaldson, K.; MacNee, W. Systemic oxidative stress in asthma, COPD, and smokers. Am. J. Respir. Crit. Care Med. 1996, 154, 1055–1060. [Google Scholar] [CrossRef]
- Beck, L.A.; Leung, D.Y. Allergen sensitization through the skin induces systemic allergic responses. J. Allergy Clin. Immunol. 2000, 106, S258–S263. [Google Scholar] [CrossRef] [Green Version]
- Tanou, K.; Koutsokera, A.; Kiropoulos, T.S.; Maniati, M.; Papaioannou, A.I.; Georga, K.; Zarogiannis, S.; Gourgoulianis, K.I.; Kostikas, K. Inflammatory and oxidative stress biomarkers in allergic rhinitis: The effect of smoking. Clin. Exp. Allergy 2009, 39, 345–353. [Google Scholar] [CrossRef]
- Riedl, M.A.; Nel, A.E. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 49–56. [Google Scholar] [CrossRef]
- Nassenstein, C.; Kwong, K.; Taylor-Clark, T.; Kollarik, M.; Macglashan, D.M.; Braun, A.; Undem, B.J. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. 2008, 586, 1595–1604. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; De Siena, G.; De Cesaris, F.; Geppetti, P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr. Opin. Investig. Drugs 2010, 11, 535–542. [Google Scholar] [PubMed]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.D.; Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef] [Green Version]
- Mazzone, S.B.; Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Patacchini, R. The rising role of TRPA1 in asthma. Open Drug Discov. J. 2010, 2, 71–80. [Google Scholar] [CrossRef]
- Bessac, B.F.; Sivula, M.; von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, E.; Campi, B.; Materazzi, S.; Trevisani, M.; Amadesi, S.; Massi, D.; Creminon, C.; Vaksman, N.; Nassini, R.; Civelli, M.; et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Investig. 2008, 118, 2574–2582. [Google Scholar] [CrossRef]
- Huber, G.L.; First, M.W.; Grubner, O. Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacol. Biochem. Behav. 1991, 40, 629–636. [Google Scholar] [CrossRef]
- Witschi, H. Carcinogenic activity of cigarette smoke gas phase and its modulation by beta-carotene and N-acetylcysteine. Toxicol. Sci. 2005, 84, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernandez-Pena, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Bhatia, M. Substance P at the neuro-immune crosstalk in the modulation of inflammation, asthma and antimicrobial host defense. Inflamm. Allergy Drug Targets 2014, 13, 112–120. [Google Scholar] [CrossRef]
- Caceres, A.I.; Brackmann, M.; Elia, M.D.; Bessac, B.F.; del Camino, D.; D’Amours, M.; Witek, J.S.; Fanger, C.M.; Chong, J.A.; Hayward, N.J.; et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. USA 2009, 106, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Reese, R.M.; Dourado, M.; Anderson, K.; Warming, S.; Stark, K.L.; Balestrini, A.; Suto, E.; Lee, W.; Riol-Blanco, L.; Shields, S.D.; et al. Behavioral characterization of a CRISPR-generated TRPA1 knockout rat in models of pain, itch, and asthma. Sci. Rep. 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Dijk, F.N.; Holloway, J.W.; Ring, S.M.; Koppelman, G.H.; Postma, D.S.; Strachan, D.P.; Granell, R.; de Jongste, J.C.; Jaddoe, V.W.; et al. TRPA1 gene polymorphisms and childhood asthma. Pediatr. Allergy Immunol. 