The Role of Inflammasomes in Glomerulonephritis
Abstract
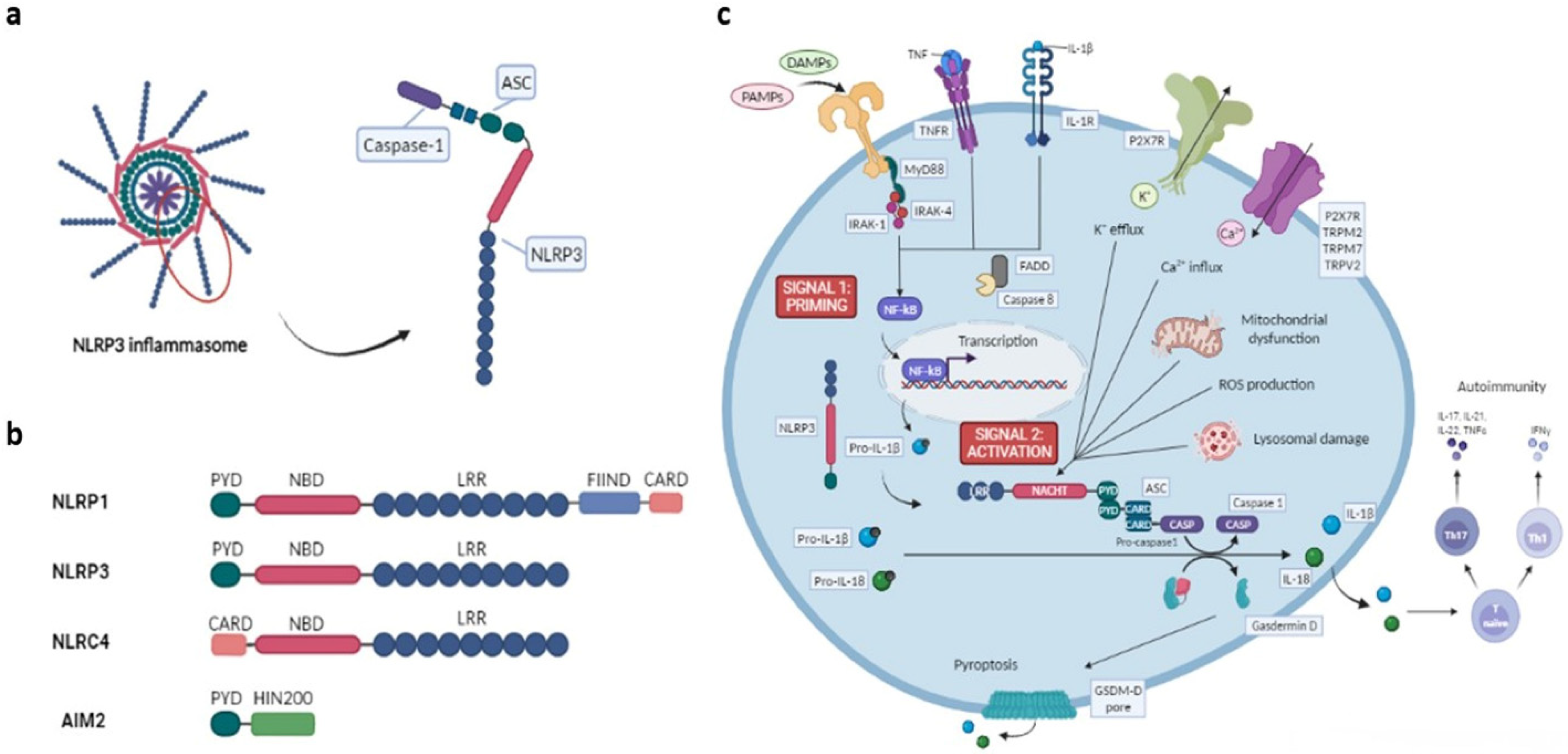
1. The Inflammasome
2. NLR Family Inflammasomes
2.1. NLRP Subfamily
2.2. IPAF-NAIP Subfamily
3. Non-NLR Family Inflammasomes
4. Mechanisms of NLRP3 Inflammasome Activation
5. Inflammasome Effector Functions
6. The Role of the Inflammasome in Adaptive Immunity and Autoimmunity
7. Inflammasome Involvement in Autoimmune Kidney Diseases
7.1. Lupus Nephritis
7.1.1. In Vitro Model
7.1.2. Animal Model
7.1.3. Human Model
7.2. ANCA Glomerulonephritis
7.2.1. In Vitro Model
7.2.2. Animal Model
7.2.3. Human Model
7.3. IgA Nephropathy
7.3.1. Animal Model
7.3.2. Human/In Vitro Model
7.4. Anti-Glomerular Basement Membrane Glomerulonephritis
Animal Model
8. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathinam, V.; Chan, F.K. Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Kanneganti, T.D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef]
- Zhang, K.-J.; Wu, Q.; Jiang, S.-M.; Ding, L.; Liu, C.-X.; Xu, M.; Wang, Y.; Zhou, Y.; Li, L. Pyroptosis: A New Frontier in Kidney Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 6686617. [Google Scholar] [CrossRef]
- Chi, K.; Geng, X.; Liu, C.; Cai, G.; Hong, Q. Research Progress on the Role of Inflammasomes in Kidney Disease. Mediat. Inflamm. 2020, 2020, 8032797. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Sharma, M.; De Alba, E. Structure, activation and regulation of NLRP3 and AIM2 inflammasomes. Int. J. Mol. Sci. 2021, 22, 872. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.-S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef]
- Wen, H.; Miao, E.A.; Ting, J.P.-Y. Mechanisms of NOD-like Receptor-Associated Inflammasome Activation. Immunity 2013, 39, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Taabazuing, C.Y.; Griswold, A.R.; Bachovchin, D.A. The NLRP1 and CARD8 inflammasomes. Immunol. Rev. 2020, 297, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bauernfried, S.; Scherr, M.; Pichlmair, A.; Duderstadt, K.E.; Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 2021, 371, eabd0811. [Google Scholar] [CrossRef] [PubMed]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. The Role of NLRP1, NLRP3, and AIM2 Inflammasomes in Psoriasis: Review. Int. J. Mol. Sci. 2021, 22, 5898. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, C.; Wang, S.; Mo, L.; Yang, G.D.; Hu, J.; Zhang, F. Role of NLRP3 and NLRP1 inflammasomes signaling pathways in pathogenesis of rheumatoid arthritis. Asian Pac. J. Trop. Med. 2014, 7, 827–831. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, C.; Yang, Z.; Wei, Q.; Mu, K.; Zhang, Y.; Zhao, W.; Wang, X.; Huai, W.; Han, L. Deregulated NLRP3 and NLRP1 Inflammasomes and Their Correlations with Disease Activity in Systemic Lupus Erythematosus. J. Rheumatol. 2013, 41, 444–452. [Google Scholar] [CrossRef]
- Haneklaus, M.; O’Neill, L.; Coll, R. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: Recent developments. Curr. Opin. Immunol. 2013, 25, 40–45. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef]
- De Torre-Minguela, C.; del Castillo, P.M.; Pelegrín, P. The NLRP3 and pyrin inflammasomes: Implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, H.; Huang, Y.; Zhang, H.; Wang, S.; Gaskin, F.; Yang, N.; Fu, S.M. Lupus Nephritis: Glycogen Synthase Kinase 3β Promotion of Renal Damage Through Activation of the NLRP3 Inflammasome in Lupus-Prone Mice. Arthritis Rheumatol. 2014, 67, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Canna, S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018, 281, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Rauch, I. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Asp. Med. 2020, 76, 100863. [Google Scholar] [CrossRef]
