Abstract
CHRFAM7A is a relatively recent and exclusively human gene arising from the partial duplication of exons 5 to 10 of the α7 neuronal nicotinic acetylcholine receptor subunit (α7 nAChR) encoding gene, CHRNA7. CHRNA7 is related to several disorders that involve cognitive deficits, including neuropsychiatric, neurodegenerative, and inflammatory disorders. In extra-neuronal tissues, α7nAChR plays an important role in proliferation, differentiation, migration, adhesion, cell contact, apoptosis, angiogenesis, and tumor progression, as well as in the modulation of the inflammatory response through the “cholinergic anti-inflammatory pathway”. CHRFAM7A translates the dupα7 protein in a multitude of cell lines and heterologous systems, while maintaining processing and trafficking that are very similar to the full-length form. It does not form functional ion channel receptors alone. In the presence of CHRNA7 gene products, dupα7 can assemble and form heteromeric receptors that, in order to be functional, should include at least two α7 subunits to form the agonist binding site. When incorporated into the receptor, in vitro and in vivo data showed that dupα7 negatively modulated α7 activity, probably due to a reduction in the number of ACh binding sites. Very recent data in the literature report that the presence of the duplicated gene may be responsible for the translational gap in several human diseases. Here, we will review the studies that have been conducted on CHRFAM7A in different pathologies, with the intent of providing evidence regarding when and how the expression of this duplicated gene may be beneficial or detrimental in the pathogenesis, and eventually in the therapeutic response, to CHRNA7-related neurological and non-neurological diseases.
1. Introduction
CHRFAM7A (dupα7) is a human-lineage-specific gene [1,2,3] that first appeared about 3.5 million years ago during evolution: this can be confirmed by its absence in other primates [4], and its absence in rodents excludes the possibility of gene loss [5]. It arose from the duplication of CHRNA7 gene exons 5 to 10 (Figure 1A), encoding the α7 subunit of the neuronal nicotinic acetylcholine receptor (nAChR) on chromosome 15q13-q14, 1.6 Mb apart from CHRNA7, and has an inverted orientation with respect to the parental gene (Figure 1B) [2,6,7]. Here, the CHRNA7-derived cassette fused in-frame to a cluster (FAM7A) of seven exons (A to F), which are present four times on chromosome 15q13.3, located both upstream and downstream of CHRNA7, in the sense and antisense directions (Figure 1A). In particular, exons A, B, C, and E derive from the unc-51 like kinase 4 gene (ULK4), located at chromosome 3p22.1, whereas exons D and F are homologous to the GOLGA8B gene, which is located 2.5 Mb 3′ from CHRNA7 [8].
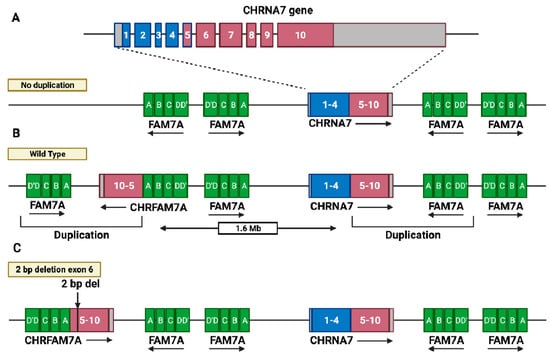
Figure 1.
The CHRNA7 and CHRFAM7A locus on Chr. 15q13.3. (A) Scheme of the genomic structure of the ten-exon CHRNA7 gene, flanked by four FAM7A cassettes. Numbers indicate exons (1 to 4 in blue, 5 to 10 in red). (B) CHRNA7 exons 5 to 10 (red) are duplicated and fused in-frame with one FAM7A cassette (green), 1.6 Mb from, and in the opposite orientation to, the CHRNA7 gene, giving rise to the hybrid CHRFAM7A gene (wild-type allele). (C) A deletion of two base pairs in exon 6 (2 bp del), generating the Δ2bp polymorphic allele, which is associated with CHRFAM7A gene inversion with the same orientation as the CHRNA7 gene. (Created with BioRender.com (accessed on 23 January 2022)).
More recently, a polymorphism (c.497-498delTG; rs67158670) and a deletion of two base pairs (Δ2bp) in exon 6, occurred in CHRFAM7A (dupΔα7; Figure 1C), which was determined as being associated with gene inversion, with the same orientation as the CHRNA7 gene [6]. The CHRFAM7A gene varies in terms of its number of copies in each individual, with 5–10% of individuals being non-carriers of CHRNA7 duplication and approximately 30% only having one copy [2,9,10]. The absence, as well as the presence of one or two copies of the gene, and the presence of Δ2bp deletion, results in six different genotypes (see Box 1). Moreover, the frequency of the Δ2bp allele varies in different ethnic groups (Table 1), and this polymorphism has been associated with an increased susceptibility to psychiatric disorders (see below).

Table 1.
Allelic frequency of 2bp deletion (-/TG) from the 1000 Genomes Project. EAS = East Asian; EUR = European; AFR = African; AMR = Ad-mixed Americans; SAS = South Asian.
Box 1. The six possible combinations of the CHRFAM7A genotype.
• CHRFAM7A null/null
• CHRFAM7A dupα7/null
• CHRFAM7A dupα7/dupα7
• CHRFAM7A dupα7/dupΔα7
• CHRFAM7A dupΔα7/dupΔα7
• CHRFAM7A dupΔα7/null
Recently, data in the literature have reported that the presence of the duplicated gene may be responsible for the translational gap in several human diseases [11,12,13,14]. However, it is important to address whether this isoform is indeed expressed in vivo. Moreover, clinical and pre-clinical data neither seem to indicate a unique role of CHRFAM7A, nor whether it is protective or toxic, because of confounding factors caused by the disease being studied, and the model (clinical or pre-clinical) used. In this review, we will summarize all of the biochemical studies containing evidence of the formation of a heteromeric receptor composed of CHRNA7 and CHRFAM7A encoded subunits for the first time. Moreover, we present all of the available data on the role of CHRFAM7A in different pathologies, with the intent of providing evidence regarding when and how the expression of this duplicated gene may be beneficial or detrimental in the pathogenesis, and eventually in the therapeutic response, to both neurological and non-neurological diseases. As a common trait, the deregulated expression of both genes (up- and down-regulated), due to the presence of the Δ2bp polymorphism in CHRFAM7A and/or the CNV of both genes, alter the CHRNA7/CHRFAM7A ratio, thus contributing to the beneficial/detrimental effects of dupα7 on α7 nAChR function.
2. Structure of Neuronal Nicotinic Receptors (nAChRs)
Nicotinic Acetylcholine Receptors (nAChRs) are part of the Cys-loop receptor superfamily. These receptors are transmembrane ion channels activated by neurotransmitters that are mainly expressed in the central and peripheral nervous systems and are responsible for the regulation and transmission of excitatory and inhibitory signals [15]. The ionotropic GABAergic receptors GABAA and GABAC, glycinergic receptors, serotonin (5-HTn) receptors, and the aforementioned nAChRs, which include muscle- and neuronal- subtypes, are the best-known members of the Cys-loop receptor superfamily [16]. Cys-loop receptors share a common pentameric structure, with their subunits being arranged in a pseudo-symmetrical rosette, forming a central pore [15,16]. Each subunit consists of an extracellular hydrophilic N-terminal domain that contains the binding site for the ligand, three hydrophobic transmembrane domains (M1–M3), a highly variable cytoplasmic domain between the different subunits, a fourth hydrophobic domain (M4), and an extracellular C-terminal domain (Figure 2). The M2 domains of the five subunits form the central pore and possess amino acids that are important for ion selectivity, permeability, and channel gating [16]. Nicotinic receptors are selective cationic channels for Na+, K+, and Ca2+, and have a high affinity for nicotine and the endogenous ligands choline (Ch) and acetylcholine (ACh); the relative affinity of the ligands varies according to the subunit composition [16]. The combination of nine α (α2–α10) and three β (β2–β4) subunits form both hetero- and homo-pentamer receptors that have different structural, functional, and pharmacological properties (Figure 2). Homo-pentameric receptors have five identical orthosteric ACh binding sites that are located in the extracellular interfaces between two adjacent subunits (Figure 2), whereas hetero-pentameric receptors, which have two α/three β and three α/two β subunits, have two orthosteric binding sites that are located at the interfaces between the α subunit and the β subunit (Figure 2).
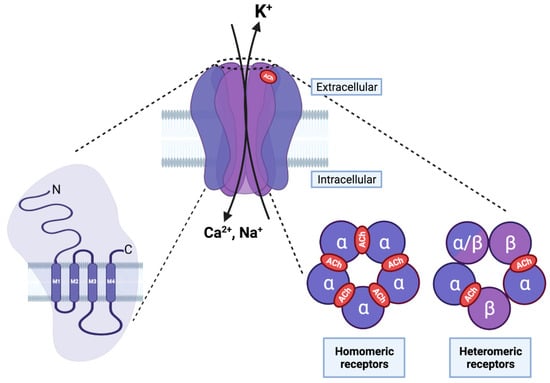
Figure 2.