2017, 28, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, M.G.; Birrell, M.A.; Khalid, S.; Wortley, M.A.; Dockry, R.; Coote, J.; Holt, K.; Dubuis, E.; Kelsall, A.; Maher, S.A.; et al. Neurophenotypes in airway diseases. Insights from translational cough studies. Am. J. Respir. Crit. Care Med. 2016, 193, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Straub, R.H. Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 244. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. Ther. 2015, 17, 321. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Pongratz, G.; Straub, R.H. The synthetic cannabinoid WIN55,212-2 mesylate decreases the production of inflammatory mediators in rheumatoid arthritis synovial fibroblasts by activating CB2, TRPV1, TRPA1 and yet unidentified receptor targets. J. Inflamm. 2016, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Pereira, I.; Mendes, S.J.; Pereira, D.M.; Muniz, T.F.; Colares, V.L.; Monteiro, C.R.; Martins, M.M.; Grisotto, M.A.; Monteiro-Neto, V.; Monteiro, S.G.; et al. Transient receptor potential ankyrin 1 channel expression on peripheral blood leukocytes from rheumatoid arthritic patients and correlation with pain and disability. Front. Pharmacol. 2017, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Bleck, J.; Schneider, M.; Pongratz, G. Selective killing of proinflammatory synovial fibroblasts via activation of transient receptor potential ankyrin (TRPA1). Biochem. Pharmacol. 2018, 154, 293–302. [Google Scholar] [CrossRef]
- Batai, I.Z.; Sar, C.P.; Horvath, A.; Borbely, E.; Bolcskei, K.; Kemeny, A.; Sandor, Z.; Nemes, B.; Helyes, Z.; Perkecz, A.; et al. TRPA1 ion channel determines beneficial and detrimental effects of GYY4137 in murine serum-transfer arthritis. Front. Pharmacol. 2019, 10, 964. [Google Scholar] [CrossRef] [Green Version]
- Hatano, N.; Suzuki, H.; Muraki, Y.; Muraki, K. Stimulation of human TRPA1 channels by clinical concentrations of the antirheumatic drug auranofin. Am. J. Physiol. Cell Physiol. 2013, 304, C354–C361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020, 11, 714. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hamalainen, M.; Pemmari, A.; Moilanen, L.J.; Tuure, L.; Nieminen, R.M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Transient receptor potential ankyrin 1 (TRPA1) is involved in upregulating interleukin-6 expression in osteoarthritic chondrocyte models. Int. J. Mol. Sci. 2020, 22, 87. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases 11th Revision. Available online: https://icd.who.int/browse11/l-m/en (accessed on 24 January 2022).
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkley, K.J.; Rapkin, A.J.; Papka, R.E. The pains of endometriosis. Science 2005, 308, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.A.; Costantini, R.; Affaitati, G.; Fabrizio, A.; Lapenna, D.; Tafuri, E.; Mezzetti, A. Viscero-visceral hyperalgesia: Characterization in different clinical models. Pain 2010, 151, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Onon, T.S.; Agrawal, A.; Shekhar, C.; Morris, J.; Hamdy, S.; Whorwell, P.J. Visceral hypersensitivity in endometriosis: A new target for treatment? Gut 2012, 61, 367–372. [Google Scholar] [CrossRef]