- Lee, B.L.; Mirrashidi, K.; Stowe, I.B.; Kummerfeld, S.K.; Watanabe, C.; Haley, B.; Cuellar, T.L.; Reichelt, M.; Kayagaki, N. ASC- A nd caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci. Rep. 2018, 8, 3788. [Google Scholar] [CrossRef]
- Cho, S.X.; Vijayan, S.; Yoo, J.S.; Watanabe, T.; Ouda, R.; An, N.; Kobayashi, K. MHC class I transactivator NLRC5 in host immunity, cancer and beyond. Immunology 2021, 162, 252–261. [Google Scholar] [CrossRef]
- Davis, B.K.; Roberts, R.A.; Huang, M.T.; Willingham, S.B.; Conti, B.J.; Brickey, W.J.; Barker, B.R.; Kwan, M.; Taxman, D.J.; Accavitti-Loper, M.-A.; et al. Cutting Edge: NLRC5-Dependent Activation of the Inflammasome. J. Immunol. 2011, 186, 1333–1337. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Walker, J. Innate Immune Receptors. NLR Proteins; Springer Nature: Hatfield, UK, 2016. [Google Scholar]
- Carneiro, L.; Magalhaes, J.; Tattoli, I.; Philpott, D.; Travassos, L. Nod-like proteins in inflammation and disease. J. Pathol. 2008, 214, 136–148. [Google Scholar] [CrossRef]
- Velloso, F.J.; Lima, M.T.; Anschau, V.; Sogayar, M.C.; Correa, R.G. NOD-like receptors: Major players (and targets) in the interface between innate immunity and cancer. Biosci. Rep. 2019, 39, BSR20181709. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T. AIM2 inflammasome in infection, cancer and autoimmunity: Role in DNA sensing, inflammation and innate immunity. Eur. J. Immunol. 2016, 46, 269–280. [Google Scholar] [CrossRef]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Lilue, J.; Stavrou, S.; Moran, E.A.; Ross, S.R. AIM2-Like Receptors Positively and Negatively Regulate the Interferon Response Induced by Cytosolic DNA. mBio 2017, 8, e00944-17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Karki, R.; Kanneganti, T. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur. J. Immunol. 2019, 49, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Xue, Y.; Tuipulotu, D.E.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Patel, M.N.; Carroll, R.G.; Galván-Peña, S.; Mills, E.L.; Olden, R.; Triantafilou, M.; Wolf, A.I.; Bryant, C.E.; Triantafilou, K.; Masters, S.L. Inflammasome Priming in Sterile Inflammatory Disease. Trends Mol. Med. 2017, 23, 165–180. [Google Scholar] [CrossRef]
- Tschopp, J. Mitochondria: Sovereign of inflammation? Eur. J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J.; Behl, B.; Banerjee, I.; Rathinam, V.A. Emerging Insights into Noncanonical Inflammasome Recognition of Microbes. J. Mol. Biol. 2017, 430, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.; Stowe, I.B.; Ramani, S.; Gonzalez, L.C.; Akashi-takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Forsberg, L.S.; et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.B.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, L.; Wu, T.; Xi, J.; Han, Y.; Yang, X.; Zhang, D.; Fang, Q.; Tang, B. NLRP3 Activation Was Regulated by DNA Methylation Modification during Mycobacterium tuberculosis Infection. BioMed. Res. Int. 2016, 2016, 4323281. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Fabi, C.; Bellet, M.M.; Costantini, C.; Nunziangeli, L.; Romani, L.; Brancorsini, S. Epigenetic Mechanisms of Inflammasome Regulation. Int. J. Mol. Sci. 2020, 21, 5758. [Google Scholar] [CrossRef]