Structure of nAChRs. On the left, each nAChR subunit is composed of an extracellular amino terminal portion, followed by three hydrophobic transmembrane domains (M1–M3), a large intracellular loop, a fourth transmembrane domain (M4), and an extracellular carboxy–terminus. In the middle, the pentameric arrangement of nAChR subunits is shown in an assembled receptor. The M2 transmembrane domain of the five subunits forms the central pore and possesses amino acids that are important for ion selectivity, permeability, and channel gating. On the right, five subunits can assemble to form homo- (five α subunits) or hetero-pentameric (α and β subunits) receptors. The orthosteric ligand binding site is formed between two α subunits (in red) in homomeric receptors, and between the α and β subunits in an heteromeric receptor. (Created with BioRender.com (accessed on 23 January 2022)).
nAChRs, as modulators of the release of neurotransmitters, play a fundamental role in cognitive functions, and their decline or dysfunction leads to neuropsychiatric and neurodegenerative diseases. Genetic and knock-out studies on mouse models have confirmed a link between the malfunction or mutation of these genes and pathologies such as epilepsy, schizophrenia, depression, and nicotine addiction [16,17].
2.1. The α7 nAChR
α7 nAChR is a homo-pentamer whose subunit, α7, is encoded by the ten-exon CHRNA7 gene (Chr 15q13-q14), which allows the translation of a protein of about 57 kDa (Figure 3A). Exons 1–7 encode the N-terminal portion that contains the ACh binding site; exons 7–8 comprise the M1–M3 transmembrane regions; and exons 9–10 comprise the cytoplasmic loop, the fourth transmembrane domain (M4), and the C-terminal portion (Figure 3A). α7 nAChR exhibits a low affinity for nicotine, a high affinity for α-bungarotoxin (α-Bgtx), and is highly permeable to Ca2+, thus suggesting that it plays a role not only as an ion channel, but also as a regulator of the calcium-activated signaling pathways [16]. The receptor is expressed in the brain, in excitatory and inhibitory neurons, both pre-synaptically, where it modulates the release of neurotransmitters, and post-synaptically, as well as in astrocytes and microglia [18,19,20]. In the periphery, it is expressed in neuroendocrine cells [21], sperm acrosome [22], epithelial cells [23], microvascular endothelial cells [24], pulmonary fibroblasts [25], bone marrow [26], chondrocytes [27], T and B lymphocytes [28,29], and macrophages [30]. Here, it plays an essential role in the “cholinergic anti-inflammatory” response [31,32,33]. Its nature as a modulator of intracellular calcium concentration, which is one of the most widespread second messengers, explains its almost ubiquitous expression. CHRNA7 is related to several disorders with cognitive deficits, including schizophrenia [9], P50 auditory gating deficits [34], autism [35], epilepsy [36], bipolar disorder [37], attention deficit hyperactivity disorder (ADHD) [38], Down syndrome [39], Parkinson’s disease (PD) [40], and Alzheimer’s disease (AD) [41,42]. In extra-neuronal tissues, α7 nAChR plays important roles in proliferation, differentiation, migration, adhesion, cell contact, apoptosis, angiogenesis, and tumor progression [43,44,45,46,47,48].
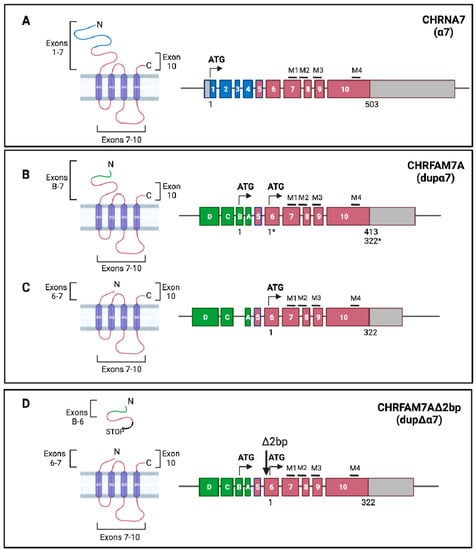
Figure 3.
The α7 and dupα7 receptor. Relative protein products of CHRNA7 and CHRFAM7A. (A), CHRNA7 encodes the 503 aa α7 subunit: exons 1–7 encode the extracellular N-terminus domain, including the signal peptide and the acetylcholine binding domain; exons 7–10 encode the M1–M3 transmembrane domains, the long M3–M4 intracellular loop, the M4 transmembrane domain, and the small extra-cellular C-terminal domain. (B,C), CHRFAM7A encodes the dupα7 subunit via two alternatively spliced mRNAs that differ in terms of the presence of exon B. Translation starting from exon B (B) results in a 413 aa protein that differs from α7 for the N-terminal domain, and a 27 amino acid-long peptide that is encoded by ExB–Ex6. Translation starting from exon 6 (C) results in a 322 aa protein that can be considered a truncated α7 subunit with a short N-terminus encoded by exons 6–7. (D), CHRFAM7AΔ2bp. The presence of the Δ2bp polymorphism (-/TG) in exon 6 results in a short, truncated protein that is encoded by exons B–6 because of the insertion of a translation stop codon and a receptor encoded by exons 6–10 (dupΔα7), due to the translational start site in exon 6. This protein is similar to the one obtained in (C). (Created with BioRender.com (accessed on 23 January 2022)).
2.2. The dupα7 Receptor
The CHRFAM7A gene transcribes for two isoforms (isoform 1, NM_139320.2/NP_647536.1; isoform 2, NM_148911.1/NP_683709.1) that differ in terms of the presence of exon B [49], due to alternative splicing. The transcript of isoform 1 presents two open reading frames (ORF) (Figure 3B); translation starting from exon B (ORF 1) results in a protein that is 412 amino acids (aa) long (46 KDa; dupα7) and is different from the α7 nAChR subunit for the N-terminal domain which is formed by a 27 aa long peptide (Figure 3B), but it still includes the disulfide bridge and the vicinal cysteines. The resulting peptide will lose a putative glycosylation site and part of the agonist binding site. A shorter polypeptide (38 KDa) may putatively originate from the second ORF starting at exon 6 (Figure 3B). This translation start site is the only start site that exists in isoform 2 (Figure 3C) and results in the 38 KDa protein, which lacks the signal peptide and the entire binding site, but contains all of the α7 transmembrane domain sequences. The expression of both transcripts and proteins has been confirmed in both heterologous expression systems [50,51,52] and in vivo [49,50,53,54,55,56]. The Δ2bp deletion polymorphism (Figure 3D) introduces a stop codon in ORF 1, generating a truncated subunit that is formed by a short 40 aa long peptide that is codified by exon B–exon 5. It is possible to synthesize the 38 KDa protein (dupΔα7) if translation restarts from exon 6, downstream from the Δ2bp polymorphism (Figure 3D) [51,52].
In leukocytes, a ~2 Kb long promoter, located upstream and that overlaps with part of exon D, drives CHRFAM7A expression [49].
Numerous attempts have been made to understand the nature and function of this duplicated gene. The homology of the full-length gene suggests that the duplicated gene may have a new function, but always in the context of cholinergic biology. The fact that this endogenous duplicated gene translates a subunit [49,53,57,58], reinforces the hypothesis that dupα7 may interact with and regulate α7 nAChR.
2.3. The Heteromeric α7/dupα7 Receptor
CHRFAM7A gene products (mRNA and protein) are present in different brain areas in different proportions compared to CHRNA7 (up to 10–20% of the α7 transcript) [1,8,44,50,55,59,60,61], as well as in at least thirty tissues in the periphery, including in leukocytes [11,45,49,53,54,56,57,62]. In leukocytes, CHRFAM7A expression overcomes that of CHRNA7 by two orders of magnitude [49,50], and seminal electrophysiological studies [57] have failed to measure an ACh or a nicotine-evoked current, probably due to the missing ACh binding sites. On the other hand, the presence of the transmembrane domains in the translated dupα7 subunits (in particular M2, forming the ion pore) indicates that a hypothetical dupα7 receptor could trigger Ca2+ influx in response to a specific ligand. An alternative hypothesis is that the dupα7 subunit does not form homomeric receptors, but is instead assembled with conventional α7 subunits to form a new class of heteromeric receptors. The role of dupα7 in these receptors may include the formation of the pore as well as many other regulatory functions.
To address this issue, several groups have used both functional and structural approaches [50,51,52,58,63,64], most of which have been based on the heterologous expression of the α7 and dupα7 proteins in oocytes and cell lines. In oocytes, the expression of dupα7 alone has failed to produce any ACh-induced currents [50], an effect that has also been observed in mammalian cell lines [51,52,63], and that has been hypothesized as being due to the missing agonist binding sites and the lack of β1β2 loop, both of which are required for channel opening [65,66]. When the receptors were co-expressed, the increased concentration of dupα7 (α7/dupα7 ratio 1:5/1:10) reduced the nicotine- and ACh-induced α7 currents, but not receptor sensitivity to ACh [50,51,52], thus suggesting that dupα7 has a dominant negative effect on the α7 current. The overexpression of the construct with the Δ2bp in exon 6, dupΔα7, further reduced the current amplitude by an additional 10% [51]. This effect has not been replicated in the Neuro2a cell line, which may be due to poor dupα7 translation [52], something that was recently confirmed in SH-SY5Y cells [58].