- Lemaire, G.S. More than just menstrual cramps: Symptoms and uncertainty among women with endometriosis. J. Obstet. Gynecol. Neonatal. Nurs. 2004, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Grieve, K.; Horne, A.W.; Saunders, P.T. Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1738–E1743. [Google Scholar] [CrossRef] [PubMed]
- Bohonyi, N.; Pohoczky, K.; Szalontai, B.; Perkecz, A.; Kovacs, K.; Kajtar, B.; Orban, L.; Varga, T.; Szegedi, S.; Bodis, J.; et al. Local upregulation of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 ion channels in rectosigmoid deep infiltrating endometriosis. Mol. Pain 2017, 13, 1744806917705564. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Franklin, N.S.; Gonzalez-Cano, R.; Peterse, D.; Ghalali, A.; Madrian, E.; Verri, W.A., Jr.; Andrews, N.; Woolf, C.J.; Rogers, M.S. Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain 2020, 161, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Schirbel, A.; Reichert, A.; Roll, S.; Baumgart, D.C.; Büning, C.; Wittig, B.; Wiedenmann, B.; Dignass, A.; Sturm, A. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 3168–3177. [Google Scholar] [CrossRef]
- Halpin, S.J.; Ford, A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Greenwood-Van Meerveld, B.; Prusator, D.K.; Johnson, A.C. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: Pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G885–G903. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Zuo, X.; Zhen, Y.; Yu, Y.; Gao, L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci. Lett. 2008, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Wang, S.; Tanaka, K.; Hao, Y.; Yamamoto, S.; Nishiyama, N.; Noguchi, K.; Dai, Y. Elevated H2O2 levels in trinitrobenzene sulfate-induced colitis rats contributes to visceral hyperalgesia through interaction with the transient receptor potential ankyrin 1 cation channel. J. Gastroenterol. Hepatol. 2016, 31, 1147–1153. [Google Scholar] [CrossRef]
- Jain, P.; Materazzi, S.; De Logu, F.; Rossi Degl’Innocenti, D.; Fusi, C.; Li Puma, S.; Marone, I.M.; Coppi, E.; Holzer, P.; Geppetti, P.; et al. Transient receptor potential ankyrin 1 contributes to somatic pain hypersensitivity in experimental colitis. Sci. Rep. 2020, 10, 8632. [Google Scholar] [CrossRef]
- Kun, J.; Szitter, I.; Kemény, A.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, A.; Gódi, S.; Szabó, I.; Szolcsányi, J.; et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef]
- Engel, M.A.; Leffler, A.; Niedermirtl, F.; Babes, A.; Zimmermann, K.; Filipović, M.R.; Izydorczyk, I.; Eberhardt, M.; Kichko, T.I.; Mueller-Tribbensee, S.M.; et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 2011, 141, 1346–1358. [Google Scholar] [CrossRef]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’Armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Stocco, G.; et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Bodkin, J.V.; Brain, S.D. Transient receptor potential ankyrin 1: Emerging pharmacology and indications for cardiovascular biology. Acta Physiol. 