- Moretti, J.; Blander, J.M. Increasing complexity of NLRP3 inflammasome regulation. J. Leukoc. Biol. 2021, 109, 561–571. [Google Scholar] [CrossRef]
- Niu, T.; De Rosny, C.; Chautard, S.; Rey, A.; Patoli, D.; Groslambert, M.; Cosson, C.; Lagrange, B.; Zhang, Z.; Visvikis, O.; et al. NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat. Commun. 2021, 12, 5862. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef]
- Chauhan, D.; Walle, L.V.; Lamkanfi, M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 2020, 297, 123–138. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Kanneganti, T. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int. Immunol. 2017, 29, 201–210. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, V.; Thygesen, S.J.; Sester, D.P.; Idris, A.; Cridland, J.A.; Vajjhala, P.R.; Roberts, T.L.; Schroder, K.; Vince, J.E.; Hill, J.M.; et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013, 20, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Fitzgerald, K.A. Inflammasome complexes: Emerging mechanisms and effector functions Inflammasome complex formation and canonical functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Ciraci, C.; Janczy, J.R.; Sutterwala, F.S.; Cassel, S. Control of innate and adaptive immunity by the inflammasome. Microbes Infect. 2012, 14, 1263–1270. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, W.; Meng, G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 2011, 11, 549–554. [Google Scholar] [CrossRef]
- Hutton, H.L.; Ooi, J.; Holdsworth, S.R.; Kitching, A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016, 21, 736–744. [Google Scholar] [CrossRef]
- Papp, G.; Boros, P.; Nakken, B.; Szodoray, P.; Zeher, M. Regulatory immune cells and functions in autoimmunity and transplantation immunology. Autoimmun. Rev. 2017, 16, 435–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Li, W.; Zhao, Y. NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases. Front. Immunol. 2021, 12, 4166. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J. Autoimmun. 2019, 103, 102299. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Z.; Zhou, Z. miRNAs: Novel regulators of autoimmunity-mediated pancreatic β-cell destruction in type 1 diabetes. Cell. Mol. Immunol. 2017, 14, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Jin, G.; Fang, J.; Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 2019, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Reizis, B. Dendritic cells: Arbiters of immunity and immunological tolerance. Cold Spring Harb. Perspect. Biol. 2012, 4, a007401. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Leal, V.N.C.; De Lima, D.S.; Reis, E.C.; Pontillo, A. Inflammasome genetics and complex diseases: A comprehensive review. Eur. J. Hum. Genet. 2020, 28, 1307–1321. [Google Scholar] [CrossRef]
- Davidson, S.; Steiner, A.; Harapas, C.R.; Masters, S.L. An Update on Autoinflammatory Diseases: Interferonopathies. Curr. Rheumatol. Rep. 2018, 20, 38. [Google Scholar] [CrossRef]
- Yang, C.A.; Chiang, B.L. Inflammasomes and human autoimmunity: A comprehensive review. J. Autoimmun. 2015, 61, 1–8. [Google Scholar] [CrossRef]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Thacker, S.G.; Berthier, C.C.; Cohen, C.D.; Kretzler, M.; Kaplan, M. Inflammasome Activation of IL-18 Results in Endothelial Progenitor Cell Dysfunction in Systemic Lupus Erythematosus. J. Immunol. 2011, 187, 6143–6156. [Google Scholar] [CrossRef]