Confocal images using both an antibody, which detects both receptors, and FITC conjugated α-Bungarotoxin (α-Bgtx), an α7 antagonist that only binds to the N-terminal domain of the α7 receptor, demonstrated that this effect was due to a reduced number of α7 receptors at the plasma membrane [11,50,63,64]. The dupα7 protein seemed to preferentially localize within the endoplasmic reticulum (ER) [50,52,64], making it likely that the dominant negative effect may be exerted by increased α7 receptor retention in the ER (Figure 4). This model does not exclude the possibility of a heteromeric receptor α7/dupα7 being formed. In the presence of 125I-α-Bgtx, surface receptor binding assays showed a reduced number of 125I-α-Bgtx binding sites and an increase in the equilibrium dissociation constant (Kd), thus suggesting the formation of a less functional receptor [50,51]. On the other hand, higher CHRFAM7A expression versus CHRNA7 expression can result in a potentiation of the effect of a positive allosteric modulator that is able to bind to the transmembrane domains of the receptor (PNU-120596) [51]. This finding proved that a heteromeric receptor that incorporates CHRFAM7A subunits, and with properties that are different from those of α7 nAChR, can be formed, although this result has not been confirmed by other groups [50,58,63].
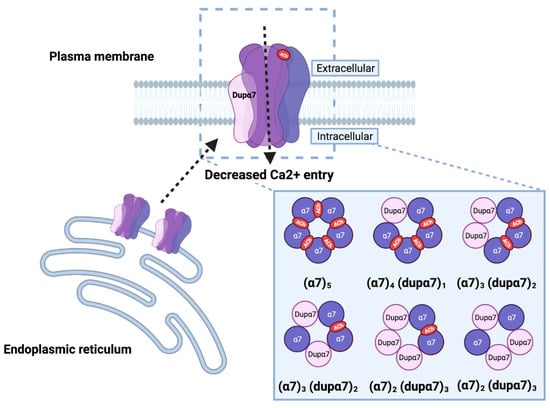
Figure 4.
The heteromeric α7/dupα7 receptor. Dupα7 can assemble and interact with α7 subunits to form heteropentameric receptors, causing reduced function of the receptor at the cell membrane (dashed line), as demonstrated by a net reduction in the macroscopic current, that is inversely proportional to the amount of dupα7 and/or retention (dashed line) at the endoplasmic reticulum (ER). In order for the heteromeric receptor to be functional, it should include at least two α7 subunits to form the agonist binding site. The possible stoichiometries of the heteromeric receptor are the result of experimental data and computational model simulations. (Created with BioRender.com (accessed on 23 January 2022)).
The putative assembly of the subunits was investigated using Forster Resonance Energy Transfer (FRET) by Donor Recovery after Acceptor Photobleaching (DRAP), and FRET by Fluorescence Lifetime Imaging Microscopy (FLIM), in the Neuro2a [52], GH4C1 and RAW264.7 [64], and SH-SY5Y [58] cell lines. Both approaches revealed and confirmed the assembly of duplicated subunits with full-length α7, forming several types of hetero-pentamers between α7 and dupα7/dupΔα7 (α7 with α7; α7 with dupα7; α7 with dupΔα7; dupα7 with dupα7; dupΔα7 with dupΔα7). According to these data, a limited number of dupα7 subunits (mainly one) may be incorporated and are likely adjacent to a α7 subunit in the heteromeric receptor. Interestingly, the duplicated isoforms can interact with other nAChRs subunits, namely α4 and α3 [52].
The formation of α7/dupα7 or α7/dupΔα7 hetero-pentamers in cell lines does not imply that they are functional. This question has been addressed by means of the substituted cysteine accessibility method (SCAM), a technique that allows the measurement of altered ion channel function, following the substitution of key amino acids with cysteine, and treatment with sulfhydryl-specific reagents [52]. The leucine at the 247 -position resides nine residues downstream from the cytoplasmic beginning of the TM2 (9′ position), which forms the ion channel pore of α7 and the duplicated isoform. A whole-patch clamp of Neuro2a cells, transfected with α7 along with substituted L9′C subunits (α7 L9′C; dupα7L9′C; dupΔα7L9′C), showed a reduction in the ACh-evoked inward current (IACh) in the presence of the dupα7 subunit isoforms that was similar to the reduction measured on cells expressing α7/α7L9′C. These data suggest that both dupα7 and dupΔα7 could form heteromeric functional receptors with α7. Despite the expression of dupα7 and dupΔα7, there was no effect on the ACh-evoked current amplitude in Neuro2a [52]. The inclusion of dupα7 induced a higher sensitivity to a high concentration of choline compared to cells that were only expressing α7 or α7/dupΔα7, whereas brief exposure to a physiological concentration of choline led to a faster desensitization of both the α7/dupα7 and α7/dupΔα7 receptors. Conversely, the inclusion of dupΔα7 increased the sensitivity of the α7 nAChR to the full α7 agonist varenicline [52], which is commonly used for smoking cessation [67]. However, genetic studies did not reveal any significant association between dupΔα7 and smoking abstinence [67].
Patch clamp analyses to measure single-channel function, coupled with the electric fingerprinting strategy, have provided the first evidence of the actual formation of functional hetero-pentamers [63]. This strategy relies on the possibility of distinguishing the activity of the α7 subunit with three arginine substitutions at the intracellular M3–M4 loop region (α7LC, α7 low-conductance). α7LC incorporation generates multiple and discrete current amplitude clusters, each one corresponding to a different population of receptors with a fixed number of LC subunits. LC receptors, when expressed individually, do not display detectable currents at the single-channel level. Single channel currents activated by ACh and 1 μM PNU-1205696, from cells expressing α7/α7LC, distribute into clusters of different amplitudes, something that is indicative of the formation of pentameric receptors that incorporate from zero up to three α7LC subunits. When co-expressed, α7LC + dupα7, or the reverse combination dupα7LC + α7, reveal that dupα7 can assemble with α7, forming functional heteromeric receptors containing one, two, or three dupα7 subunits (Figure 4). Mutagenesis analyses of the conserved Tyr-93 in the α7 subunit, which is important for ACh binding, in the context of the (α7)2(dupα7)3 receptor, revealed that the heteromeric receptor has to contain at least two adjacent α7 subunits, and no more than three dupα7 subunits to be functional (Figure 4) [63,68]. Moreover, the channel kinetics elicited by the ACh + PNU-120596 of the (α7)2(dupα7)3 receptor does not differ from that of the α7 homomeric receptor [63], as previously reported [51].
These findings have been confirmed by computational model studies [69] on all of the possible combinations of α7 and dupα7 pentamers. The authors of this study have shown that receptors with dupα7/α7 interfaces are more stable and less detrimental for ion conductance than dupα7/dupα7 interfaces, and that the most stable stoichiometry is 1: dupα7/4: α7; however, functional heteromeric receptors can be formed with no more than three dupα7 subunits (Figure 4). Moreover, the heteromeric receptors showed a very low affinity for α-Bgtx, thus explaining the reduction observed in previous studies [50,51,52,70], and are less sensitive to Aβ1–42, which supports recent experimental data [12].
In conclusion, these studies demonstrated that the duplicated form of the CHRNA7 gene, CHRFAM7A, translates for the dupα7 (or dupΔα7) protein in a multitude of cell lines and heterologous systems with processing and trafficking that are very similar to the full-length form without affecting CHRNA7 transcription. It does not form functional ion channel receptors alone. In the presence of CHRNA7 gene products, dupα7 can assemble and form heteromeric receptors that, in order to be functional, should include at least two α7 subunits to form the agonist binding site. When incorporated into the receptor, dupα7 negatively modulates α7 activity, as demonstrated by a net reduction in the macroscopic current, which is inversely proportional to the amount of dupα7 and is probably due to a reduction in the number of ACh binding sites.
This observation can be of fundamental importance, especially when considering the different dupα7 and α7 expression levels in different tissues, and the modulation of their expression by various stimuli (IL-1β, LPS, nicotine, donepezil, HIV-1 gp120) [49,50,53,61], although evidence of the formation of a heteromeric dupα7/α7 receptor in vivo is still missing. Recently, CHRFAM7A transgenic mice showed decreased α-Bgtx at the neuromuscular junction and in brain tissue compared to wild-type (WT) animals, showing for the first time that dupα7 expression decreases α7 nAChR ligand binding in vivo [70,71]. Silencing CHRFAM7A expression in SH-SY5Y cells significantly increased α7 nAChR-induced neurotransmitter release, which was reduced by the overexpression of the dupα7 protein [58].
The involvement of α7 nAChR in physiological mechanisms, and its association with neurological, neurodegenerative, and inflammatory disorders, confirmed the importance of understanding whether an heteromeric receptor is formed in vivo (i.e., in neurons), and how these isoforms are regulated and eventually deregulated in different pathologies and in response to therapies targeting the cholinergic system.
3. CHRNA7 and CHRFAM7A Role in Diseases
3.1. Schizophrenia and Neuropsychiatric Disorders
Chromosomal defects (deletion, duplication) and polymorphisms in the 15q13-q14 region are associated with several neuropsychiatric disorders, including schizophrenia [72,73,74], epilepsy [10,36,75,76], psychosis [77,78,79], intellectual disability (ID) [80,81], and autism [35,82]. A direct genetic association of CHRNA7 with these disorders is controversial; many markers (i.e., the dinucleotide marker D15S1360 in intron 2 of the CHRNA7 gene and other microsatellite markers) in the vicinity of the CHRNA7 gene at 15q13-q14, which have a strong association with schizophrenia, have been reported [74]. Other studies have failed to find any genetic linkage to this region [83,84,85,86], thus supporting the idea that schizophrenia is a genetically heterogeneous disease, and that the loci involved may differ depending on ethnicity [87,88]. A stronger linkage with CHRNA7 was obtained when the P50 auditorily evoked response deficits [89,90], an endophenotype of both schizophrenia and bipolar disorders, and nicotine, transiently normalized the P50 deficit in schizophrenic and bipolar disorder patients, thus confirming the involvement of a nicotinic receptor [91]. The finding that schizophrenic and depressed patients showed the highest prevalence of smoking [92,93] reinforced the hypothesis that smoking may be a form of “self-medication” for these patients [92,94]. Indeed, evidence from animal and human studies have indicated that targeting α7 nAChRs improves cognition, memory, and sensory gating deficits in schizophrenia [95,96,97,98]. It is worth noting that in peripheral blood lymphocytes, CHRFAM7A expression is lower in cotinine, in self-reported smokers versus in non-smokers, and is negatively correlated to cotinine (nicotine metabolite) levels, but not with the diagnosis of schizophrenia [99]. Conversely, individuals with two or three copies of the CHRFAM7A gene who also smoked showed a higher rate of smoking cessation success under varenicline therapy [67].
