Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway
Abstract
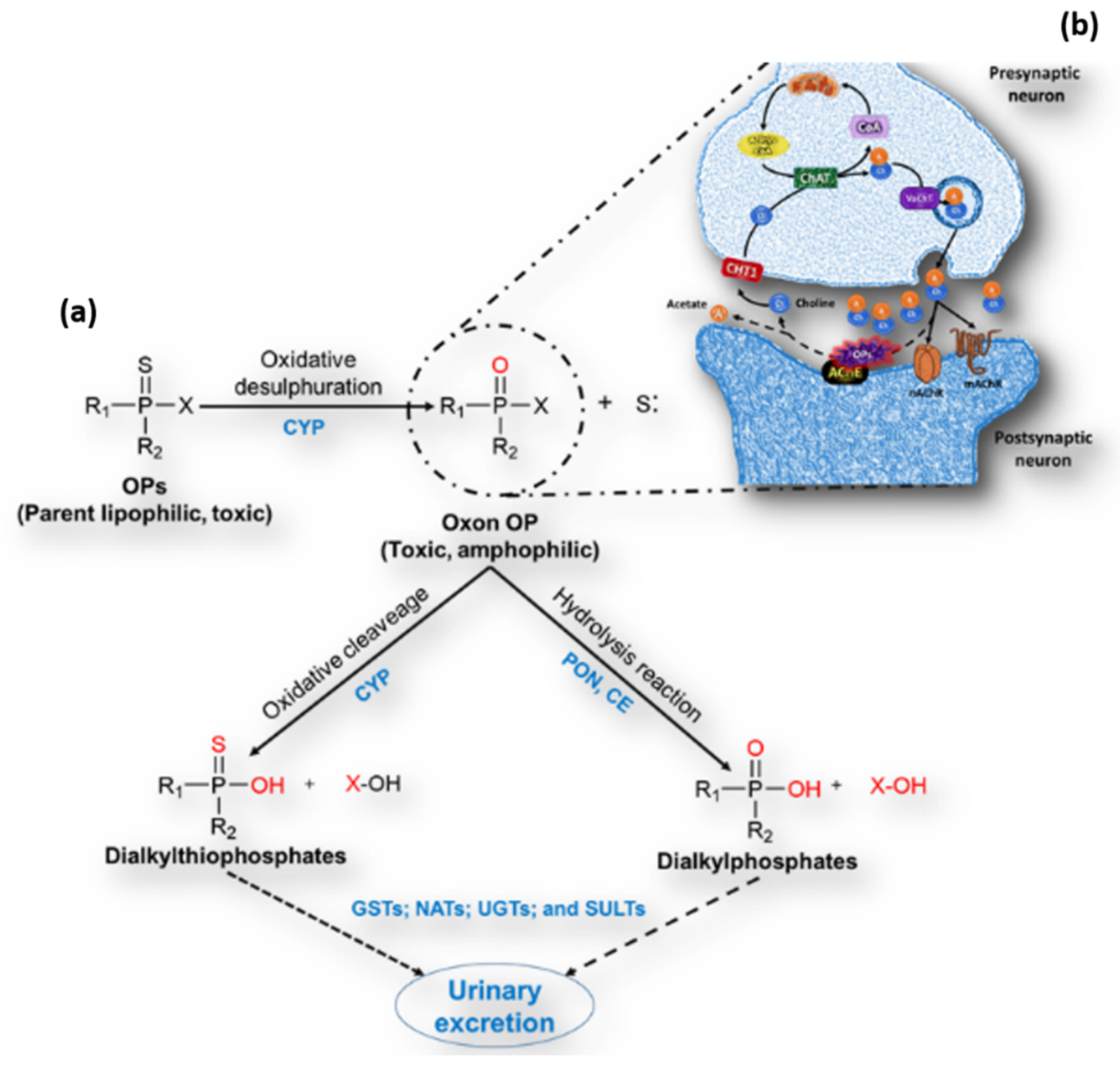
1. Organophosphorus Pesticides
Mechanism of Action of OPs and Toxicity
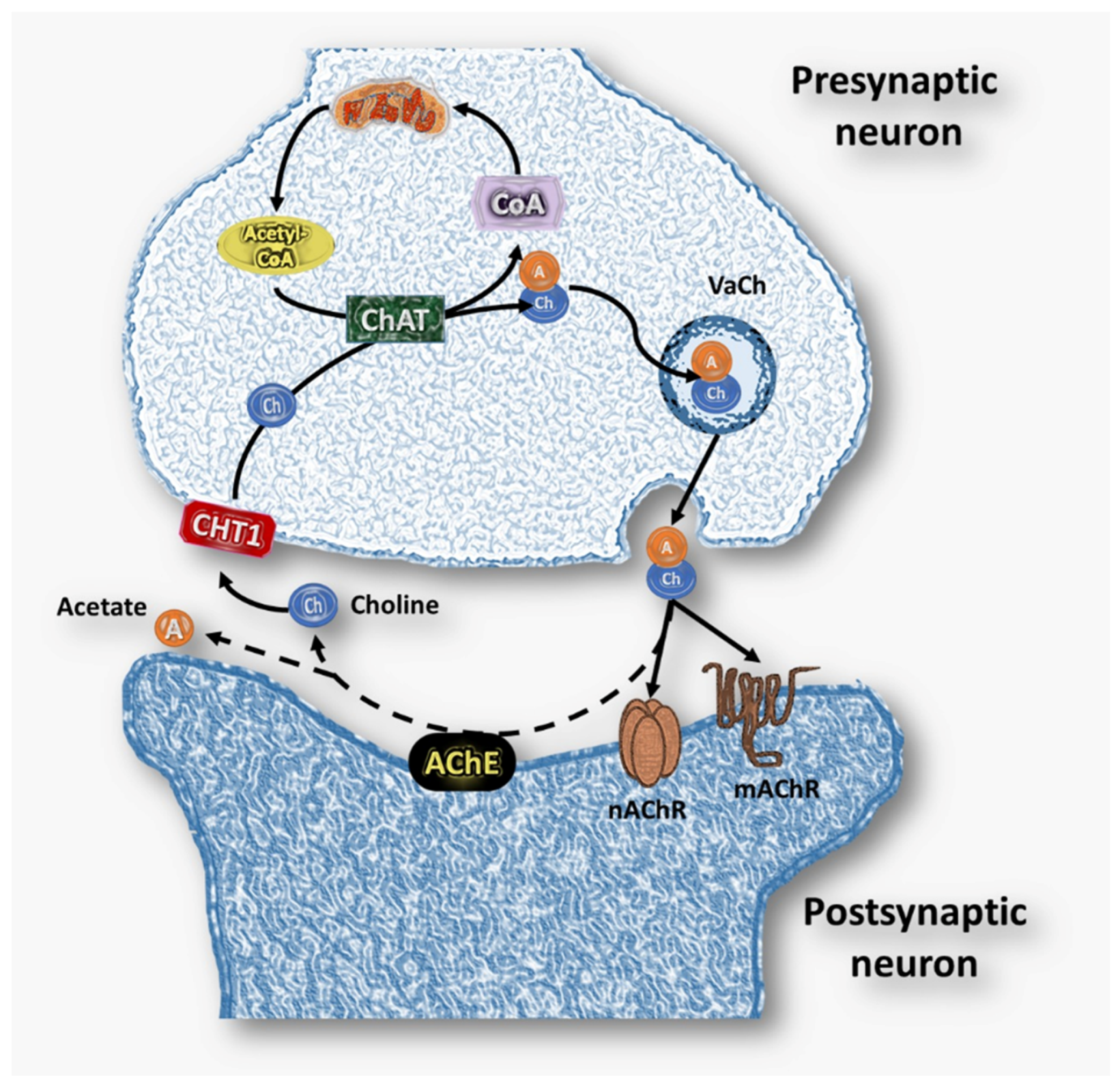
2. Cholinergic System
3. Immunotoxicity of OPs through the Cholinergic System
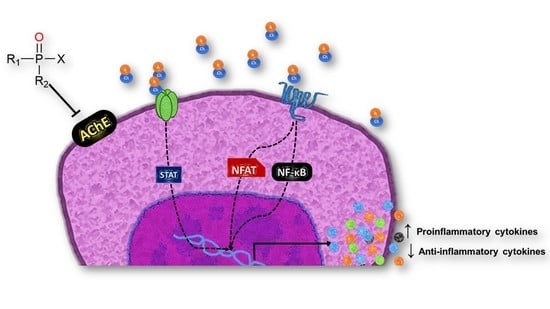
4. Cytokine-Mediated Modulation of the Inflammatory Process by OP Exposure
5. Therapeutic Strategies to Mitigate the Long-Term Inflammatory Effects of Acute OP Intoxication
6. Lower Vertebrates as a Biomedical Model
| OPs | Dose | Exposure Time | Effects | Tissue/Cell Line | Organism Model | References |
|---|---|---|---|---|---|---|
| Diazinon | LC50-7.830 ppm, ½ LC50-3.915 ppm | 96 h | ↓ AChE activity ↑ ACh levels | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [22]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 6, 12 and 24 h | ↓ AChE activity ↓mAChR, nAChR concentration and ↑ ACh levels. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [23]. |
| Diazoxon | 1 nm, 1 µM, and 10 µM | 24 h | ↓ (M3, M4, M5) receptors and nAChR-β2 expression. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [24]. |
| Diazinon | 1.96 mg/L | 96 h | ↑ Respiratory burst and IgM concentration | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [61]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 6 and 24 h | Alterations in Ca2+ flux and pERK 1/2. ↑ Cellular senescence ↓ mitchondrial membrane. potential ↑ apoptotic cells. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [107]. |
| Chlorpyrifos | 0.422 and 0.211 mg/L) | 96 h | ↓ Phagocytic Capacity. | Peripheral blood | Nile tilapia (O. niloticus) | [145]. |
| Diazinon | LC50-7.830 ppm | 96 h | ↓ Phagocytic capacity and cellular proliferation. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [146]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 6 and 24 h | ↑ Reactive oxygen species ↓ Phagocytic activity | Peripheral blood mononuclear cells | Nile tilapia (O. niloticus) | [147]. |
| Chlorpyrifos | 0.051 mg/L | 96 h | ↓ IgM levels and deregulation in lysozyme activity. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [148]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 12 and 24 h | ↑ Protein oxidative damage. | Liver and gills | Nile tilapia (O. niloticus) | [149]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 6 and 24 h | ↑ Neutrophil extracellular traps (NETs) induction. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [150]. |
| Diazoxon | M | 1 h and 2 h | ↓ Ca2+ flux against PMA and ionomycin stimulation. ↓ ERK1/2 phosphorylation. ↓ Mitochondrial membrane potential. ↑Apoptotic and cellular senescence. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [151]. |
| Temephos | 10 mg/L | 7 and 21 days | AChE inhibition ↑ ACh levels | Smooth muscle | Guppy fish (Poecilia reticulata) | [153]. |