- Hatef, M.R.; Sahebari, M.; Rezaieyazdi, Z.; Nakhjavani, M.R.; Mahmoudi, M. Stronger Correlation between Interleukin 18 and Soluble Fas in Lupus Nephritis Compared with Mild Lupus. ISRN Rheumatol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Perez-Alamino, R.; Cuchacovich, R.; Espinoza, L.R.; Porretta, C.P.; Zea, A. Role of Inflammasome Activation in Systemic Lupus Erythematosus: Are Innate Immune Cells Activated? Reum. Clin. 2021, 17, 187–191. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinder, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M. Neutrophil Extracelullar Trap-associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Yalavarthi, S.; Zhao, W.; Hodgin, J.B.; Reed, T.J.; Tsuji, N.M.; Kaplan, M. An essential role for caspase-1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 2014, 66, 152–162. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Dai, C.; Wang, H.; Zhang, H.; Huang, Y.; Wang, S.; Gaskin, F.; Yang, N.; Fu, S.M. P2X7Blockade Attenuates Murine Lupus Nephritis by Inhibiting Activation of the NLRP3/ASC/Caspase 1 Pathway. Arthritis Care Res. 2013, 65, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.M.; Tam, F.W.K.; Lai, P.-C.; Tarzi, R.M.; Burnstock, G.; Pusey, C.D.; Cook, H.T.; Unwin, R.J. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol. Dial. Transplant. 2006, 22, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Jia, S.; Yao, P.; Yang, Q.; Zhang, Y. High-Mobility Group Box 1 Inhibition Alleviates Lupus-Like Disease in BXSB Mice. Scand. J. Immunol. 2014, 79, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Zheng, L.; Sinniah, R.; Hsu, S. Pathogenic role of NF-κB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J. Histochem. Cytochem. 2008, 56, 517–529. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Huang, Y.; Wang, H.; Wang, S.; Zhao, C.; Liang, Y.; Yang, N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int. Immunopharmacol. 2013, 17, 116–122. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Xu, W.; Yin, Z.; Gao, X.; Xiong, S. AIM2 facilitates the apoptotic DNA-induced systemic lupus erythematosus via arbitrating macrophage functional maturation. J. Clin. Immunol. 2013, 33, 925–937. [Google Scholar] [CrossRef]
- Yin, Q.; Sester, D.P.; Tian, Y.; Hsiao, Y.-S.; Lu, A.; Cridland, J.A.; Sagulenko, V.; Thygesen, S.J.; Choubey, D.; Hornung, V.; et al. Molecular Mechanism for p202-Mediated Specific Inhibition of AIM2 Inflammasome Activation. Cell Rep. 2013, 4, 327–339. [Google Scholar] [CrossRef]
- Pontillo, A.; Girardelli, M.; Kamada, A.J.; Pancotto, J.A.T.; Donadi, E.A.; Crovella, S.; Sandrin-Garcia, P. Polimorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity 2012, 45, 271–278. [Google Scholar] [CrossRef]
- Pontillo, A.; Vendramin, A.; Catamo, E.; Fabris, A.; Crovella, S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Off. J. Am. Coll. Gastroenterol. ACG 2011, 106, 539–544. [Google Scholar] [CrossRef]