The presence of CHRNA7 and CHRFAM7A, in this locus, could increase the probability that the deregulation (gene dosage; altered gene expression) of even one of the two genes could lead to the onset of neuropsychiatric pathologies [55,100,101]. The expression of α7 is reduced in multiple areas of postmortem brain specimens from schizophrenic patients and from patients with major psychiatric disorders [55,102,103,104,105,106], and 21 polymorphisms have been identified in the promoter region, most of which decrease transcription and are strongly associated with schizophrenia and P50 deficits [34,107,108]. Polymorphisms in both the coding region and the introns that result in splice variants were identified in CHRNA7 and CHRFAM7A genes [109], but none of them were associated with schizophrenia, thus reinforcing the assumption that the receptor is functionally normal and that the causative defect may rely on the expression level of CHRNA7. Deletions involving CHRNA7 are rare but are strongly associated with schizophrenia [72,73]: these patients only have one copy of CHRNA7 and two copies of CHRFAM7A, leading to an altered CHRNA7/CHRFAM7A ratio, whereas 15q13.3 duplication has been linked to childhood-onset schizophrenia [110]. A reduced CHRNA7:CHRFAM7A ratio, due to increased CHRFAM7A and/or reduced CHRNA7 expression, has been reported in the prefrontal cortex of patients with bipolar disorder and schizophrenia [55,59].
Conversely, reduced CHRFAM7A expression has been measured in the peripheral blood lymphocytes of schizophrenic patients [111,112] and was determined to be related to illness severity. A recent study [100] examined the expression of CHRFAM7A in the peripheral blood lymphocytes of schizophrenic patients, and found a significant negative correlation between CHRFAM7A expression and a negative psychopathology score (SANS), but not with a positive score (SAPS). Pairwise analyses before and after antipsychotic treatment revealed an increase in CHRFAM7A gene expression during follow-ups compared to the baseline, thus suggesting that CHRFAM7A has a role in schizophrenia pathogenesis and treatment. The effect of antipsychotics on CHRFAM7A expression has not been found in previous studies [55]. This discrepancy may be explained by limitations in both studies: there is missing knowledge about CHRNA7 expression (not detected in lymphocytes), the genotype of the analyzed patients in Kalmady’s study, and the use of postmortem human brain samples in Kunii’s analyses, where confounding factors, such as the antemortem use of antipsychotic and antidepressant drugs as well as of substance of abuse, may contribute to the changes observed in the patients in those studies.
Several studies have investigated whether the Δ2bp allelic variant of CHRFAM7A could confer susceptibility to schizophrenia [8,9,84,113,114,115,116,117,118,119]. Similar to CHRNA7, controversial data have been obtained, probably due to differences in ethnic groups, in the phenotype considered (schizophrenia, P50 deficit, antisaccade performance), and in small sample sizes. Sinkus and colleagues [9] reported that despite the deleted CHRFAM7A allele being more frequent in Caucasians than in African-Americans, its presence is significantly associated with schizophrenia in both ethnic groups, but the number of CHRFAM7A alleles [8], and the presence of at least one copy of the deleted allele [113,117], is sufficient to have a P50 sensory gating deficit. These data have not been confirmed by another study [55], which reported no differences in the allelic frequency of Δ2bp and no association with schizophrenia, but instead reported an association with decreased CHRFAM7A expression in all subjects, including in the control groups. Moreover, statistical analyses [109] revealed that Δ2bp is less frequent in individuals with CHRNA7 promoter variants [34], which are responsible for decreased α7 expression and are linked to schizophrenia. Other studies have linked this polymorphism to major depressive disorder [78], bipolar disorder [114], and deficits in episodic memory [116], but not to antisaccade performance [119], the latter two being endophenotypes of schizophrenia.
These studies seem to conclude that, overall, the mutations in the CHRNA7/CHRFAM7A “gene cluster” which are associated with neuropsychiatric disorders, fall into the CHRNA7 promoter, the Δ2bp deletion of CHRFAM7A and, although very rare, deletion of the CHRNA7 gene. Moreover, the deregulated expression of both genes (up- and down-regulated) could also contribute to altering the CHRNA7/CHRFAM7A ratio, thus contributing to an increased detrimental effect of dupα7 on α7 nAChR function.
3.2. Epilepsy and Neurodevelopmental Disorders
The impact of CHRFAM7A gene expression and the presence of the Δ2bp polymorphism has also been evaluated in several neurological disorders, including in epilepsy and autism spectrum disorders (ASD). A susceptibility locus for the common idiopathic generalized epilepsies (IGEs) has been mapped to the 15q13-q14 region, with a strong association with microdeletions of Chr 15q13.3 [10,36,120,121,122,123,124]. Rozycka et al. [120] determine an association between the Δ2bp polymorphism and IGE. In a population-based study, they reported that the frequency of Δ2bp carriers was lower in the cohort of IGE patients versus in the cohort of healthy controls, thus providing a protective effect against IGE. This finding is in contrast to what was found in schizophrenic patients [8] and the authors were unable to exclude the possibility that the Δ2bp is in linkage disequilibrium (LD) with the true causative polymorphism contributing to IGE [120]. The protective effect could only be explained by considering the negative effect of dupα7 when translated from a wild-type allele, something that is limited in Δ2bp allele carriers, and by considering the CHRFAM7A Δ2bp allele as a null allele that is unable to be translated into a functional protein. This would lead to an increase in functional α7 receptors.
The study by Rozycka [120] does not report any indication regarding CHRFAM7A expression levels. However, independent studies reported a 1.5 Mb microdeletion of the region encompassing the CHRNA7 gene in a small fraction of IGE patients [121,123,124,125], thus confirming that α7 nAChR may play a fundamental role in IGE pathogenesis. On the other hand, a 15q13.3 duplication involving CHRNA7 has been implicated as being a risk factor for attention deficit hyperactivity disorder (ADHD) [38]. The presence of CNV and the Δ2bp in the CHRFAM7A gene, along with the microduplication in the CHRNA7 gene, has been associated with phenotypic variation in a family with Tourette syndrome, ADHD, and obsessive-compulsive disorders (OCD) [126]. It is worth noting that the expression levels of duplicated genes do not always follow the gene copy number, as shown in brain samples with maternal 15q duplication [127], and for CHRNA7, in a neuronal cell model of 15q duplication [128], because of epigenetic alterations that can contribute to defining the CHRNA7/CHRFAM7A ratio. Indeed, significantly reduced CHRNA7 expression has been reported in the frontal cortex of individuals with Rett syndrome or with typical ASD [60]. It was hypothesized that MeCP2 modulates both CHRNA7 and CHRFAM7A expression through long-range chromatin interactions with the 15q11.2–13.3 region, which includes the Prader-Willi/Angelman syndrome’s imprinting center (PWS-IC). MeCP2 deficiency caused by mutations, such as in Rett syndrome [129], or decreased expression, such as in autism samples [130], influences chromatin loop organization, thus leading to altered gene expression.
A lack of CHRFAM7A expression in the CD4+ T-lymphocytes of autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) patients [131], a form of epilepsy that is mainly associated with mutations in other nAChR subunits (CHRNA2, CHRNA4 and CHRNB2), suggests that there might be a link between the expression of CHRFAM7A and the occurrence of ADNFLE symptoms [131].
3.3. Inflammatory Diseases: The “Cholinergic Anti-Inflammatory Pathway”
Neural reflex circuits [132,133] regulate the immune system response to pathogens during the inflammatory process. The central nervous system (CNS) senses inflammation by means of specialized cells in the brain vasculature, choroid plexus, and circumventricular organs as well as through Toll-like receptors (TLRs) and cytokine receptors in the brain. The observation that vagal nerve stimulation attenuated the systemic inflammatory response in rats under endotoxemia, and that the inhibition of the pro-inflammatory cytokines was due to the acetylcholine stimulation of the nicotinic receptors expressed on macrophages, led to the discovery of the “cholinergic anti-inflammatory pathway (CAIP)” [134]. Later studies, reviewed in Hoover, 2017 [33], defined the anatomy of this inflammatory reflex and showed that α7 nAChR plays a major role in the vagal inhibition of the inflammatory response to endotoxemia [32], despite the presence of other nAChRs on immune cells [33,135].