| Temephos | 10 mg/L | 7 days | ↓ Phagocytic capacity | Spleen mononuclear cells | Guppy fish (P. reticulata) | [154]. |
| Temephos | 10 mg/L | 7, 14, and 21 days | ↑ Leucocytes death | Spleen mononuclear cells | Guppy fish (P. reticulata) | [155]. |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Ghanim, K.A. Acute toxicity and effects of sub-lethal malathion exposure on biochemical and haematological parameters of Oreochromis niloticus. Sci. Res. Essays 2012, 7, 1674–1680. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- International Code of Conduct on the Distribution and Use of Pesticides. Available online: http://apps.who.int/iris/bitstream/handle/10665/70602/WHO_HTM_NTD_WHOPES_2010.7_spa.pdf;jsessionid=6BF8CBB80C94FB71C65499FC33ACB281?sequence=1 (accessed on 28 February 2022).
- Cancer Clusters. Available online: https://www.cdc.gov/nceh/clusters/fallon/organophosfaq.htm#:~:text=Organophosphates%20are%20the%20most%20widely,in%20the%20body%20called%20acetylcholinesterase (accessed on 28 February 2022).
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Torres-Palma, R.; Serna-Galvis, E. Sonolysis; Academic Press: Cambridge, MA, USA, 2018; pp. 177–213. [Google Scholar] [CrossRef]
- Fernández, A.; Mancipe, L.; Fernández, A. Intoxicación por organofosforados. Rev. Med. 2010, 18, 84–92. [Google Scholar] [CrossRef]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. Acute organophosphorus poisoning. Clin. Chim. Acta 2014, 431, 66–76. [Google Scholar] [CrossRef]
- Nkinsa, P.N.; Muckle, G.; Ayotte, P.; Lanphear, B.P.; Arbuckle, T.E.; Fraser, W.D.; Bouchard, M.F. Organophosphate pesticides exposure during fetal development and IQ scores in 3 and 4-year old Canadian children. Environ. Res. 2020, 190, 110023. [Google Scholar] [CrossRef]
- Abbas, R.; Leister, C.; El-Gaaloul, M.; Chalon, S.; Sonnichsen, D. Ascending Single-Dose Study of the Safety Profile, Tolerability, and Pharmacokinetics of Bosutinib Coadministered with Ketoconazole to Healthy Adult Subjects. Clin. Therap. 2012, 34, 2011–2019. [Google Scholar] [CrossRef]
- Kaur, K.; Besnier, F.; Glover, K.A.; Nilsen, F.; Aspehaug, V.T.; Fjørtoft, H.B.; Horsberg, T.E. The mechanism (Phe362Tyr mutation) behind resistance in Lepeophtheirus salmonis pre-dates organophosphate use in salmon farming. Sci. Rep. 2017, 7, 12349. [Google Scholar] [CrossRef]
- Oruc, E.; Usta, D. Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ. Toxicol. Pharmacol. 2007, 23, 48–55. [Google Scholar] [CrossRef]
- Fulton, M.H.; Key, P.B. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 2001, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Profile for Diazinon. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp86.pdf (accessed on 28 February 2022).