- Da Cruz, H.L.A.; Cavalcanti, C.A.J.; Silva, J.D.A.; De Lima, C.A.D.; Fragoso, T.S.; Barbosa, A.D.; Dantas, A.T.; Mariz, H.D.A.; Duarte, A.L.B.P.; Pontillo, A.; et al. Differential expression of the inflammasome complex genes in systemic lupus erythematosus. Immunogenetics 2020, 72, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Nachman, P. ANCA glomerulonephritis and vasculitis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, J.; Martínez-Valenzuela, L.; Martin-Capon, I.; Bordignon-Draibe, J. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: Toward an Individualized Approach. Nephron 2022, 146, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Hewins, P.; Morgan, M.D.; Holden, N.; Neil, D.; Williams, J.M.; Savage, C.; Harper, L. IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int. 2006, 69, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cannetti, C.A.; Leung, B.P.; Culshaw, S.; McInnes, I.B.; Cunha, F.Q.; Liew, F. IL-18 Enhances Collagen-Induced Arthritis by Recruiting Neutrophils Via TNF-α and Leukotriene B 4. J. Immunol. 2003, 171, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Shochet, L.; Holdsworth, S.; Kitching, A.R. Animal Models of ANCA Associated Vasculitis. Front. Immunol. 2020, 11, 525. [Google Scholar] [CrossRef]
- Schreiber, A.; Pham, C.T.N.; Hu, Y.; Schneider, W.; Luft, F.C.; Kettritz, R. Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2012, 23, 470–482. [Google Scholar] [CrossRef]
- Adkison, A.M.; Raptis, S.Z.; Kelley, D.G.; Pham, C. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Investig. 2002, 109, 363–371. [Google Scholar] [CrossRef]
- Schreiber, A.; Luft, F.C.; Kettritz, R. Phagocyte NADPH Oxidase Restrains the Inflammasome in ANCA-Induced GN. J. Am. Soc. Nephrol. 2014, 26, 411–424. [Google Scholar] [CrossRef]
- Gerderman, K.A.; Hultqvist, M.; Pizzola, A.; Zhao, M.; Nandakumar, K.S.; Mattsson, R.; Holmdahl, R. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Investig. 2007, 117, 3020–3028. [Google Scholar] [CrossRef]
- Hultgren, O.; Andersson, B.; Hahn-Zoric, M.; Almroth, G. Serum concentration of interleukin-18 is up-regulated in patients with ANCA-associated vasculitis. Autoimmunity 2007, 40, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Sumiishi, A.; Sano, K.; Fujioka, Y.; Yamada, A.; Karube, M.; Koji, H.; Arimura, Y.; Nagasawa, T. Tubulointerstitial nephritis without glomerular lesions in three patients with myeloperoxidase-ANCA-associated vasculitis. Clin. Exp. Nephrol. 2009, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Plafkin, C.; Zhong, W.; Singh, T. ANCA vasculitis presenting with acute interstitial nephritis without glomerular involvement. Clin. Nephrol.—Case Stud. 2019, 7, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Sasatomi, Y.; Watanabe, R.; Watanabe, M.; Miyake, K.; Abe, Y.; Yasuno, T.; Ito, K.; Ueki, N.; Hamauchi, A.; et al. IL-1ß promotes tubulointerstitial injury in MPO-ANCA-associated glomerulonephritis. Clin. Nephrol. 2016, 86, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Sun, X.-J.; Chen, M.; Zhao, M.-H. The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis. J. Transl. Med. 2019, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; Haas, M.; Reich, H. IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef]
- Wardle, E. Cytokine growth factors and glomerulonephritis. Nephron 1991, 57, 257–261. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2020, 361, 176–194. [Google Scholar] [CrossRef]
- Yoshioka, K.; Takemura, T.; Murakami, K.; Okada, M.; Yagi, K.; Miyazato, H.; Matsushima, K.; Maki, S. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993, 44, 825–833. [Google Scholar] [CrossRef][Green Version]
- Zou, J.-N.; Xiao, J.; Hu, S.-S.; Fu, C.-S.; Zhang, X.-L.; Zhang, Z.-X.; Lu, Y.-J.; Chen, W.-J.; Ye, Z.-B. Toll-like Receptor 4 Signaling Pathway in the Protective Effect of Pioglitazone on Experimental Immunoglobulin A Nephropathy. Chin. Med. J. 2017, 130, 906–913. [Google Scholar] [CrossRef]