Functional studies on the dupα7 gene in leukocytes revealed the essential role that it plays in CAIP activation [53]. In the macrophages, where dupα7 is expressed at higher levels compared to α7 [49], pro-inflammatory stimuli down-regulate its expression, which is counterbalanced by an increase in CHRNA7 [49,53], with an NF-κB dependent mechanism. These findings incite the hypothesis that in physiological situations, the high CHRFAM7A/CHRNA7 ratio allows for the formation of hetero-pentamers due to a large contribution of dupα7, or due to the intracellular retention of α7 [31,50], thus contributing to blocking CAIP activation. In the presence of inflammatory signals, the down-regulation of CHRFAM7A, which is paralleled by increased CHRNA7 expression, will result in the formation of functional α7 nAChRs on the cell membrane and activation of CAIP, subsequently dampening inflammation. Due to pathological status, the altered regulation of both subunits will lead to an altered CHRFAM7A/CHRNA7 ratio, consequently deregulating CAIP response.
Uncontrolled systemic inflammation leads to sepsis [136], the leading cause of death in intensive care units (ICUs). One of the main causes of its pathogenesis may rely on an imbalance between excessive inflammation and anti-inflammatory mechanisms, and was reviewed in van der Poll et al. 2021 [137]. During an inflammatory challenge imposed by sepsis, α7 mRNA levels increased during acute illness and returned to the levels found in the controls once the patient recovered [138]. An inverse correlation between the CHRNA7 expression level and CAIP activity, and disease severity and mortality, was reported in a cohort of patients with sepsis [138] that had no deaths among patients expressing high levels of CHRNA7, thus suggesting that measuring the α7 expression level in PBMC may serve as a prognostic marker for sepsis. A later analysis [64] showed that patients showing a high CHRFAM7A/CHRNA7 ratio have a poor prognosis compared to patients expressing higher CHRNA7 levels. A similar conclusion was drawn by Baird and colleagues [139], who showed that CHRFAM7A expression was increased, and that CHRNA7 expression was decreased in Inflammatory Bowel Disease (IBD), thus suggesting that CHRFAM7A may be an unrecognized target for the development of therapeutics for IBD. These data are in accordance with previously reported increases of CHRFAM7A caused by the LPS in gut epithelial cells [56], suggesting a different role for dupα7 in this tissue type.
α7 nAChR activation plays a protective role in osteoarthritis (OA), since its absence was associated with severe cartilage degradation in a murine meniscectomy α7 KO model, whereas α7 nAChR activation decreased the IL-1β-induced chondrocyte inflammation [27]. The chondrocytes of OA patients showed elevated CHRFAM7A expression [27], which was positively correlated with increased Metalloproteinase-3 (MMP-3) expression, thus suggesting it has a role in the pathophysiology of OA. α7 nAChR silencing, and treatment with specific agonists, revealed a role for α7 nAChR in controlling joint inflammation in a collagen-induced arthritis rat model of rheumatoid arthritis (RA) [140], and in the synovial tissues and fibroblast-like synoviocytes of patients with RA [62]. These tissues express α7and dupα7 receptors, but since there is no information regarding their expression levels in RA compared to in healthy tissues, no conclusions about the role of CHRFAM7A in RA can be drawn.
A protective role for dupα7 has also been proposed. Cerebral ischemia/reperfusion (I/R) injury [141] is an inflammatory-related disorder that is caused by the abnormal activation of the nod-like receptor protein 3 (NLRP3) inflammasome, and microglia pyroptosis, following the disruption to blood supply after an ischemic event [142]. In a case-control study [141], in patients with cerebral I/R injury, the CHRFAM7A level was negatively related to the expression of inflammatory cytokines. These data are in accordance with the reported down-regulation of CHRFAM7A by inflammatory stimuli [53]. The CHRFAM7A overexpression in OGD/R-treated microglia human cell lines, a model of I/R injury, reverted the clinical observation by increasing cell proliferation, decreasing the release of pro-inflammatory cytokines, and promoting M1 to M2 microglia polarization [141], thus suggesting a protective role for CHRFAM7A in the attenuation of inflammatory injury of microglia.
CHRFAM7A is also down-regulated in hypertrophic scars (HTS) [143]. In a human HTS-like SCID mouse model, transfected CHRFAM7A played a positive role in the amelioration of HTS formation [143] by decreasing TGF-β and CTFG expression and by increasing MMP-1 expression, and conversely to the I/R injury model, attenuating M2 macrophage polarization via the activation of the Notch pathway. An increase in CHRFAM7A expression has been measured in radiotherapy-induced lacrimal gland injury, resulting in the inhibition of the p38/JNK signaling pathway and oxidative stress [144].
An imbalanced inflammatory response is responsible for tissue damage and COVID-19 severity [145]. The cholinergic system has been proposed as one of the regulators of COVID-19 induced hypercytokinemia [146,147,148,149,150]. CHRFAM7A expression showed a slight tendency to decrease its level in a cohort of SARS-CoV-2 patients compared with healthy controls [14]. When patients were stratified according to disease severity, CHRFAM7A expression was significantly lower in critical COVID-19 patients. Again, this is in accordance with inflammatory-induced CHRFAM7A down-regulation [53]. Absence of CHRFAM7A expression was also associated with increased inflammatory biomarkers, including the C-reactive protein, lymphopenia, extension pulmonary lesions, and plasma viral load, as well as the enhanced expression of genes in the TNF signaling pathway [14]. Ongoing trials are evaluating the usefulness of nicotine to determine the risk of developing the SARS-CoV-2 infection and severe disease; however, these data have led to the hypothesis that individuals with no CHRFAM7A duplication, and/or who are carriers of Δ2bp, may develop symptomatic and severe COVID-19. This aspect is challenging and deserves further investigation.
All these studies suffer from the lack of CHRNA7 expression analysis, making it difficult to draw conclusions about the significance of a CHRFAM7A increase in I/R injury. It is possible to hypothesize that the increase in CHRFAM7A is a step in the inflammation resolution process. In accordance with this hypothesis, is a study on the effect of donepezil [49], an acetylcholine esterase inhibitor (AChEi) used as a symptomatic therapy in AD. Besides its inhibitory effect, it has been proposed as an anti-inflammatory mechanism for this class of compounds [151]. In macrophages, donepezil synergizes with LPS to increase CHRNA7 expression, resulting in the potentiation of CAIP. The parallel increase in CHRFAM7A expression may suggest a role in controlling excessive CAIP activation, with the consequence of an impaired response to inflammatory stimuli [31]. In COVID-19 patients, such deregulation in the cholinergic system may explain severe prognosis. In view of this, CHRFAM7A may be considered as a target for intervention in the fine modulation of CAIP and in the restoration of a homeostatic state.
In individuals with traumatic spinal cord injury (SCI), the presence of the Δ2bp polymorphism may affect clinical outcomes [13,152] depending on the severity of the injury. In severe SCI, Δ2bp carriers show higher levels of circulating inflammatory molecules (TNF-α, INF-γ, IL-13, CCL11, IL-12p70, IL-8, CXCL10, CCL4, IL-12p40, IL-1β, IL-15 and IL-2), whereas in mild SCI, IL-15 is lower in Δ2bp carriers than it is Δ2bp non-carriers. Temporal variations in inflammatory mediator analyses have revealed that severe SCI Δ2bp carriers had higher levels of IL-8 and CCL2 during the acute phase post-SCI, but no variations were observed in mild SCI. These two molecules are important for macrophage and neutrophil migration and infiltration modulators, as theyplay an important role in the early phases of inflammation post-SCI. Moreover, neuropathic pain was positively associated with IL-13 in Δ2bp carriers, but only in severe SCI. Conversely, Δ2bp carriers with mild SCI showed that a higher risk of pressure ulcers was positively correlated with the circulating levels of IFN-γ, CXCL10, and CCL4, and negatively associated with IL-12p70 levels.
Animal studies on the association between IL-13 and neuropathic pain produced opposite results [153], thus confirming the importance of including CHRFAM7A genotyping in human studies, and that the presence of dupΔα7 can modify the anti-inflammatory function of α7 receptor after SCI and the response to α7 ligands for inflammatory and pain control [154].
Other Roles for CHRFAM7A
The higher expression of CHRFAM7A in macrophages compared to CHRNA7 suggests other roles for dupα7 in regulating monocyte/macrophage activation other than the regulation of α7 nAChR activity. The transgenic expression of CHRFAM7A, in a model of human systemic inflammatory response syndrome (SIRS) due to severe injury and sepsis, showed an increase in the hematopoietic stem cell (HSC) reservoir in bone marrow, and increased immune cell mobilization, myeloid cell differentiation, and inflammatory monocytes in inflamed lungs [11]. These data confirmed the importance of CHRFAM7A in physiological, pathophysiological, and species-specific responses, and point to its role as a doubled-edge sword, being both protective and detrimental in an emergency myelopoiesis model upon injury. Indeed, limiting HSC exhaustion upon injury can increase the responsiveness to a second stimulus, thus improving, for example, survival.
The overexpression of CHRFAM7A in monocyte-like cell lines has shown reduced cell migration, chemotaxis to the monocyte chemo-attractant protein (MCP-1), and the inhibition of anchorage-independent colony formation [155]. The mechanism of this effect is still unknown and is in contrast with previous data from the same group [11,54], which reported that the increased expression of gene encoding for proteins favors cell migration, adhesion, and the formation of cell clusters upon CHRFAM7A overexpression. Other affected pathways tied to pro-inflammatory conditions [156,157] include Type L interferon and cancer pathways [54]. Unexpectedly, CHRFAM7A overexpression increased the CHRNA7 level [54], resulting in increased α-Bgtx binding. This is in contrast with previously discussed data [50,52,63,64], but it corroborates the finding that CHRNA7 and CHRFAM7A expression are co-regulated [49,53,56]. Thus, we cannot rule out that many of the direct effects of CHRFAM7A overexpression in the modulation of different signaling pathways are indeed the result of the modulation of the metabotropic function of α7 nAChR [158].