- Elersek, T.; Filipič, M. Organophosphorous Pestidices Mechanism of Their Toxicity Pesticides—The Impact of Pesticides Expusure; Intech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Robb, E.L.; Baker, M.B. Organophosphathe Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021; PMID: 29261901. [Google Scholar]
- Vásquez, M.O. Intoxicación por organofosforados. Rev. Med. Sinerg. 2020, 5, e558. [Google Scholar] [CrossRef]
- King, A.M.; Aaron, C.K. Organophosphate and Carbamate Poisoning. Emerg. Med. Clin. N. Am. 2015, 33, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Mileson, B.E.; Chambers, J.E.; Chen, W.L.; Dettbarn, W.; Ehrich, M.; Eldefrawi, A.T.; Gaylor, D.W.; Hamernik, K.; Hodgson, E.; Karczmar, A.G.; et al. Common Mechanism of Toxicity: A Case Study of Organophosphorus Pesticides. Toxicol. Sci. 1998, 41, 8–20. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Life Sci. 2012, 1261, 1027–1032. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Zaitseva, G.; Casas-Solis, J.; Santerre, A. Effects of diazinon and diazoxon on the lymphoproliferation rate of splenocytes from Nile tilapia (Oreochromis niloticus): The immunosuppresive effect could involve an increase in acetylcholine levels. Fish Shellfish Immunol. 2008, 25, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibarra, G.; Díaz-Resendiz, K.; Pavón-Romero, L.; Rojas-García, A.; Medina-Díaz, I.; Girón-Pérez, M. Effects of diazinon on the lymphocytic cholinergic system of Nile tilapia fish (Oreochromis niloticus). Vet. Immunol. Immunopathol. 2016, 176, 58–63. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.; Girón-Pérez, M.; Covantes-Rosales, C.; Ventura-Ramón, G.; Pérez-Sánchez, G.; López-Torres, A.; Diaz-Resendiz, K.; Becerril-Villanueva, E.; Pavón, L. Alterations in the non-neuronal cholinergic system induced by in-vitro exposure to diazoxon in spleen mononuclear cells of Nile tilapia (O. niloticus). Fish Shellfish Immunol. 2021, 108, 134–141. [Google Scholar] [CrossRef]
- Proskocil, B.; Bruun, D.A.; Thompson, C.M.; Fryer, A.; Lein, P.J. Organophosphorus Pesticides Decrease M2 Muscarinic Receptor Function in Guinea Pig Airway Nerves via Indirect Mechanisms. PLoS ONE 2010, 5, e10562. [Google Scholar] [CrossRef]
- Poojary, A.; Basha, P.M. Cold stress interaction on organophosphate insecticide poisoning: Age-related assessment in rat cerebral cortex. Indian J. Exp. Biol. 2012, 50, 110–116. [Google Scholar]
- Basaure, P.; Guardia-Escote, L.; Cabré, M.; Peris-Sampedro, F.; Sánchez-Santed, F.; Domingo, J.L.; Colomina, M.T. Postnatal chlorpyrifos exposure and apolipoprotein E (APOE) genotype differentially affect cholinergic expression and developmental parameters in transgenic mice. Food Chem. Toxicol. 2018, 118, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Wang, C.; Tian, H.; Wang, W.; Ru, S. Effects of monocrotophos pesticide on cholinergic and dopaminergic neurotransmitter systems during early development in the sea urchin Hemicentrotus pulcherrimus. Toxicol. Appl. Pharmacol. 2017, 328, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, X.-G.; Yang, X.; He, Y.-Z. The diagnostic value of butyrylcholinesterase in acute organophosphorus pesticide poisoning. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue= Chin. Crit. Care Med. = Zhongguo Weizhongbing Jijiuyixue 2010, 22, 193–196. [Google Scholar]
- Figueiredo, T.H.; Apland, J.P.; Braga, M.F.M.; Marini, A.M. Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia 2018, 59, 92–99. [Google Scholar] [CrossRef]
- Bayrami, M.; Hashemi, T.; Malekirad, A.A.; Ashayeri, H.; Faraji, F.; Abdollahi, M. Electroencephalogram, cognitive state, psychological disorders, clinical symptom, and oxidative stress in horticulture farmers exposed to organophosphate pesticides. Toxicol. Ind. Health 2012, 28, 90–96. [Google Scholar] [CrossRef]
- Perry, J.; Cotton, J.; Rahman, M.; Brumby, S. Organophosphate exposure and the chronic effects on farmers: A narrative review. Rural Remote Health 2020, 20, 4508. [Google Scholar] [CrossRef]
- De Silva, H.J.; Samarawickrema, N.A.; Wickremasinghe, A.R. Toxicity due to organophosphorus compounds: What about chronic exposure? Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 803–806. [Google Scholar] [CrossRef]
- Thrasher, J.D.; Heuser, G.; Broughton, A. Immunological Abnormalities in Humans Chronically Exposed to Chlorpyrifos. Arch. Environ. Health Int. J. 2002, 57, 181–187. [Google Scholar] [CrossRef]
- Zafar, R.; Munawar, K.; Nasrullah, A.; Haq, S.; Ghazanfar, H.; Sheikh, A.B.; Khan, A.Y. Acute Renal Failure due to Organophosphate Poisoning: A Case Report. Cureus 2017, 9, e1523. [Google Scholar] [CrossRef]
- Cavari, Y.; Landau, D.; Sofer, S.; Leibson, T.; Lazar, I. Organophosphate Poisoning-Induced Acute Renal Failure. Pediatr. Emerg. Care 2013, 29, 646–647. [Google Scholar] [CrossRef]