- Chen, A.; Sheu, L.F.; Chou, W.Y.; Tsai, S.C.; Chang, D.M.; Liang, S.C.; Lin, F.G.; Lee, W. Interleukin-1 receptor antagonist modulates the progression of a spontaneously occurring IgA nephropathy in mice. Am. J. Kidney Dis. 1997, 30, 693–702. [Google Scholar] [CrossRef]
- Chun, J.; Chung, H.; Wang, X.; Barry, R.; Taheri, Z.M.; Platnich, J.; Ahmed, S.B.; Trpkov, K.; Hemmelgarn, B.; Benediktsson, H.; et al. NLRP3 Localizes to the Tubular Epithelium in Human Kidney and Correlates With Outcome in IgA Nephropathy. Sci. Rep. 2016, 6, 24667. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Hua, K.-F.; Chen, A.; Wei, C.-W.; Chen, W.-S.; Wu, C.-Y.; Chu, C.-L.; Yu, Y.-L.; Lo, C.-W.; Ka, S.-M. NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy. Sci. Rep. 2017, 7, srep41123. [Google Scholar] [CrossRef] [PubMed]
- McAdoo, S.P.; Pusey, C. Anti-glomerular basement membrane disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1162–1172. [Google Scholar] [CrossRef]
- Gulati, K.; McAdoo, S.P. Anti–Glomerular Basement Membrane Disease. Rheum. Dis. Clin. N.Am. 2018, 44, 651–673. [Google Scholar] [CrossRef]
- Timoshanko, J.R.; Kitching, A.R.; Iwakura, Y.; Holdsworth, S.R.; Tipping, P. Contributions of IL-1β and IL-1α to Crescentic Glomerulonephritis in Mice. J. Am. Soc. Nephrol. 2004, 15, 910–918. [Google Scholar] [CrossRef]
- Kitching, A.R.; Turner, A.L.; Wilson, G.R.A.; Semple, T.; Odobasic, D.; Timoshanko, J.R.; O’Sullivan, K.M.; Tipping, P.G.; Takeda, K.; Akira, S.; et al. IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J. Am. Soc. Nephrol. 2005, 16, 2023–2033. [Google Scholar] [CrossRef]
- Lan, H.Y.; Nikolic-Paterson, D.J.; Zarama, M.; Vannice, J.L.; Atkins, R. Suppression of experimental crescentic glomerulonephritis by deoxyspergualin. J. Am. Soc. Nephrol. 1993, 3, 1765–1774. [Google Scholar] [CrossRef]
- Tang, W.W.; Feng, L.; Vannice, J.L.; Wilson, C.B. Interleukin-1 receptor antagonist ameliorates experimental anti-glomerular basement membrane antibody-associated glomerulonephritis. J. Clin. Investig. 1994, 93, 273–279. [Google Scholar] [CrossRef][Green Version]
- Lichtnekert, J.; Kulkarni, O.P.; Mulay, S.R.; Rupanagudi, K.V.; Ryu, M.; Allam, R.; Vielhauer, V.; Muruve, D.; Lindenmeyer, M.T.; Cohen, C.D.; et al. Anti-gbm glomerulonephritis involves il-1 but is independent of nlrp3/asc inflammasome-mediated activation of caspase-1. PLoS ONE 2011, 6, e26778. [Google Scholar] [CrossRef]
| LUPUS NEPRHITIS | |
| Significant Findings | References |
| IN VITRO MODEL | |
| LL-37: | |
| LDGs have the capacity to produce NETs which increase the externalization of immunostimulatory proteins and autoantigens as LL-37, IL-17 and dsDNA. Kidneys from SLE patients are infiltrated by netting neutrophils which show LL-37 and dsDNA explaining the role of aberrant lupus neutrophils the pathogenic role of NETs. | Villanueva et al., Journal of Immunology, 2011. |
| NLRP3 is activated by NETs and the expression of the NETs-associated protein LL-37. This stimulus contributes to the production of IL1β and IL-18 causing NETosis. | Kahlenberg et al., Journal of immunology, 2013. |
| Expression of axis’s inflammasome: | |
| Isolated fresh monocytes from SLE patients increased inflammasome activation described by the elevated expression of Caspase-1, IL-1β and IL-18. | Perez-Alamino et al., Reumatologia Clinica, 2021. |
| ANIMAL MODEL | |
| P2X7: | |
| Increased expression of P2X7 has been observed in kidney biopsies from patients with SLE. | TTurner et al., Nephrology, dialysis, transplantation, 2007. |