3.4. Neurodegenerative Diseases
Epidemiological [159] and in vitro studies indicate a protective role of nicotine assumption in Alzheimer’s (AD) and Parkinson’s (PD) disease against glutamate and β-amyloid-related cytotoxicity [160], and on dopaminergic neurons via an anti-inflammatory mechanism that is mediated by the modulation of microglial activation and astrocyte apoptosis [161,162]. α7 nAChR is expressed by microglial cells, where it plays important roles in controlling their activation [163,164], and several reports support the existence of a brain CAIP [18,165,166].
One of the hallmarks of AD and PD is the loss of cholinergic neurons and a selective decrease in the number of nicotinic receptors (reviewed in [160,167]) that is associated with an inflammatory state, which is generally caused by the hyper-activation of the microglia [168,169]. For these reasons, the cholinergic system is one of the therapeutic targets in AD. Acetylcholinesterase inhibitors (AChEi), such as galantamine or donepezil, are indeed effective, and ameliorate the cognitive symptoms of AD [170].
Genetic variations in the CHRNA7 gene, or the altered expression and function of α7 nAChR have been associated with AD [41,160,171,172] and PD [173,174], and in the case of AD, the reduced α7 expression is correlated with beta-amyloid (Aβ) plaque deposition and cognitive impairments [41,160,175]. The high-affinity binding of Aβ oligomers to α7 nAChR shows Aβ concentration-dependent opposite effects. At physiological concentrations (pM to low nM range), Aβ triggers a change in α7 to a desensitized conformation that still responds to agonists, while at higher concentrations (nM to low μM range), Aβ acts as a negative modulator [176,177,178,179]. This results in the activation of neuroprotective (pM range) or neurotoxic (nM range) signaling pathways [167,177,180,181]. Moreover, Aβ-α7 interaction leads to intraneuronal accumulation of Aβ1–42 and Aβ-induced tau protein phosphorylation [182,183].
CHRFAM7A CNV is over-represented in mild cognitive impairment (MCI) and late-onset AD patients [184,185,186]. Ordered-subset analysis (OSA) by age at AD onset confirmed CHRFAM7A as one of the loci that can confer the risk of AD [187] in a subset of patients. Here, reduced CHRFAM7A expression in human post-mortem temporal lobe tissue was correlated with the disease state (AD vs. controls) and with CNV [187]. In view of this, the increased functional α7 nAChR due to lower CHRFAM7A expression is able to sustain enhanced Aβ1–42 internalization and neuronal vulnerability, and therapies with α7 antagonists may be more effective in these AD patients.
The presence of the Δ2bp polymorphism did not confer susceptibility to Alzheimer’s disease in a case-control study [188]. Patients were stratified by three different CHRFAM7A genotypes (genotype 1: two wild-type alleles; genotype 2: one wild-type allele and one Δ2bp allele; genotype 3: two Δ2bp alleles) in four types of dementia, including AD, dementia with Lewy’s bodies (DLB), Pick’s disease (PiD), and vascular dementia (VD). The results showed that the CHRFAM7A genotype with two wild-type copies of the gene was over-represented in the AD, DLB, and PiD cases, but not in VD [189], which may be due to the heterogeneity of the underlying pathology [190]. This observation indicates that the Δ2bp polymorphism, as a protective factor against the development of these forms of dementia, which are associated with abnormal protein aggregations, and again, we have to hypothesize that this may occur if dupΔα7 behaves as a null allele, results in increased α7 nAChR assembly. The protective role of nicotine in AD and PD reinforces this hypothesis since nicotine, apart from stimulating α7 nAChR, down-regulates the mRNA of CHRFAM7A [50].
These apparently controversial results may depend on multiple confounding factors, such as the age of onset, the duration of the disease from diagnosis, and the type of disease.
However, therapeutic approaches aimed at both stimulating or antagonizing α7 nAChR failed to translate into humans [191].
By using median ganglionic eminence (MGE) neuronal progenitors, obtained by the neuronal differentiation of human induced pluripotent stem cells (iPSC) from AD patients with 0 or 1 copies of CHRFAM7A, it has been shown [12,192] that dupα7 decreases the probability of the α7 nAChR channel opening in the presence of the positive allosteric modulator (PAM) PNU 120,596 (PNU) with currents that desensitize faster [192], and this desensitization increases as a function of the CHRFAM7A dosage. These data suggest that the number of CHRFAM7A copies may affect the response to α7 PAM, agonists, and antagonists in different individuals. Moreover, dupα7 mitigates Aβ1–42 uptake at a higher concentration, as well as the α7-dependent Aβ-induced inflammatory response, suggesting a protective role in AD during the Aβ1–42 accumulation phase. At higher Aβ concentrations, the presence of dupα7 increases IL-1β and TNF-α release as an ultimate signal for the activation of an anti-inflammatory response. In this sense, the absence of CHRFAM7A is a risk factor for AD [192]. In this iPSC model, dupΔα7 behaves as a null allele [12], as its expression does not affect PNU-modulated α7 nAChR currents and Aβ1–42 uptake.
Of greater interest, is that dupα7 expression affects the response to AChEi (donepezil and rivastigmine) and encenicline, a selective α7 agonist used in AD therapy [12]. In CHRFAM7A non-carriers, the drugs reduced Aβ1–42 uptake, and Aβ1–42 induced toxicity and apoptosis. On the other hand, in CHRFAM7A carrier cells, the neurotoxicity caused by donepezil and encenicline was unchanged, and donepezil increased apoptosis. These data suggest that CHRFAM7A may affect drug response.
Non-carriers of the functional CHRFAM7A allele comprise 25% of the human population, whereas 75% are carriers, with no differences being observed between normal aged controls and AD patients, thus suggesting that CHRFAM7A is not associated with a disease phenotype, which is in contrast with the data discussed above [184,185,186,187,189]. A double-blind pharmacogenetics study [12] showed that non-carriers of the functional CHRFAM7A allele had a superior response to the AChEi, which is in agreement with the iPSC model, and this lasted over a 7-year observation period. The CHRFAM7A carriers did not demonstrate a treatment response effect.
To demonstrate the role of CHRFAM7A in Aβ-associated neuroinflammation, the same group [193] showed that dupα7 also mitigates Aβ1–42 uptake in iPSC-derived microglial-like cells, but unlike the iPSC-derived neurons, the presence of CHRFAM7A induced a high NF-κB-mediated innate immune response by prolonging its nuclear presence, resulting in microglia activation. In these cells, nicotine paradoxically increases the proinflammatory response to LPS in CHRFAM7A carrier cell lines, thus suggesting that the presence of dupα7 antagonizes the anti-inflammatory role of homomeric α7. These data are in line with earlier results obtained from the overexpression of CHRFAM7A in RAW264.7 murine macrophages [64], but not with the findings of other groups [141,143]. A limit of these studies is that most of the conclusions rely on data obtained from comparing a CHRFAM7A non-carrier cell line and its isogenic counterpart, which expresses CHRFAM7A under the control of a constitutive promoter. LPS, via an NF-κB dependent mechanism, and nicotine, down-regulate CHRFAM7A expression [50,53], an aspect that might lead to different conclusions, and it would be interesting to know whether Aβ1–42 plays a similar role. Similar data on the LPS/nicotine synergism obtained in a native CHRFAM7A carrier cell line [193] may suffer from long-term LPS treatment (24 h), as it has been shown that 3–6 h of LPS down-regulates CHRFAM7A expression [53] that returns to basal level after 24 h, but no data on CHRFAM7A expression have been presented. On the other hand, long-term exposure to LPS may resemble a chronic inflammatory insult, such as that caused by Aβ in AD, where an excessive inflammatory insult may lead to CAIP dysregulation.
Proteomic profiling in a CHRFAM7A transgenic mouse brain [71] showed that dupα7 overexpression modulates the expression of proteins involved in the α7 nAChR signaling pathways, and that they are related to the pathogenesis of neurological and neuropsychiatric disorders, including Parkinson’s and Alzheimer’s disease. In particular, most of these genes are mitochondrial components, and are involved in the maintenance of the anti-oxidative response, whose deregulation is one of the main causes of neurodegenerative and neuropsychiatric disorders.
The HIV-1 (human immunodeficiency virus type-1) envelope protein gp120IIIB induces α7 nAChR in neuronal cells and in the brain, particularly in the striatum, the basal ganglia’s primary input, and promotes cell death in a calcium-dependent manner, an effect abrogated by α7 antagonists [194]. Conversely, CHRFAM7A is down-regulated in some patients [61], thus increasing the CHRNA7/CHRFAM7A ratio. α7/dupα7 deregulation may thus play a role in the development of HIV-1-associated neurocognitive disorders (HAND) [61].