- Ortega-Miller, J.G.; Yezioro-Rubinsky, S.; Benavides-Pinto, B.C.; Báez-Quintero, L.C. Efectos teratogénicos de insecticidas organofosforados en la etiología de labio y paladar hendido: Revisión de literatura. Rev. Nac. De Odontol. 2017, 13, 13. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Diaz, R.; Dando, R.; Jacques-Silva, M.C.; Fachado, A.; Molina, J.; Abdulreda, M.H.; Ricordi, C.; Roper, S.D.; Berggren, P.-O.; Caicedo, A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011, 17, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kirkpatrick, C.J.; Racké, K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: Expression and function in humans. Pharmacol. Ther. 1998, 77, 59–79. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Murad, F.; Fant, M. The placental cholinergic system: Localization to the cytotrophoblast and modulation of nitric oxide. Cell Commun. Signal. 2006, 4, 4–7. [Google Scholar] [CrossRef]
- Lev-Lehman, E.; Deutsch, V.; Eldor, A.; Soreq, H. Immature Human Megakaryocytes Produce Nuclear-Associated Acetylcholinesterase. Blood 1997, 89, 3644–3653. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. The lymphocytic cholinergic system and its biological function. Life Sci. 2003, 72, 2101–2109. [Google Scholar] [CrossRef]
- Resendiz, K.J.G.D.; Toledo-Ibarra, G.A.; Girón-Pérez, M.I. Modulation of Immune Response by Organophosphorus Pesticides: Fishes as a Potential Model in Immunotoxicology. J. Immunol. Res. 2015, 2015, 213836. [Google Scholar] [CrossRef]
- Banks, C.N.; Lein, P.J. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. NeuroToxicology 2012, 33, 575–584. [Google Scholar] [CrossRef]
- Koureas, M.; Rachiotis, G.; Tsakalof, A.; Hadjichristodoulou, C. Increased Frequency of Rheumatoid Arthritis and Allergic Rhinitis among Pesticide Sprayers and Associations with Pesticide Use. Int. J. Environ. Res. Public Health 2017, 14, 865. [Google Scholar] [CrossRef]
- Meyer, A.; Sandler, D.P.; Freeman, L.E.B.; Hofmann, J.; Parks, C.G. Pesticide Exposure and Risk of Rheumatoid Arthritis among Licensed Male Pesticide Applicators in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 077010. [Google Scholar] [CrossRef]
- Andrew, P.M.; Lein, P.J. Neuroinflammation as a Therapeutic Target for Mitigating the Long-Term Consequences of Acute Organophosphate Intoxication. Front. Pharmacol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Parrón, T.; Alarcón, R. Pesticides and asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Benka-Coker, W.; Loftus, C.; Karr, C.; Magzamen, S. Association of Organophosphate Pesticide Exposure and a Marker of Asthma Morbidity in an Agricultural Community. J. Agromed. 2020, 25, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-L.; Hou, Y.-C.; Wang, I.-K.; Lu, K.-C.; Yen, T.-H. Organophosphate pesticides and new-onset diabetes mellitus: From molecular mechanisms to a possible therapeutic perspective. World J. Diabetes 2021, 12, 1818–1831. [Google Scholar] [CrossRef]
- Wessler, I.K.; Kirkpatrick, C.J. The Non-neuronal Cholinergic System: An Emerging Drug Target in the Airways. Pulm. Pharmacol. Ther. 2001, 14, 423–434. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Filgueiras, C.; Manhães, A.C. Developmental aspects of the cholinergic system. Behav. Brain Res. 2011, 221, 367–378. [Google Scholar] [CrossRef]
- Nizri, E.; Brenner, T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 2011, 45, 73–85. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. J. Cereb. Blood Flow Metab. 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M. A comprehensive review of pesticides and the immune dysregulation: Mechanisms, evidence and consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Xue, B.; Shi, H. Activation of the Cholinergic Antiinflammatory Pathway Ameliorates Obesity-Induced Inflammation and Insulin Resistance. Endocrinology 2011, 152, 836–846. [Google Scholar] [CrossRef] [PubMed]
- El-Bouhy, Z.; El-Hakim, Y.A.; Mohamed, E. Chronic Effect of Chlorpyrifos on Biochemical, Immunological Changes and DNA Damage in Juvenile Nile Tilapia (Oreochromis niloticus). Zagazig Vet. J. 2018, 46, 51–59. [Google Scholar] [CrossRef][Green Version]
- Ahmadi, K.; Mirvaghefei, A.R.; Banaee, M.; Vosoghei, A.R. Effects of long-term diazinon exposure on some immunological and haematological parameters in rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Toxicol. Environ. Health Sci. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Castillo-Sosa, Y.; Sierra-Fonseca, A.; Martínez-Martínez, A.; Plenge-Tellechea, F. Efecto del diazinón sobre el cultivo de linfocitos de sangre periférica de human. Tecnoscience 2009, 3, 97–106. Available online: https://vocero.uach.mx/index.php/tecnociencia/article/view/734 (accessed on 2 March 2022).