2011, 203, 87–98. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Y.; Chen, J.; Hu, Y.; Jia, P.; Wang, X.; Zhao, Q.; Deng, Y.; Li, N.; Zang, Y.; et al. Pathogenic CARD11 mutations affect B cell development and differentiation through a noncanonical pathway. Sci. Immunol. 2019, 4, eaaw5618. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Liu, L.; Wei, D.; Lv, Y.; Wang, G.; Xiong, W.; Wang, X.; Altaf, A.; Wang, L.; He, D.; et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int. J. Mol. Med. 2015, 35, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Janks, L.; Sprague, R.S.; Egan, T.M. ATP-gated P2X7 receptors require chloride channels to promote inflammation in human macrophages. J. Immunol. 2019, 202, 883–898. [Google Scholar] [CrossRef] [Green Version]
- Stachon, P.; Geis, S.; Peikert, A.; Heidenreich, A.; Michel, N.A.; Ünal, F.; Hoppe, N.; Dufner, B.; Schulte, L.; Marchini, T.; et al. Extracellular ATP induces vascular inflammation and atherosclerosis via purinergic receptor Y2 in mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Han, X.; He, L.; Tang, F.; Huang, R.; Lin, Z.; Li, S.; Deng, S.; Xu, J.; Huang, H.; et al. Transient receptor potential ankyrin 1 contributes to the ATP-elicited oxidative stress and inflammation in THP-1-derived macrophage. Mol. Cell. Biochem. 2020, 473, 179–192. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, B.R.; Lynch, K.J.; Touma, E.; Niforatos, W.; Burgard, E.C.; Alexander, K.M.; Park, H.S.; Yu, H.; Metzger, R.; Kowaluk, E.; et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur. J. Pharmacol. 1999, 376, 127–138. [Google Scholar] [CrossRef]
- Zhao, J.F.; Shyue, S.K.; Kou, Y.R.; Lu, T.M.; Lee, T.S. Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage-foam cell formation. Int. J. Biol. Sci. 2016, 12, 812–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chen, K.; Zhang, F.; Peng, K.; Wang, Z.; Yang, D.; Yang, Y. TRPA1 regulates macrophages phenotype plasticity and atherosclerosis progression. Atherosclerosis 2020, 301, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.; Silverberg, J.I. Epidemiology of childhood atopic dermatitis. Clin. Dermatol. 2015, 33, 281–288. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Kahremany, S.; Hofmann, L.; Harari, M.; Gruzman, A.; Cohen, G. Pruritus in psoriasis and atopic dermatitis: Current treatments and new perspectives. Pharmacol. Rep. 2021, 73, 443–453. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Saarnilehto, M.; Chapman, H.; Savinko, T.; Lindstedt, K.; Lauerma, A.I.; Koivisto, A. Contact sensitizer 2,4-dinitrochlorobenzene is a highly potent human TRPA1 agonist. Allergy 2014, 69, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, C.; Zhou, W.; Ma, X.; Pu, X.; Zeng, Y.; Zhou, W.; Lv, F. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. 2021, 266, 118906. [Google Scholar] [CrossRef]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A.; et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Wheeler, J.J.; Pitake, S.; Ding, H.; Jiang, C.; Fukuyama, T.; Paps, J.S.; Ralph, P.; Coyne, J.; Parkington, M.; et al. Periostin activation of integrin receptors on sensory neurons induces allergic itch. Cell Rep. 2020, 31, 107472. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, D.; Follansbee, T.; Wu, X.; Yu, S.; Wang, B.; Shi, Z.; Domocos, D.T.; Carstens, M.; Carstens, E.; et al. Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice. J. Cell. Mol. Med. 2019, 23, 4819–4828. [Google Scholar] [CrossRef] [Green Version]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The genetics of chronic itch: Gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [Green Version]
- Kemény, Á.; Kodji, X.; Horváth, S.; Komlódi, R.; Szőke, É.; Sándor, Z.; Perkecz, A.; Gyömörei, C.; Sétáló, G.; Kelemen, B.; et al. TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice. J. Investig. Dermatol. 2018, 138, 1774–1784. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zhou, Q.; Wen, C.; Hu, J.; Li, H.; Zhao, M.; Zhao, H. Mustard seed (Sinapis Alba Linn) attenuates imiquimod-induced psoriasiform inflammation of BALB/c mice. J. Dermatol. 2013, 40, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Saghy, E.; Sipos, E.; Acs, P.; Bolcskei, K.; Pohoczky, K.; Kemeny, A.; Sandor, Z.; Szoke, E.; Setalo, G., Jr.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination-A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Lee, H.T.; Lin, H.C.; Tsay, H.J.; Tsai, F.C.; Shyue, S.K.; Lee, T.S. Role of transient receptor potential ankyrin 1 channels in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural alterations and morphological changes are attenuated by the lack of TRPA1 receptors in the cuprizone-induced demyelination model in mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef]
- Dalenogare, D.P.; Theisen, M.C.; Peres, D.S.; Fialho, M.F.P.; Luckemeyer, D.D.; Antoniazzi, C.T.D.; Kudsi, S.Q.; Ferreira, M.A.; Ritter, C.D.S.; Ferreira, J.; et al. TRPA1 activation mediates nociception behaviors in a mouse model of relapsing-remitting experimental autoimmune encephalomyelitis. Exp. Neurol. 2020, 328, 113241. [Google Scholar] [CrossRef]
- Ritter, C.; Dalenogare, D.P.; de Almeida, A.S.; Pereira, V.L.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.; Kudsi, S.Q.; Ferreira, J.; et al. Nociception in a progressive multiple sclerosis model in mice is dependent on spinal TRPA1 channel activation. Mol. Neurobiol. 2020, 57, 2420–2435. [Google Scholar] [CrossRef]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.; Oulidi, A.; Boop, F.A.; Feng, Y.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015, 8, ra2. [Google Scholar] [CrossRef] [Green Version]
- Pires, P.W.; Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. eLife 2018, 7, e35316. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Nabissi, M. TRP channels: New potential therapeutic approaches in CNS neuropathies. CNS Neurol. Disord. Drug Targets 2013, 12, 274–293. [Google Scholar] [CrossRef]
- Probst, A.; Langui, D.; Ulrich, J. Alzheimer’s disease: A description of the structural lesions. Brain Pathol. 1991, 1, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Muller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef]