3.5. Cancer
The dysregulation of nAChRs gene expression and/or the dysfunction of these receptors may be involved in the development of lung tumors [45]. α7 nAChR is overexpressed in squamous cells, but not in adenocarcinoma lung tumors, and is a determinant for the progression of this type of cancer. Nicotine further increases CHRNA7 expression and function, mediating its proliferative, survival, and angiogenic effects. In both cell types, increased α5 and β4 accompanies dupα7 and β3 down-regulation. In particular, reduced CHRFAM7A expression may facilitate the α7-mediated oncogenic process by relieving the negative effect of dupα7. Indeed, its overexpression in two NSCLC cell lines (A549 and SK-MES-1) blocked nicotine- or NKK-induced tumor progression, both in vitro and in vivo, in an athymic mouse model implanted with A549dupα7 or A549 xenografts [195]. These data suggest that dupα7 can be considered as a therapeutic target in smoking-related cancers.
4. Conclusions
Ever since its discovery in 1998, understanding the nature and function of the CHRFAM7A gene has become a great challenge to respond to the failure of translating α7-directed therapeutic approaches into humans, with treatments aimed at both stimulating or antagonizing α7 nAChR. Its assembly with the α7 subunit leads to the formation of heteromeric receptors, which show reduced ion conductance and a reduced affinity for α-Bgtx, thus suggesting a negative role of dupα7 on α7 nAChR function.
The great amount of data on CHRFAM7A involvement in neurological and non-neurological disorders reported here, and summarized in Table 2, showed a contrasting effect, being protective in some cases and related to a poor prognosis or a susceptibility gene in the pathogenesis of that disorder in others, but these studies have highlighted the impact of this gene in a variety of α7 nAChR-related disorders. These confounding results may suffer from being clinical studies based on small sample sizes and from the lack of expression levels detected from both CHRNA7 and CHRFAM7A and/or CHRFAM7A genotyping. Most importantly, the CHRFAM7A copy number and the presence of the Δ2bp polymorphism influence the quality of protein products, and therefore, the possible effect on α7 nAChR. Moreover, dupα7 can play a different role depending on the tissue type, thus shifting the ionotropic feature of α7 nAChR to that of a metabotropic receptor, as indicated by the modulation of signaling pathways upon CHRFAM7A overexpression. One important issue to take into account is that the expression levels of dupα7 and α7 vary in different tissues and can be modulated by various stimuli. The deregulated expression of both genes (up- and down-regulated) thus contribute to altering the CHRNA7/CHRFAM7A ratio, paving the way to an increased detrimental effect of dupα7 on α7 nAChR function.

Table 2.
CHRFAM7A involvement in neurological and non-neurological disorders. ↓ Reduced expression; ↑ increased expression; =, unchanged; n.d., not determined; und, undetectable. AD, Alzheimer’s disease; DLB, Dementia with Lewy’s bodies; PiD, Pick’s disease; PD, Parkinson’s disease; HAND, HIV-1-associated neurocognitive disorder; HSC, hematopoietic stem cell; NSCLC, non-small cell lung carcinoma. Numbers in square brackets indicate references.
Many questions are still pending; in particular, the use of the CHRFAM7A expression level, which can be measured via blood samples, as a prognostic marker even in neurological disorders, or as therapeutic target, but whether to reduce or increase its expression, remains to be elucidated. On the other hand, computational model studies will be very important for the design of drugs targeting heteromeric receptors.
Author Contributions
Conceptualization, R.B., S.D.L. and D.F.; writing, R.B.; artworks, S.D.L.; writing-review and editing, R.B., S.D.L. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gault, J.; Robinson, M.; Berger, R.; Drebing, C.; Logel, J.; Hopkins, J.; Moore, T.; Jacobs, S.; Meriwether, J.; Choi, M.J.; et al. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics 1998, 52, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.; Williamson, M.; Collier, D.; Wilkie, H.; Makoff, A. A 3-Mb map of a large Segmental duplication overlapping the alpha7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics 2002, 79, 197–209. [Google Scholar] [CrossRef] [PubMed]
- O’Bleness, M.; Searles, V.B.; Varki, A.; Gagneux, P.; Sikela, J.M. Evolution of genetic and genomic features unique to the human lineage. Nat. Rev. Genet. 2012, 13, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Locke, D.P.; Archidiacono, N.; Misceo, D.; Cardone, M.F.; Deschamps, S.; Roe, B.; Rocchi, M.; Eichler, E.E. Refinement of a chimpanzee pericentric inversion breakpoint to a segmental duplication cluster. Genome Biol. 2003, 4, R50. [Google Scholar] [CrossRef]
- Locke, D.P.; Jiang, Z.; Pertz, L.M.; Misceo, D.; Archidiacono, N.; Eichler, E.E. Molecular evolution of the human chromosome 15 pericentromeric region. Cytogenet. Genome Res. 2005, 108, 73–82. [Google Scholar] [CrossRef]
- Flomen, R.H.; Davies, A.F.; Di Forti, M.; La Cascia, C.; Mackie-Ogilvie, C.; Murray, R.; Makoff, A.J. The copy number variant involving part of the alpha7 nicotinic receptor gene contains a polymorphic inversion. Eur. J. Hum. Genet. 2008, 16, 1364–1371. [Google Scholar] [CrossRef]
- Szafranski, P.; Schaaf, C.P.; Person, R.E.; Gibson, I.B.; Xia, Z.; Mahadevan, S.; Wiszniewska, J.; Bacino, C.A.; Lalani, S.; Potocki, L.; et al. Structures and molecular mechanisms for common 15q13.3 microduplications involving CHRNA7: Benign or pathological? Hum. Mutat. 2010, 31, 840–850. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 2015, 96, 274–288. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Lee, M.J.; Gault, J.; Logel, J.; Short, M.; Freedman, R.; Christian, S.L.; Lyon, J.; Leonard, S. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009, 1291, 1–11. [Google Scholar] [CrossRef]
- Taske, N.L.; Williamson, M.P.; Makoff, A.; Bate, L.; Curtis, D.; Kerr, M.; Kjeldsen, M.J.; Pang, K.A.; Sundqvist, A.; Friis, M.L.; et al. Evaluation of the positional candidate gene CHRNA7 at the juvenile myoclonic epilepsy locus (EJM2) on chromosome 15q13-14. Epilepsy Res 2002, 49, 157–172. [Google Scholar] [CrossRef]
- Costantini, T.W.; Chan, T.W.; Cohen, O.; Langness, S.; Treadwell, S.; Williams, E.; Eliceiri, B.P.; Baird, A. Uniquely human CHRFAM7A gene increases the hematopoietic stem cell reservoir in mice and amplifies their inflammatory response. Proc. Natl. Acad. Sci. USA 2019, 116, 7932–7940. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, K.; Ihnatovych, I.; Birkaya, B.; Chen, Z.; Ouf, A.; Indurthi, D.C.; Bard, J.E.; Kann, J.; Adams, A.; Chaves, L.; et al. CHRFAM7A: A human specific fusion gene, accounts for the translational gap for cholinergic strategies in Alzheimer’s disease. EBioMedicine 2020, 59, 102892. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Huang, W.; Kabbani, N.; Theiss, M.M.; Hamilton, J.F.; Ecklund, J.M.; Conley, Y.P.; Vodovotz, Y.; Brienza, D.; Wagner, A.K.; et al. Effect of CHRFAM7A Δ2bp gene variant on secondary inflammation after spinal cord injury. PLoS ONE 2021, 16, e0251110. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Boussier, J.; Hadjadj, J.; Yatim, N.; Barnabei, L.; Péré, H.; Veyer, D.; Kernéis, S.; Carlier, N.; Pène, F.; et al. Regulation of the acetylcholine/α7nAChR anti-inflammatory pathway in COVID-19 patients. Sci. Rep. 2021, 11, 11886. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef]
- Fasoli, F.; Gotti, C. Structure of neuronal nicotinic receptors. Curr. Top. Behav. Neurosci. 2015, 23, 1–17. [Google Scholar] [CrossRef]
- Schaaf, C.P. Nicotinic acetylcholine receptors in human genetic disease. Genet. Med. 2014, 16, 649–656. [Google Scholar] [CrossRef]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front. Cell. Neurosci. 2020, 14, 577912. [Google Scholar] [CrossRef]
- Patel, H.; McIntire, J.; Ryan, S.; Dunah, A.; Loring, R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J. Neuroinflamm. 2017, 14, 192. [Google Scholar] [CrossRef]
- Song, P.; Spindel, E.R. Basic and clinical aspects of non-neuronal acetylcholine: Expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J. Pharmacol. Sci. 2008, 106, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Meizel, S. Evidence suggesting that the mouse sperm acrosome reaction initiated by the zona pellucida involves an alpha7 nicotinic acetylcholine receptor. Biol. Reprod. 2003, 68, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.E.; Whelan, C.J.; Parsons, M.E. Nicotinic acetylcholine receptor subunits and receptor activity in the epithelial cell line HT29. Life Sci. 2003, 72, 2091–2094. [Google Scholar] [CrossRef]