| Upregulation of P2X7/NLRP3 in kidneys of MRL/lpr mice associates an increase in IL-1β and renal damage developing LN.P2X7 inhibition decreases autoantibodies and immune complexes deposited in the kidneys. | Zhao et al., Arthritis Rheumatology, 2013. |
| NFκB and NLPR3: | |
| The inhibition of NFκB and NLPR3 by Bay11-7082 in MRL/lpr mice reduces nephritis, the levels of IL-1β, TNF-α and anti-dsDNA and the deposition of immune complexes. | Zhao et al., International Immunopharmacology, 2013. |
| AIM2: | |
| AIM2 is augmented in macrophages induced by lymphocyte-derived apoptotic DNA. Its knock-down by siRNA ameliorates infiltration of macrophages in tissues. | Zhang et al., Journal of Clinical Immunology, 2013. |
| p202 limits AIM2. This increases INF causing susceptibility to murine lupus. | Yin et al., Cell reports, 2014. |
| Caspasa-1: | |
| The caspase-1 −/− mouse model exposed to pristane protected against the development of autoantibodies related to SLE, nephritis and the action of type I INF. | Kahlenberg et al., Arthritis and Rheumatology, 2014. |
| HMGB1: | |
| Blocking HMGB1 in BXSB mice reduces the machinery of NLPR3 and improves renal inflammation. | Zhang et al., Scandinavian Journal of immunology, 2014. |
| HUMAN MODEL | |
| NLRP1: | |
| The NLRP1 rs2670660 and NLRP1 rs12150220-rs2670660 A-G haplotype polymorphisms were associated with SLE and the event of nephritis, arthritis and rash. | Pontillo et al., Autoimmunity, 2012. |
| NLPR3, NLRP1, Caspasa-1, AIM2: | |
| The variant rs10754558 NLRP3 was more common in SLE patients with nephritis. The stimulus with LPS+ATP generated the expression of NLRP1, AIM2, CASP1 and IL1β genes, indicating that NLRP1 is responsible for the IL-β production reflected in monocytes. | da Cruz et al., Immunogenetics, 2020. |
| ANCA GLOMERULONEPHRITIS | |
| Significant Findings | References |
| IN VITRO MODEL | |
| IL-18: | |
| Il-18 expression is upregulated in patients with ANCA vasculitis. | Hewins et al., Kidney International, 2006. |
| ANIMAL MODEL | |
| NPS: | |
| NE-/PR3- mice in anti-MPO antibody-induced model reduce local cytokines and induction of NCGN. | Schreiber et al., Journal of the American Society of Nephrology, 2012. |
| NADPH oxidase: | |
| An antibody-mediated anti-MPO model, gp91phox-deficient or p47phox-deficient mice had worsening NCGN. Gp91phox-deficient/caspase-1 double-deficient mice improved NCGN, suggesting that Phox limits the activity of caspase-1 and thus of the inflammasome. | Schreiber et al., Journal of the American Nephrology, 2015. |
| HUMAN MODEL | |
| IL-18: | |
| IL-18 is elevated in the serum from patients diagnosed with ANCA vasculitis compared to healthy controls. The increase in IL-18 is regardless of MPO/PR3 levels. | Hultren et al., Autoimmunity, 2007. |
| NLRP3, NOD2, NLRC5: | |
| The investigators glimpsed the role of NLRP3 in the tubulointerstitial compartment and the correlation of IL-1β levels with the severity of tubulointerstitial injury in the glomerulus. | Tashiro et al., Clinical Nephrology, 2016. |
| NOD2, NLRP3 and NLRC5 were mostly expressed in podocytes and in infiltrating monocytes and macrophages, but barely expressed in glomeruli. | Wang et al., Journal of Translational Medicine, 2019. |
| IGA NEPHROPATHY | |
| Significant Findings | References |
| ANIMAL MODEL | |
| IL-1ra: | |