- Ferreiro, E.; Oliveira, C.R.; Pereira, C.M.F. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008, 30, 331–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabrook, T.J.; Jiang, L.; Maier, M.; Lemere, C.A. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 2006, 53, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Yang, E.; Kaddoumi, A. Role of endothelial TRPA1 expression in blood-brain barrier dysfunction. Alzheimer’s Dement. 2021, 17 (Suppl. S2), e058550. [Google Scholar] [CrossRef]
- Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Pernet-Gallay, K.; Buisson, A.; Albrieux, M. Astrocyte-neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 2021, 145, 388–405. [Google Scholar] [CrossRef]
- Surathi, P.; Jhunjhunwala, K.; Yadav, R.; Pal, P.K. Research in Parkinson’s disease in India: A review. Ann. Indian Acad. Neurol. 2016, 19, 9–20. [Google Scholar] [CrossRef]
- Cacabelos, R. Parkinson’s disease: From pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Mandel, S.; Grunblatt, E.; Riederer, P.; Gerlach, M.; Levites, Y.; Youdim, M.B. Neuroprotective strategies in Parkinson’s disease: An update on progress. CNS Drugs 2003, 17, 729–762. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, L.; Compagnion, A.C.; Sauvage, M.C.; Bemelmans, A.P.; Leclere-Turbant, S.; Cirotteau, V.; Tohme, M.; Beke, A.; Trichet, M.; Bazin, V.; et al. TLR9 activation via microglial glucocorticoid receptors contributes to degeneration of midbrain dopamine neurons. Nat. Commun. 2018, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, Y. TRPC channels and programmed cell death. Adv. Exp. Med. Biol. 2017, 976, 47–60. [Google Scholar] [CrossRef]
- Duitama, M.; Vargas-Lopez, V.; Casas, Z.; Albarracin, S.L.; Sutachan, J.J.; Torres, Y.P. TRP channels role in pain associated with neurodegenerative diseases. Front. Neurosci. 2020, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, A.; Zheng, L.; Tambe, M.A.; Strathearn, K.E.; Acosta, G.; Hubers, S.A.; Liu, F.; Herr, S.A.; Tang, J.; Truong, A.; et al. Acrolein-mediated neuronal cell death and alpha-synuclein aggregation: Implications for Parkinson’s disease. Mol. Cell. Neurosci. 2018, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Shi, R. Dimercaprol is an acrolein scavenger that mitigates acrolein-mediated PC-12 cells toxicity and reduces acrolein in rat following spinal cord injury. J. Neurochem. 2017, 141, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Lin, Y.; Jiao, Y.; Herr, S.A.; Tang, J.; Rogers, E.; Chen, Z.; Shi, R. Acrolein scavenger dimercaprol offers neuroprotection in an animal model of Parkinson’s disease: Implication of acrolein and TRPA1. Transl. Neurodegener. 2021, 10, 13. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lin, H.C.; Zhao, W.Z.; Huang, H.J.; Lo, Y.L.; Wang, H.T.; Lin, A.M. Acrolein acts as a neurotoxin in the nigrostriatal dopaminergic system of rat: Involvement of alpha-synuclein aggregation and programmed cell death. Sci. Rep. 2017, 7, 45741. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.P.; Zhang, Z.H.; Zhang, L.H.; Rui, H.C. Neuroprotective role of MicroRNA-22 in a 6-hydroxydopamine-induced cell model of parkinson’s disease via regulation of its target gene TRPM7. J. Mol. Neurosci. 2016, 60, 445–452. [Google Scholar] [CrossRef]