- Girón-Pérez, M.; Velázquez-Fernández, J.B.; Díaz-Resendiz, K.J.G.; Díaz-Salas, F.; Canto-Montero, C.; Medina-Díaz, I.; Robledo-Marenco, M.; Rojas-García, A.; Zaitseva, G. Immunologic parameters evaluations in Nile tilapia (Oreochromis niloticus) exposed to sublethal concentrations of diazinon. Fish Shellfish Immunol. 2009, 27, 383–385. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.; Ahmadi, K. Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic. Biochem. Physiol. 2011, 99, 1–6. [Google Scholar] [CrossRef]
- Alluwaimi, A.M.; Hussein, Y. Diazinon immunotoxicity in mice: Modulation of cytokines level and their gene expression. Toxicology 2007, 236, 123–131. [Google Scholar] [CrossRef]
- Khoshbavar-Rostami, H.; Soltani, M.; Hassan, H. Immune response of great sturgeon (Huso huso) subjected to long-term exposure to sublethal concentration of the organophosphate, diazinon. Aquaculture 2006, 256, 88–94. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Rojas-Mayorquín, A.E.; Girón-Pérez, M.I. Influence of the Cholinergic System on the Immune Response of Teleost Fishes: Potential Model in Biomedical Research. Clin. Dev. Immunol. 2013, 2013, 536534. [Google Scholar] [CrossRef]
- Naughton, S.X.; Terry, A.V. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The Cholinergic Anti-inflammatory Pathway: A Missing Link in Neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, D.; Balasch, J.C.; Criado, M.; Tort, L.; Mackenzie, S.; Roher, N. Functional evidence for the inflammatory reflex in teleosts: A novel α7 nicotinic acetylcholine receptor modulates the macrophage response to dsRNA. Dev. Comp. Immunol. 2018, 84, 279–291. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, E.; Davel, L.; Jasnis, M.A.; Gotoh, T.; de Lustig, E.S.; Sales, M.E. Muscarinic receptors participation in angiogenic response induced by macrophages from mammary adenocarcinoma-bearing mice. Breast Cancer Res. 2005, 7, R345–R352. [Google Scholar] [CrossRef]
- Kawashima, K. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front. Biosci. 2004, 9, 2063–2085. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Ochani, M.; Yang, L.-H.; Gallowitsch-Puerta, M.; Ochani, K.; Lin, X.; Levi, J.; Parrish, W.R.; Rosas-Ballina, M.; Czura, C.J.; et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007, 35, 1139–1144. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Kostoff, R.N.; Briggs, M.B.; Porter, A.L.; Hernández, A.F.; Abdollahi, M.; Aschner, M.; Tsatsakis, A. The under-reported role of toxic substance exposures in the COVID-19 pandemic. Food Chem. Toxicol. 2020, 145, 111687. [Google Scholar] [CrossRef]
- Galloway, T.; Handy, R. Immunotoxicity of Organophosphorous Pesticides. Ecotoxicology 2003, 12, 345–363. [Google Scholar] [CrossRef]
- Mitra, A.; Sarkar, M.; Chatterjee, C. Modulation of immune response by organophosphate pesticides: Mammals as potential model. Proc. Zoologic. Soc. 2019, 72, 13–24. [Google Scholar] [CrossRef]
- Bakry, N.M.S.; El-Rashidy, A.H.; Eldefrawi, A.T.; Eldefrawi, M.E. Direct actions of organophosphate anticholinesterases on nicotinic and muscarinic acetylcholine receptors. J. Biochem. Toxicol. 1988, 3, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Trailović, S.M.; Marjanović, D.S.; Uzelac, T.V.; Milovanović, M.; Trailović, J.N. Two opposite dose-dependent effects of diazinon on the motor activity of the rat ileum. Res. Vet. Sci. 2017, 112, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, H.; Golmanesh, L. Changes in mRNA and protein levels of nicotinic acetylcholine receptors in Diazoxon exposed pC12 cells. Toxicol. In Vitro 2008, 22, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Smulders, C.J.G.M.; Bueters, T.J.H.; Vailati, S.; Van Kleef, R.G.D.M.; Vijverberg, H.P.M. Block of Neuronal Nicotinic Acetylcholine Receptors by Organophosphate Insecticides. Toxicol. Sci. 2004, 82, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, T.; Suriyo, T.; Thiantanawat, A.; Chaiyaroj, S.C.; Parkpian, P.; Satayavivad, J. Effects of paraoxon on neuronal and lymphocytic cholinergic systems. Environ. Toxicol. Pharmacol. 2011, 31, 119–128. [Google Scholar] [CrossRef]
- Tarkowski, M.S.; Lutz, W.; Birindelli, S. The lymphocytic cholinergic system and its modulation by organophosphorus pesticides. Int. J. Occup. Med. Environ. Health 2004, 17, 325–337. [Google Scholar]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Starkov, A.A. Calcium and Mitochondrial Reactive Oxygen Species Generation: How to Read the Facts. J. Alzheimer’s Dis. 2010, 20, S413–S426. [Google Scholar] [CrossRef]
- Buccafusco, J.J.; Beach, J.W.; Terry, A. Desensitization of Nicotinic Acetylcholine Receptors as a Strategy for Drug Development. J. Pharmacol. Exp. Ther. 2008, 328, 364–370. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Tsan, Y.; Hsu, J.; Hung, D.-Z.; Wang, C.-H. Early onset pneumonia in patients with cholinesterase inhibitor poisoning. Respirology 2010, 15, 961–968. [Google Scholar] [CrossRef]
- Faustini, A.; Settimi, L.; Pacifici, R.; Fano, V.; Zuccaro, P.; Forastiere, F. Immunological changes among farmers exposed to phenoxy herbicides: Preliminary observations. Occup. Environ. Med. 1996, 53, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Luebke, B. Pesticide-Induced Immunotoxicity: Are Humans at Risk? Hum. Ecol. Risk Assess. Int. J. 2002, 8, 293–303. [Google Scholar] [CrossRef]
- Dietert, R.R. Developmental immunotoxicology (DIT): Windows of vulnerability, immune dysfunction and safety assessment. J. Immunotoxicol. 2008, 5, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Koppe, F.; Stenger, B.; Brochhausen, C.; Schmidt, A.; Steinritz, D.; Thiermann, H.; Kirkpatrick, C.J.; Pohl, C. Influence of organophosphate poisoning on human dendritic cells. Chem. Interact. 2013, 206, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, M.; Liang, Q.; Yun, L.; Kang, H.; Fan, L.; Wang, D.; Zhang, G. Changes in monoclonal HLA-DR antigen expression in acute organophosphorus pesticide-poisoned patients. Exp. Ther. Med. 2014, 7, 137–140. [Google Scholar] [CrossRef]
- Costa, C.; Briguglio, G.; Catanoso, R.; Giambò, F.; Polito, I.; Teodoro, M.; Fenga, C. New perspectives on cytokine pathways modulation by pesticide exposure. Curr. Opin. Toxicol. 2020, 19, 99–104. [Google Scholar] [CrossRef]