| The use of Il-1 receptor antagonist in a IgAN’s mouse model (ddY mice) ceases the exacerbation of the disease. | Chen et al., American Journal of Kidney Diseases, 1997. |
| HUMAN/IN VITRO MODEL | |
| NLRP3 was mostly expressed in the tubules with no staining in the glomerulus of normal kidneys. Nevertheless, in patients with IgAN, NLRP3 expression was detected in the glomerulus, though it was more increased in the tubules. In human kidney biopsies and in low passage human cells, they established that NLRP3 was decreased during tubular damage. Equally the immunostaining results and the NLRP3 mRNA expression confirmed the presence of NLRP3 and its subsequent loss after renal injury. | Chun et al., Scientific reports, 2016. |
| IgAN knockout NLRP3 mice model was generated. The production of IgA immune complexes was inhibited by knockout mice. NLRP3 knockout mice and the kidney-targeting delivery of shRNA of NLRP3 improve renal function. | Tsai et al., scientific reports, 2017. |
| ANTIGLOMERULAR BASEMENT MEMBRANE GLOMERULONEPHRITIS | |
| Significant Findings | References |
| ANIMAL MODEL | |
| IL-1ra: | |
| IL-1ra protects against clinical and histological worsening in a rat anti-GBM model. | Lan et al., Kidney International, 1993. |
| In a rat anti-GBM model, IL-1ra diminishs proteinuria and the expression of adhesion molecules of PMN, such as ICAM-1. | Tang et al., The Journal of Clinical Investigation, 1994. |
| IL-18 and IL-12p40: | |
| Anti-GBM mice model with IL-12p40−/−, IL-18−/− and both IL-12p40−/− and IL-18 demonstrate IL-12p40 as a crucial cytokine chain in nephritogenic Th1 responses and IL-18 as a proinflammatory local (renal) cytokine. | Kitching et al., Journal of the American Society of Nephrology, 2005. |
| IL-1βand IL-1RI: | |
| An anti-GBM IL-1β −/− and IL-1RI −/− mouse model was formed. IL-1β −/− mice demonstrated a reduction in crescent formation and cell recruitment. IL-1RI −/− mice presented less serum titers antibodies, less proteinuria and reduced serum creatinine. | Timoshanko et al., Journal of the American Society of Nephrology, 2004. |
| Anti-GBM nephritis develops independently of the NLRP3-caspase-1 axis due to the inability of glomerular cells to generate IL-1β. | Lichtnekert et al., Plos One, 2011. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton-Pampols, P.; Diaz-Requena, C.; Martinez-Valenzuela, L.; Gomez-Preciado, F.; Fulladosa, X.; Vidal-Alabro, A.; Torras, J.; Lloberas, N.; Draibe, J. The Role of Inflammasomes in Glomerulonephritis. Int. J. Mol. Sci. 2022, 23, 4208. https://doi.org/10.3390/ijms23084208
Anton-Pampols P, Diaz-Requena C, Martinez-Valenzuela L, Gomez-Preciado F, Fulladosa X, Vidal-Alabro A, Torras J, Lloberas N, Draibe J. The Role of Inflammasomes in Glomerulonephritis. International Journal of Molecular Sciences. 2022; 23(8):4208. https://doi.org/10.3390/ijms23084208
Chicago/Turabian StyleAnton-Pampols, Paula, Clara Diaz-Requena, Laura Martinez-Valenzuela, Francisco Gomez-Preciado, Xavier Fulladosa, Anna Vidal-Alabro, Joan Torras, Núria Lloberas, and Juliana Draibe. 2022. "The Role of Inflammasomes in Glomerulonephritis" International Journal of Molecular Sciences 23, no. 8: 4208. https://doi.org/10.3390/ijms23084208
APA StyleAnton-Pampols, P., Diaz-Requena, C., Martinez-Valenzuela, L., Gomez-Preciado, F., Fulladosa, X., Vidal-Alabro, A., Torras, J., Lloberas, N., & Draibe, J. (2022). The Role of Inflammasomes in Glomerulonephritis. International Journal of Molecular Sciences, 23(8), 4208. https://doi.org/10.3390/ijms23084208