- Chung, K.K.; Freestone, P.S.; Lipski, J. Expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J. Neurophysiol. 2011, 106, 2865–2875. [Google Scholar] [CrossRef]
- Thapak, P.; Vaidya, B.; Joshi, H.C.; Singh, J.N.; Sharma, S.S. Therapeutic potential of pharmacological agents targeting TRP channels in CNS disorders. Pharmacol. Res. 2020, 159, 105026. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; Freeman, C.; Hunt, S.E.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, L.; Prone, V. Headache in multiple sclerosis and autoimmune disorders. Neurol. Sci. 2015, 36 (Suppl. S1), 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Caminero, A.B.; Herbert, J.; Lipton, R.B. Tension-type headache and migraine in multiple sclerosis. Curr. Pain Headache Rep. 2010, 14, 441–448. [Google Scholar] [CrossRef]
- La Mantia, L. Headache and multiple sclerosis: Clinical and therapeutic correlations. Neurol. Sci. 2009, 30 (Suppl. S1), S23–S26. [Google Scholar] [CrossRef]
- De Logu, F.; Landini, L.; Janal, M.N.; Li Puma, S.; De Cesaris, F.; Geppetti, P.; Nassini, R. Migraine-provoking substances evoke periorbital allodynia in mice. J. Headache Pain 2019, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Hohlfeld, R.; Dornmair, K.; Meinl, E.; Wekerle, H. The search for the target antigens of multiple sclerosis, part 1: Autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2016, 15, 198–209. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T cells driving multiple sclerosis: Identity, mechanisms and potential triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 2008, 18, 86–95. [Google Scholar] [CrossRef]
- van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.D.; van der Valk, P.; de Vries, H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudi, V.; Škuljec, J.; Yildiz, Ö.; Frichert, K.; Skripuletz, T.; Moharregh-Khiabani, D.; Voss, E.; Wissel, K.; Wolter, S.; Stangel, M. Spatial and temporal profiles of growth factor expression during CNS demyelination reveal the dynamics of repair priming. PLoS ONE 2011, 6, e22623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Z.; Liu, L.; Spangler, R.; Spear, C.; Wang, C.; Gulen, M.F.; Veenstra, M.; Ouyang, W.; Ransohoff, R.M.; Li, X. IL-17-induced Act1-mediated signaling is critical for cuprizone-induced demyelination. J. Neurosci. 2012, 32, 8284–8292. [Google Scholar] [CrossRef] [PubMed]
- Zeger, M.; Popken, G.; Zhang, J.; Xuan, S.; Lu, Q.R.; Schwab, M.H.; Nave, K.A.; Rowitch, D.; D’Ercole, A.J.; Ye, P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia 2007, 55, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Mihai, D.P.; Nitulescu, G.M.; Ion, G.N.D.; Ciotu, C.I.; Chirita, C.; Negres, S. Computational drug repurposing algorithm targeting TRPA1 calcium channel as a potential therapeutic solution for multiple sclerosis. Pharmaceutics 2019, 11, 446. [Google Scholar] [CrossRef] [Green Version]
- Dalenogare, D.P.; Ritter, C.; Bellinaso, F.R.A.; Kudsi, S.Q.; Pereira, G.C.; Fialho, M.F.P.; Luckemeyer, D.D.; Antoniazzi, C.T.D.; Landini, L.; Ferreira, J.; et al. Periorbital nociception in a progressive multiple sclerosis mouse model is dependent on TRPA1 channel activation. Pharmaceuticals 2021, 14, 831. [Google Scholar] [CrossRef]