- Helali, I.; Ferchichi, S.; Maaouia, A.; Aouni, M.; Harizi, H. Modulation of macrophage functionality induced in vitro by chlorpyrifos and carbendazim pesticides. J. Immunotoxicol. 2016, 13, 745–750. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Kawada, T. Organophosphorus pesticides induce apoptosis in human NK cells. Toxicology 2007, 239, 89–95. [Google Scholar] [CrossRef]
- Lasram, M.M.; Lamine, A.J.; Dhouib, I.B.; Bouzid, K.; Annabi, A.; Belhadjhmida, N.; Ben Ahmed, M.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Antioxidant and anti-inflammatory effects of N-acetylcysteine against malathion-induced liver damages and immunotoxicity in rats. Life Sci. 2014, 107, 50–58. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Ahmed, A.A.M.; Selim, M.A.A. Cytotoxic effect of chlorpyrifos is associated with activation of Nrf-2/HO-1 system and inflammatory response in tongue of male Wistar rats. Environ. Sci. Pollut. Res. 2018, 25, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Proskocil, B.J.; Grodzki, A.C.G.; Jacoby, D.B.; Lein, P.J.; Fryer, A.D. Organophosphorus Pesticides Induce Cytokine Release from Differentiated Human THP1 Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, F.; Mohseni, M.; Beigmohamadi, M.; Yazdinezhad, A.; Ramazani, A.; Hosseini, M.-J.; Sharafi, A. The protective effects of Ziziphora tenuior L. against chlorpyrifos induced toxicity: Involvement of inflammatory and cell death signaling pathways. J. Ethnopharmacol. 2021, 272, 113959. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Wang, Y.; Hong, J.; Shi, M.; Pfaff, D.; Guo, L.; Tang, H. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. Chemosphere 2020, 252, 126470. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Arslan-Acaroz, D.; Demirel, H.H.; Varol, N.; Ozyurek, H.A.; Zemheri, F.; Kucukkurt, I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed. Pharmacother. 2017, 96, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Weis, G.C.C.; Assmann, C.E.; Mostardeiro, V.B.; Alves, A.D.O.; da Rosa, J.R.; Pillat, M.M.; de Andrade, C.M.; Schetinger, M.R.C.; Morsch, V.M.M.; da Cruz, I.B.M.; et al. Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: A role in neuroinflammation. Chemosphere 2021, 278, 130417. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Matsushima, M.; Kawamura, N.; Atsumi, K.; Yamaguchi, T.; Ochi, H.; Kusatsugu, Y.; Oyabu, S.; Hashimoto, N.; Hasegawa, Y.; et al. Modulation of immunological activity on macrophages induced by diazinon. Toxicology 2017, 379, 22–30. [Google Scholar] [CrossRef]
- Tigges, J.; Worek, F.; Thiermann, H.; Wille, T. Organophosphorus pesticides exhibit compound specific effects in rat precision-cut lung slices (PCLS): Mechanisms involved in airway response, cytotoxicity, inflammatory activation and antioxidative defense. Arch. Toxicol. 2021, 96, 321–334. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Buhrmann, C.; Pourbagher-Shahri, A.M.; Shakibaei, M.; Samarghandian, S. Organophosphorus Compounds and MAPK Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4258. [Google Scholar] [CrossRef]
- Mostafalou, S.; Eghbal, M.A.; Nili-Ahmadabadi, A.; Baeeri, M.; Abdollahi, M. Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicol. Ind. Health 2012, 28, 840–851. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Ortiz-Lazareno, P.; Rosales, C.E.C.; Trujillo-Lepe, A.; Toledo-Ibarra, G.; Ventura, H.; Girón-Pérez, M. Effect of diazinon, an organophosphate pesticide, on signal transduction and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 89, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Raheja, G.; Gill, K.D. Role of muscarinic signal transduction and CREB phosphorylation in dichlorvos-induced memory deficits in rats: An acetylcholine independent mechanism. Toxicology 2009, 256, 175–182. [Google Scholar] [CrossRef]
- Falkenburger, B.H.; Jensen, J.B.; Hille, B. Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J. Gen. Physiol. 2010, 135, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Phan, T.; Han, S.; Wang, H.; Chan, G.C.-K.; Scheiner, Z.S.; Storm, D.R. Circadian oscillation of hippocampal MAPK activity and cAMP: Implications for memory persistence. Nat. Neurosci. 2008, 11, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Caughlan, A.; Newhouse, K.; Namgung, U.; Xia, Z. Chlorpyrifos Induces Apoptosis in Rat Cortical Neurons that is Regulated by a Balance Between p38 and ERK/JNK MAP Kinases. Toxicol. Sci. 2004, 78, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Esquivel-Sentíes, M.; Barrera, I.; Ortega, A.; Vega, L. Organophosphorous pesticide metabolite (DEDTP) induces changes in the activation status of human lymphocytes by modulating the interleukin 2 receptor signal transduction pathway. Toxicol. Appl. Pharmacol. 2010, 248, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, I.D.; Clemenza, L.; Scotter, A.J.; Chen, G.; Guerra, F.M.; Rottapel, R. Putting out the fire: Coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol. Rev. 2008, 224, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Cohney, S.J.; Sanden, D.; Cacalano, N.A.; Yoshimura, A.; Mui, A.; Migone, T.S.; Johnston, J.A. SOCS-3 Is Tyrosine Phosphorylated in Response to Interleukin-2 and Suppresses STAT5 Phosphorylation and Lymphocyte Proliferation. Mol. Cell. Biol. 1999, 19, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Magnarelli, G.; Fonovich, T. Protein phosphorylation pathways disruption by pesticides. Adv. Biol. Chem. 2013, 03, 460–474. [Google Scholar] [CrossRef][Green Version]
- Lima, A.; Vega, L. Methyl-parathion and organophosphorous pesticide metabolites modify the activation status and interleukin-2 secretion of human peripheral blood mononuclear cells. Toxicol. Lett. 2005, 158, 30–38. [Google Scholar] [CrossRef]
- Malek, T.R. The Biology of Interleukin-2. Annu. Rev. Immunol. 2008, 26, 453–479. [Google Scholar] [CrossRef]
- Costa, L.G. Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol. Sci. 2018, 162, 24–35. [Google Scholar] [CrossRef]
- Eddleston, M.; Buckley, N.; Eyer, P.; Dawson, A. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Hulse, E.J.; Davies, J.; Simpson, A.J.; Sciuto, A.M.; Eddleston, M. Respiratory Complications of Organophosphorus Nerve Agent and Insecticide Poisoning. Implications for Respiratory and Critical Care. Am. J. Respir. Crit. Care Med. 2014, 190, 1342–1354. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Bird, S.B.; Gaspari, R.; Dickson, E.W. Early Death Due to Severe Organophosphate Poisoning Is a Centrally Mediated Process. Acad. Emerg. Med. 2003, 10, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Roldan-Tapia, M.; Nieto-Escamez, F.A.; del Águila, E.; Laynez, F.; Parron, T.; Sanchez-Santed, F. Neuropsychological sequelae from acute poisoning and long-term exposure to carbamate and organophosphate pesticides. Neurotoxicol. Teratol. 2006, 28, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, T.; Weerasinghe, V.; Dangahadeniya, U.; Kularatne, K.; Dawson, A.; Karalliedde, L.; Senanayake, N. Cognitive processing of visual stimuli in patients with organophosphate insecticide poisoning. Neurology 2007, 68, 2027–2030. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Organophosphate-induced brain damage: Mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. NeuroToxicology 2012, 33, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.D.A.; Rossetti, F.; Chanda, S.; Yourick, D. Exposure to nerve agents: From status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. NeuroToxicology 2012, 33, 1476–1490. [Google Scholar] [CrossRef]
- Pereira, E.F.R.; Aracava, Y.; DeTolla, L.J.; Beecham, E.J.; Basinger, G.W.; Wakayama, E.J.; Albuquerque, E.X. Animal Models That Best Reproduce the Clinical Manifestations of Human Intoxication with Organophosphorus Compounds. J. Pharmacol. Exp. Ther. 2014, 350, 313–321. [Google Scholar] [CrossRef]
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B. Resveratrol and vitamin C as antioxidants in blood platelets. Thromb. Res. 2002, 106, 143–148. [Google Scholar] [CrossRef]
- Zaidi, S.R.; Banu, N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin. Chim. Acta 2004, 340, 229–233. [Google Scholar] [CrossRef]
- Ojha, A.; Srivastava, N. Redox imbalance in rat tissues exposed with organophosphate pesticides and therapeutic potential of antioxidant vitamins. Ecotoxicol. Environ. Saf. 2012, 75, 230–241. [Google Scholar] [CrossRef]
- Deshpande, L.; Blair, R.E.; Huang, B.A.; Phillips, K.F.; DeLorenzo, R.J. Pharmacological blockade of the calcium plateau provides neuroprotection following organophosphate paraoxon induced status epilepticus in rats. Neurotoxicol. Teratol. 2016, 56, 81–86. [Google Scholar] [CrossRef] [PubMed]
- El-Ebiary, A.A.; Elsharkawy, R.E.; Soliman, N.A.; Soliman, M.A.; Hashem, A.A. N-acetylcysteine in Acute Organophosphorus Pesticide Poisoning: A Randomized, Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2016, 119, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; She, J.; Lin, F.; Wu, J.-C.; Han, R.; Sheng, R.; Wang, G.; Qin, Z.-H. RRx-001 Exerts Neuroprotection Against LPS-Induced Microglia Activation and Neuroinflammation Through Disturbing the TLR4 Pathway. Front. Pharmacol. 2022, 13, 1219. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chiang, C.-Y.; Tsai, H.-J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef]
- Wilson, A.B. MHC and adaptive immunity in teleost fishes. Immunogenetics 2017, 69, 521–528. [Google Scholar] [CrossRef]
- Saha, A.; Ghosh, R.K.; Jesna, P.-K.; Choudhury, P.P. Bioindicators of Pesticide Contaminations. Sustain. Agric. Rev. 2021, 48, 185–231. [Google Scholar] [CrossRef]
- Medina-Garza, H. Uso de Biomarcadores en Peces Como Herramienta Para Evaluar la Exposición y Efecto de Contaminantes Ambientales en Cuerpos de Agua. Master’s Thesis, Universidad Autonoma de San Luis Potosí, México, December 2012. [Google Scholar]
- Gonçalves, C.; Marins, A.T.; Amaral, A.M.B.D.; Nunes, M.E.M.; Müller, T.E.; Severo, E.; Feijó, A.; Rodrigues, C.C.; Zanella, R.; Prestes, O.D.; et al. Ecological impacts of pesticides on Astyanax jacuhiensis (Characiformes: Characidae) from the Uruguay river, Brazil. Ecotoxicol. Environ. Saf. 2020, 205, 111314. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Barcelós-García, R.; Vidal-Chavez, Z.G.; Romero-Bañuelos, C.A.; Robledo-Marenco, M.L. Effect of Chlorpyrifos on the Hematology and Phagocytic Activity of Nile Tilapia Cells (Oreochromis niloticus). Toxicol. Mech. Methods 2006, 16, 495–499. [Google Scholar] [CrossRef]
- Girón-Pérez, M.I.; Santerre, A.; Gonzalez-Jaime, F.; Casas-Solis, J.; Hernández-Coronado, M.; Peregrina-Sandoval, J.; Takemura, A.; Zaitseva, G. Immunotoxicity and hepatic function evaluation in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Fish Shellfish Immunol. 2007, 23, 760–769. [Google Scholar] [CrossRef]
- Covantes-Rosales, C.E.; Trujillo-Lepe, A.; Díaz-Resendiz, K.J.G.; Toledo-Ibarra, G.; Ventura, H.; Ortiz-Lazareno, P.; Girón-Pérez, M. Phagocytosis and ROS production as biomarkers in Nile tilapia (Oreochromis niloticus) leukocytes by exposure to organophosphorus pesticides. Fish Shellfish Immunol. 2019, 84, 189–195. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.G.J.; Girón-Pérez, M.I. Effect of chlorpyrifos on the immune response of Nile tilapia (Oreochromis niloticus). Rev. BioCiencias. 2014, 3, 59–64. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Díaz-Resendiz, K.J.G.; Ventura-Ramón, G.H.; González-Jaime, F.; Vega-López, A.; Becerril-Villanueva, E.; Pavón, L.; Girón-Pérez, M.I. Oxidative damage in gills and liver in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 200, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; González-Navarro, I.; Agraz-Cibrian, J.M.; Girón-Pérez, D.A.; Ventura-Ramon, G.H.; Diaz-Resendiz, K.J.G.; Bueno-Durán, A.Y.; Ponce-Regalado, M.D.; Girón-Pérez, M.I. Diazinon acute exposure induces neutrophil extracellular traps in Nile tilapia (Oreochromis niloticus). Food Agric. Immunol. 2020, 31, 1004–1013. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Bernal-Ortega, J.; Covantes-Rosales, C.E.; Ortiz-Lazareno, P.C.; Toledo-Ibarra, G.A.; Ventura-Ramon, G.H.; Girón-Pérez, M.I. In-vitro effect of diazoxon, a metabolite of diazinon, on proliferation, signal transduction, and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 105, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Covantes-Rosales, C.; Toledo-Ibarra, G.; Díaz-Resendíz, K.; Ventura-Ramón, G.; Girón-Pérez, M. Muscarinic acetylcholine receptor expression in brain and immune cells of Oreochromis niloticus. J. Neuroimmunol. 2019, 328, 105–107. [Google Scholar] [CrossRef]