- Peres, D.S.; Theisen, M.C.; Fialho, M.F.P.; Dalenogare, D.P.; Rodrigues, P.; Kudsi, S.Q.; Bernardes, L.B.; Ruviaro da Silva, N.A.; Luckemeyer, D.D.; Sampaio, T.B.; et al. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 2021, 175, 1–15. [Google Scholar] [CrossRef]
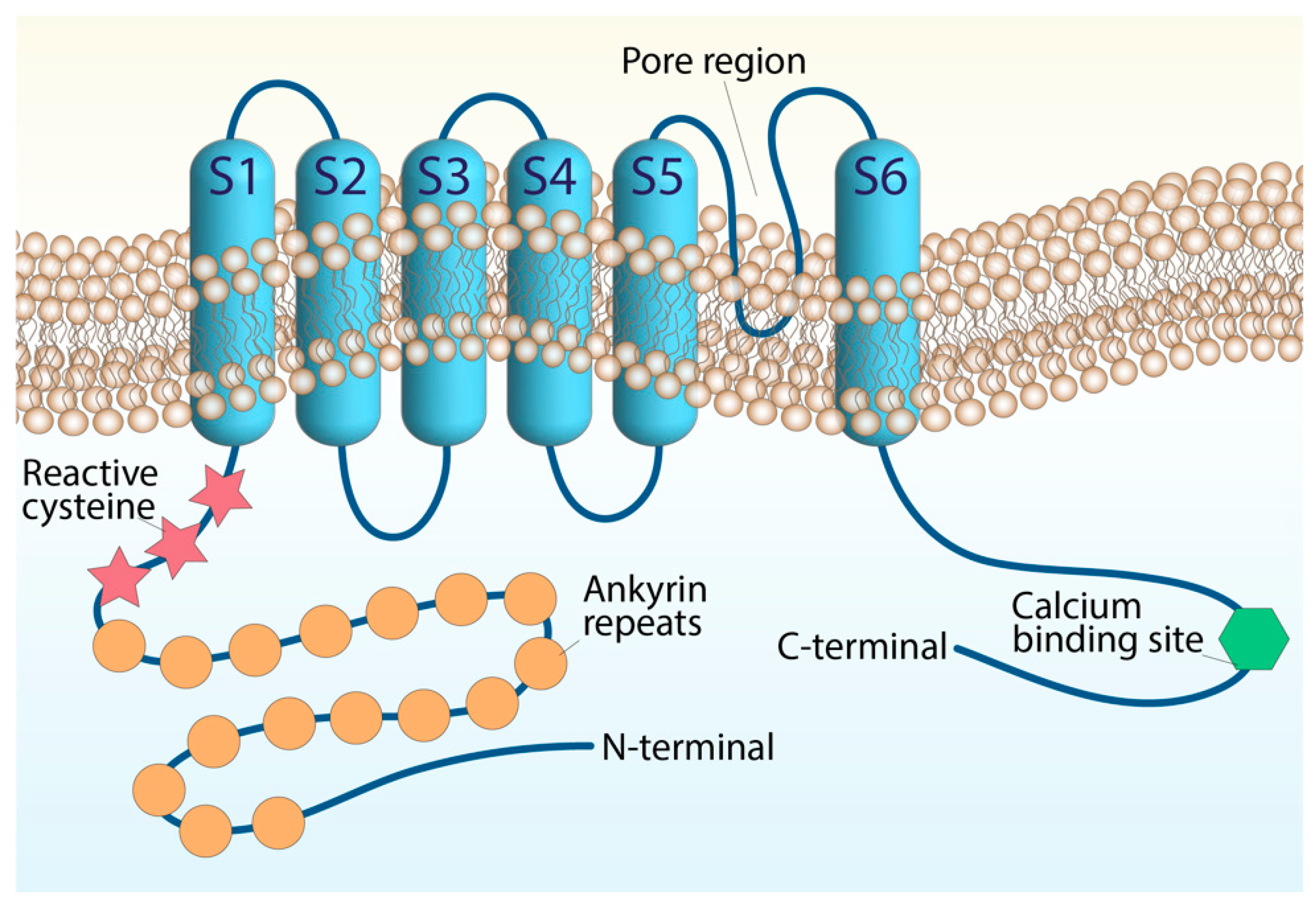
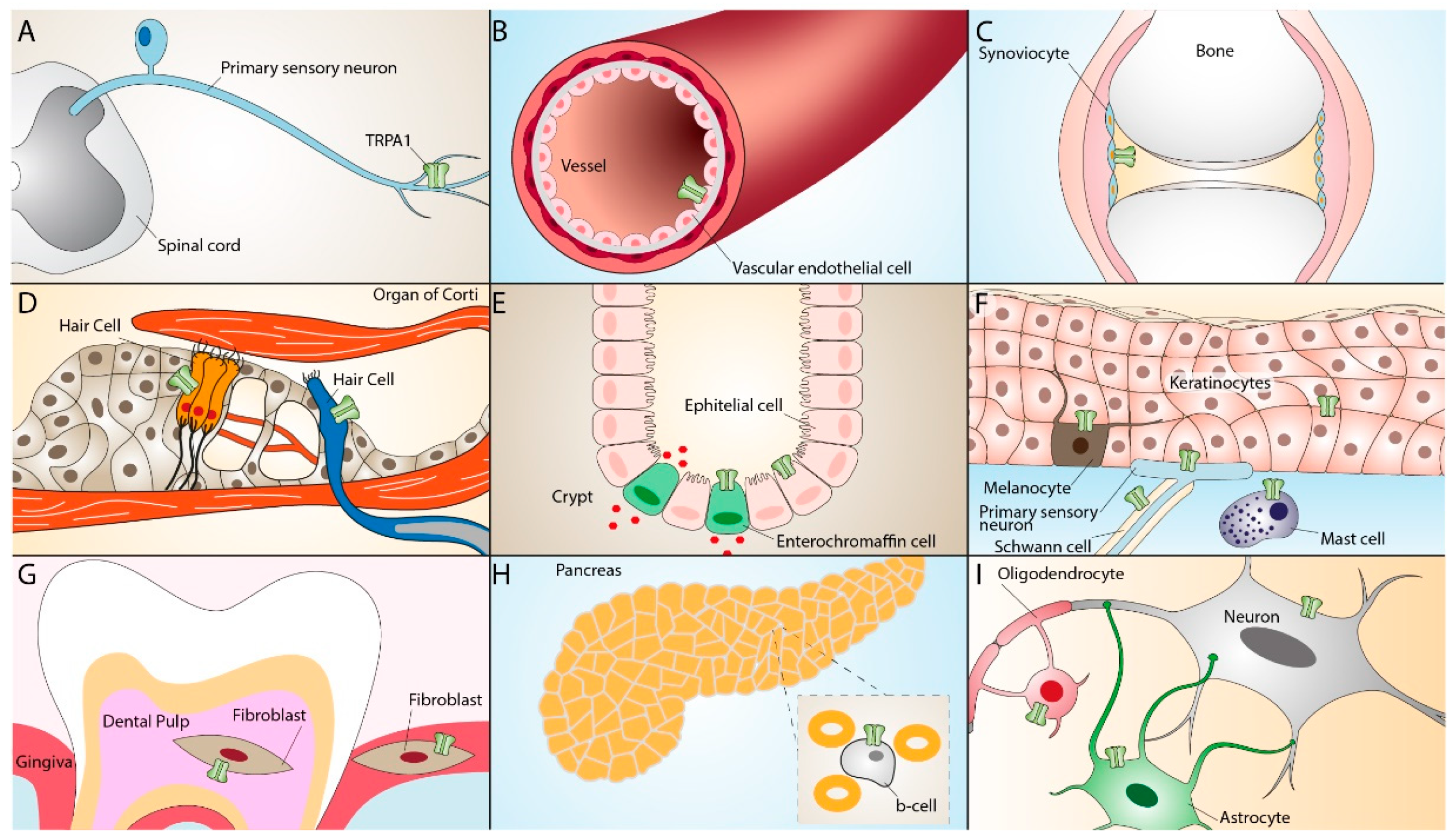
| Inflammatory Diseases | TRPA1 Distribution | TRPA1 Activation-Dependent Effect | References |
|---|---|---|---|
| Asthma and COPD | Vagal sensory neurons, lung fibroblasts, and epithelial cells | Vagal nerve activation, cough, bronchoconstriction, airway neurogenic inflammation | [40,74,75,76,77,78,79,80,84] |
| Rheumatoid arthritis | Peripheral blood leukocytes, synovial fibroblasts | Increase in cell viability and proliferation, release of inflammatory mediators | [94,95,98] |
| Endometriosis | Peritoneum nociceptive neurons, stromal and epithelial cells of ectopic endometrium | Increase in Ca2+ responses and oxidative stress, increase in pain hypersensitivity | [108,109,110] |
| Inflammatory bowel disease | Extrinsic and enteric neurons, neuroendocrine cells, colonic tissue CD4+ T cells | Increase in pain hypersensitivity, release of proinflammatory neuropeptides, cytokines, and chemokines | [120,121,122,123,124] |
| Atherosclerosis | Macrophages in atherosclerosis plaque | M1 macrophages polarization, calcium overload, mitochondria injury, increase in IL-1β secretion, and oxidative stress | [135,136,137,138,139] |
| Psoriasis and atopic dermatitis | Mast cells, dermal sensory nerve fibers, keratinocytes, melanocytes | Release of inflammatory cytokines, pruritus | [48,149,150,151,156,157] |
| Alzheimer’s and Parkinson’s diseases | Astrocytes, oligodendrcocytes, cerebral artery endothelium, dopaminergic neurons | Increase in Ca2+ response, astrocyte activation, increased levels of Aβ peptide and neuroinflammation, increase in oxidative stress | [51,158,159,163,164,165,173,179,180,181,182,183] |
| Multiple Sclerosis | Astrocytes, oligodendrocytes | Modulation of pro-apoptotic pathways, increased Ca2+ influx, neuroglial activation, periorbital allodynia | [51,158,198,199,200,201,202] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. https://doi.org/10.3390/ijms23094529
Landini L, Souza Monteiro de Araujo D, Titiz M, Geppetti P, Nassini R, De Logu F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? International Journal of Molecular Sciences. 2022; 23(9):4529. https://doi.org/10.3390/ijms23094529
Chicago/Turabian StyleLandini, Lorenzo, Daniel Souza Monteiro de Araujo, Mustafa Titiz, Pierangelo Geppetti, Romina Nassini, and Francesco De Logu. 2022. "TRPA1 Role in Inflammatory Disorders: What Is Known So Far?" International Journal of Molecular Sciences 23, no. 9: 4529. https://doi.org/10.3390/ijms23094529
APA StyleLandini, L., Souza Monteiro de Araujo, D., Titiz, M., Geppetti, P., Nassini, R., & De Logu, F. (2022). TRPA1 Role in Inflammatory Disorders: What Is Known So Far? International Journal of Molecular Sciences, 23(9), 4529. https://doi.org/10.3390/ijms23094529