- Toledo-Ibarra, G.A.; Rodríguez-Sánchez, E.J.; Ventura-Ramón, H.G.; Resendiz, K.J.G.D.; Giron, M.I. Cholinergic alterations by exposure to pesticides used in control vector: Guppies fish (Poecilia reticulta) as biological model. Int. J. Environ. Health Res. 2017, 28, 79–89. [Google Scholar] [CrossRef]
- Girón-Pérez, D.A.; Hermosillo-Escobedo, A.T.; Macias-Garrigos, K.; Díaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Girón-Pérez, M.I. Altered phagocytic capacity due to acute exposure and long-term post-exposure to pesticides used for vector-borne disease as dengue. Int. J. Environ. Health Res. 2020, 32, 455–462. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Hermosillo-Escobedo, A.T.; Ventura-Ramón, G.H.; Toledo-Ibarra, G.A.; Girón-Pérez, D.A.; Bueno-Durán, A.Y.; Girón-Pérez, M.I. Death of guppy fish (Poecilia reticulata) leukocytes induced by in vivo exposure to temephos and spinosad. Int. J. Environ. Health Res. 2020, 32, 701–711. [Google Scholar] [CrossRef]

| OPs | Dose | Exposure Time | Cholinergic Effects | Tissue/Cell Line | Organism Model | References |
|---|---|---|---|---|---|---|
| Diazinon | LC50-7.830 ppm, ½ LC50-3.915 ppm | 96 h | ↓ AChE activity ↑ ACh levels | Spleen mononuclear cells | Nile tilapia (Oreochromis niloticus) | [22]. |
| Diazinon | 0.97, 1.95 and 3.91 mg/L | 6, 12, and 24 h | ↓ AChE activity ↓ mAChR, nAChR concentration and ↑ ACh levels. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [23]. |
| Diazoxon | 1 nm, 1 µM, and 10 µM | 24 h | ↓ (M3, M4, M5) receptors and nAChR β2 expression. | Spleen mononuclear cells | Nile tilapia (O. niloticus) | [24]. |
| Paraoxon | 1 mg/kg | 6 and 24 h | ↓ mAChR M2 function ↑ ACh levels. ↑ mAChR M3 stimulation | Peripheral blood | Guinea Pig | [25]. |
| Chlorpyrifos | LD50 1/3 LD50 | 48 h | ↓ ChAT activity ↓ AChE activity | Cerebral cortex | Male Rat | [26]. |
| Chlorpyrifos | 1 mg/Kg | 1 h and 6 h | ↓ ChAT activity, nAChR α4, and α7 expression ↓ VAChT expression | Forebrain Peripheral blood | Human apoE-TR mice | [27]. |
| Monocrotophos | 0.01, 0.10, or 1.00 mg/L | N/A | ↓ ChAT activity ↓ AChE activity | Embryos | Sea urchin (Hemicentrotus pulcherrimus) | [28]. |
| OPs | Acute exposure | N/A | ↓ BuChE activity | Peripheral blood | Human | [29]. |
| OPs | Dose | Exposure Time | Effects of Cytokines | Inflammation Results | Organism Model | References |
|---|---|---|---|---|---|---|
| Chlorpyrifos, dimethoate | 0–1000 μM | 24 h | IL-10 was significantly downregulated | ↓ DC-specific cell surface markers (i.e., CD83 and CD209). Inhibition of Akt family | DC, differentiated from the monocyte cell line THP-1 | [90]. |
| Chlorpyrifos | 0, 001, 10 μM | 24 h | ↓ Expression of IL-1β and TNF-α | Biphasic responses of lysosomal enzyme activity. inhibition NO release | Macrophages from mouse peritoneum | [93]. |
| Malathion | 200 mg/kg b.w./day | 28 days | ↑ Expression of IL-1β, IL-6 and IFN-γ | ↑ Activities of hepatocellular enzymes in plasma, lipid peroxidation index, CD3+/CD4+ and CD3+/CD4+ percent | Adult male Wistar rats | [95]. |
| chlorpyrifos | 3.375–13.5 mg/kg | 28 days | ↑ Expression of IL-1β and TNF-α | ↑ Activation of NF-kB, cleaved caspase 3 and HO-1 and Nrf-2 pathway Cellular damage in organs | Male Wistar rats | [96]. |
| Parathion, chlorpyrifos, and diazinon | 1–100 μM | 24 h | ↑ Expression of TNF-α, IL-1β PDGF (platelet-derived growth factor) and TGF-β (transforming growth factor-β). the of TNF-α protein. | ↑ NF-κB activation and ↓AChE activity | THP1 cells differentiated into macrophages | [97]. |
| Chlorpyrifos | 6.75 mg/kg | 8 weeks | ↑ Expression of IL-6, TLR-2, IL-1β, TNF-α, and NLPR3 | ↑ Expression of apoptotic genes (Caspase 3, Caspase 9, Caspase 8 and Bax) | Male rats | [98]. |
| Triphenyl phosphate | 0, 50, or 150 mg/kg | 30 days | ↑ Expression of IL-6 and TNF-α | ↑ Inflammation in the thalamus and hippocampus. MAPK signaling pathways were significantly affected. | Male mice (C57/BL6) | [99]. |
| Malathion | 27 mg/kg (1/50 of LD50) | 30 days | ↑ Expression of IF-γ, IL1-β, TNF-α, and NFĸB | ↓AChE levels in serum (30%) and liver (25%) compared to the control group. Lipid peroxidation. | Rats | [100]. |
| Chlorpyrifos | 0.3–300 μM | 24 h | ↑ Expression of IL-1β and NLRP3 | ↑ Oxidative stress production (NO, MDA, and O2∙) | BV-2 microglial cells. | [101]. |
| Diazinon | 10–100 μM | 24 h | Induce expression of TNF-α and IL-6 | ↑ ROS generation. Induced expressions of COX-2, iNOS, and cell-surface molecules CD40, CD86, and MHC class II. ↓phagocytic activity | RAW264.7 cells | [102]. |
| Parathion, Malathion, paraoxon and malaoxon | 100–2000 µmol/L | 24 h | ↑ Expression of IL-6, GM-CSF and MIP-1α | ↓Viability, intracellular GSH and phosphorylation of STAT3. ↑Phosphorylated p38MAPK | Rat precision-cut lung slices | [103]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Pérez, M.R.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Mercado-Salgado, U.; Ponce-Regalado, M.D.; Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway. Int. J. Mol. Sci. 2022, 23, 4523. https://doi.org/10.3390/ijms23094523
Camacho-Pérez MR, Covantes-Rosales CE, Toledo-Ibarra GA, Mercado-Salgado U, Ponce-Regalado MD, Díaz-Resendiz KJG, Girón-Pérez MI. Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway. International Journal of Molecular Sciences. 2022; 23(9):4523. https://doi.org/10.3390/ijms23094523
Chicago/Turabian StyleCamacho-Pérez, Milton Rafael, Carlos Eduardo Covantes-Rosales, Gladys Alejandra Toledo-Ibarra, Ulises Mercado-Salgado, María Dolores Ponce-Regalado, Karina Janice Guadalupe Díaz-Resendiz, and Manuel Iván Girón-Pérez. 2022. "Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway" International Journal of Molecular Sciences 23, no. 9: 4523. https://doi.org/10.3390/ijms23094523
APA StyleCamacho-Pérez, M. R., Covantes-Rosales, C. E., Toledo-Ibarra, G. A., Mercado-Salgado, U., Ponce-Regalado, M. D., Díaz-Resendiz, K. J. G., & Girón-Pérez, M. I. (2022). Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway. International Journal of Molecular Sciences, 23(9), 4523. https://doi.org/10.3390/ijms23094523