- Saeed, R.W.; Varma, S.; Peng-Nemeroff, T.; Sherry, B.; Balakhaneh, D.; Huston, J.; Tracey, K.J.; Al-Abed, Y.; Metz, C.N. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005, 201, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Ritzenthaler, J.D.; Gil-Acosta, A.; Rivera, H.N.; Roser-Page, S. Nicotine and fibronectin expression in lung fibroblasts: Implications for tobacco-related lung tissue remodeling. FASEB J. 2004, 18, 1436–1438. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P.S.; Katz, D.A.; Rosas-Ballina, M.; Levine, Y.A.; Ochani, M.; Valdés-Ferrer, S.I.; Pavlov, V.A.; Tracey, K.J.; Chavan, S.S. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 2012, 18, 539–543. [Google Scholar] [CrossRef]
- Courties, A.; Do, A.; Leite, S.; Legris, M.; Sudre, L.; Pigenet, A.; Petit, J.; Nourissat, G.; Cambon-Binder, A.; Maskos, U.; et al. The role of the non-neuronal cholinergic system in inflammation and degradation processes in osteoarthritis. Arthritis Rheumatol. 2020, 72, 2072–2082. [Google Scholar] [CrossRef]
- Skok, M.; Grailhe, R.; Agenes, F.; Changeux, J.P. The role of nicotinic acetylcholine receptors in lymphocyte development. J. Neuroimmunol. 2006, 171, 86–98. [Google Scholar] [CrossRef]
- Skok, M.; Grailhe, R.; Changeux, J.P. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur. J. Pharmacol. 2005, 517, 246–251. [Google Scholar] [CrossRef]
- de Jonge, W.J.; Ulloa, L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef]
- Benfante, R.; Di Lascio, S.; Cardani, S.; Fornasari, D. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: A new therapeutic perspective in aging-related disorders. Aging Clin. Exp. Res. 2021, 33, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Yang, H.; Ulloa, L.; Al-Abed, Y.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017, 179, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Gault, J.; Hopkins, J.; Logel, J.; Vianzon, R.; Short, M.; Drebing, C.; Berger, R.; Venn, D.; Sirota, P.; et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry 2002, 59, 1085–1096. [Google Scholar] [CrossRef]
- Bacchelli, E.; Battaglia, A.; Cameli, C.; Lomartire, S.; Tancredi, R.; Thomson, S.; Sutcliffe, J.S.; Maestrini, E. Analysis of CHRNA7 rare variants in autism spectrum disorder susceptibility. Am. J. Med. Genet. A 2015, 167A, 715–723. [Google Scholar] [CrossRef]
- Damiano, J.A.; Mullen, S.A.; Hildebrand, M.S.; Bellows, S.T.; Lawrence, K.M.; Arsov, T.; Dibbens, L.; Major, H.; Dahl, H.H.; Mefford, H.C.; et al. Evaluation of multiple putative risk alleles within the 15q13.3 region for genetic generalized epilepsy. Epilepsy Res. 2015, 117, 70–73. [Google Scholar] [CrossRef]
- Masurel-Paulet, A.; Andrieux, J.; Callier, P.; Cuisset, J.M.; Le Caignec, C.; Holder, M.; Thauvin-Robinet, C.; Doray, B.; Flori, E.; Alex-Cordier, M.P.; et al. Delineation of 15q13.3 microdeletions. Clin. Genet. 2010, 78, 149–161. [Google Scholar] [CrossRef]
- Williams, N.M.; Franke, B.; Mick, E.; Anney, R.J.; Freitag, C.M.; Gill, M.; Thapar, A.; O’Donovan, M.C.; Owen, M.J.; Holmans, P.; et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13.3. Am. J. Psychiatry 2012, 169, 195–204. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Burket, J.A.; Benson, A.D. Targeting the α7 nicotinic acetylcholine receptor to prevent progressive dementia and improve cognition in adults with Down’s syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 131–139. [Google Scholar] [CrossRef]
- Quik, M.; Zhang, D.; McGregor, M.; Bordia, T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem. Pharmacol. 2015, 97, 399–407. [Google Scholar] [CrossRef]
- Ma, K.G.; Qian, Y.H. Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides 2019, 73, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Tropea, M.R.; Li Puma, D.D.; Melone, M.; Gulisano, W.; Arancio, O.; Grassi, C.; Conti, F.; Puzzo, D. Genetic deletion of α7 nicotinic acetylcholine receptors induces an age-dependent Alzheimer’s disease-like pathology. Prog. Neurobiol. 2021, 206, 102154. [Google Scholar] [CrossRef] [PubMed]
- Mucchietto, V.; Fasoli, F.; Pucci, S.; Moretti, M.; Benfante, R.; Maroli, A.; Di Lascio, S.; Bolchi, C.; Pallavicini, M.; Dowell, C.; et al. α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br. J. Pharmacol. 2018, 175, 1957–1972. [Google Scholar] [CrossRef]
- Pucci, S.; Fasoli, F.; Moretti, M.; Benfante, R.; Di Lascio, S.; Viani, P.; Daga, A.; Gordon, T.J.; McIntosh, M.; Zoli, M.; et al. Choline and nicotine increase glioblastoma cell proliferation by binding and activating α7- and α9- containing nicotinic receptors. Pharmacol. Res. 2021, 163, 105336. [Google Scholar] [CrossRef] [PubMed]
- Bordas, A.; Cedillo, J.L.; Arnalich, F.; Esteban-Rodriguez, I.; Guerra-Pastrián, L.; de Castro, J.; Martín-Sánchez, C.; Atienza, G.; Fernández-Capitan, C.; Rios, J.J.; et al. Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget 2017, 8, 67878–67890. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef]
- Vieira-Alves, I.; Coimbra-Campos, L.M.C.; Sancho, M.; da Silva, R.F.; Cortes, S.F.; Lemos, V.S. Role of the α7 Nicotinic Acetylcholine Receptor in the Pathophysiology of Atherosclerosis. Front. Physiol. 2020, 11, 621769. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef]
- Maroli, A.; Di Lascio, S.; Drufuca, L.; Cardani, S.; Setten, E.; Locati, M.; Fornasari, D.; Benfante, R. Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: A possible link to the cholinergic anti-inflammatory pathway. J. Neuroimmunol. 2019, 332, 155–166. [Google Scholar] [CrossRef]
- de Lucas-Cerrillo, A.M.; Maldifassi, M.C.; Arnalich, F.; Renart, J.; Atienza, G.; Serantes, R.; Cruces, J.; Sánchez-Pacheco, A.; Andrés-Mateos, E.; Montiel, C. Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: Potential implications for the cholinergic anti-inflammatory response. J. Biol. Chem. 2011, 286, 594–606. [Google Scholar] [CrossRef]
- Araud, T.; Graw, S.; Berger, R.; Lee, M.; Neveu, E.; Bertrand, D.; Leonard, S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem. Pharmacol. 2011, 82, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, C.; Indersmitten, T.; Freedman, R.; Leonard, S.; Lester, H.A. The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J. Biol. Chem. 2014, 289, 26451–26463. [Google Scholar] [CrossRef] [PubMed]
- Benfante, R.; Antonini, R.A.; De Pizzol, M.; Gotti, C.; Clementi, F.; Locati, M.; Fornasari, D. Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Neuroimmunol. 2011, 230, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Dang, X.; Yurchyshyna, M.V.; Coimbra, R.; Eliceiri, B.P.; Baird, A. A human-specific α7-nicotinic acetylcholine receptor gene in human leukocytes: Identification, regulation and the consequences of CHRFAM7A expression. Mol. Med. 2015, 21, 323–336. [Google Scholar] [CrossRef]
- Kunii, Y.; Zhang, W.; Xu, Q.; Hyde, T.M.; McFadden, W.; Shin, J.H.; Deep-Soboslay, A.; Ye, T.; Li, C.; Kleinman, J.E.; et al. CHRNA7 and CHRFAM7A mRNAs: Co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am. J. Psychiatry 2015, 172, 1122–1130. [Google Scholar] [CrossRef]
- Dang, X.; Eliceiri, B.P.; Baird, A.; Costantini, T.W. CHRFAM7A: A human-specific α7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 2015, 29, 2292–2302. [Google Scholar] [CrossRef][Green Version]
- Villiger, Y.; Szanto, I.; Jaconi, S.; Blanchet, C.; Buisson, B.; Krause, K.H.; Bertrand, D.; Romand, J.A. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J. Neuroimmunol. 2002, 126, 86–98. [Google Scholar] [CrossRef]
- Martín-Sánchez, C.; Alés, E.; Balseiro-Gómez, S.; Atienza, G.; Arnalich, F.; Bordas, A.; Cedillo, J.L.; Extremera, M.; Chávez-Reyes, A.; Montiel, C. The human-specific duplicated α7 gene inhibits the ancestral α7, negatively regulating nicotinic acetylcholine receptor-mediated transmitter release. J. Biol. Chem. 2021, 296, 100341. [Google Scholar] [CrossRef]
- De Luca, V.; Likhodi, O.; Van Tol, H.H.; Kennedy, J.L.; Wong, A.H. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 2006, 114, 211–215. [Google Scholar] [CrossRef]
- Yasui, D.H.; Scoles, H.A.; Horike, S.; Meguro-Horike, M.; Dunaway, K.W.; Schroeder, D.I.; Lasalle, J.M. 15q11.2-13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet. 2011, 20, 4311–4323. [Google Scholar] [CrossRef]
- Ramos, F.M.; Delgado-Vélez, M.; Ortiz, Á.; Báez-Pagán, C.A.; Quesada, O.; Lasalde-Dominicci, J.A. Expression of CHRFAM7A and CHRNA7 in neuronal cells and postmortem brain of HIV-infected patients: Considerations for HIV-associated neurocognitive disorder. J. Neurovirol. 2016, 22, 327–335. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, M.A.; Stoof, S.P.; van der Zanden, E.P.; de Jonge, W.J.; Janssen, R.A.; Fischer, D.F.; Vandeghinste, N.; Brys, R.; Vervoordeldonk, M.J.; Tak, P.P. The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: A possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009, 60, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Lasala, M.; Corradi, J.; Bruzzone, A.; Esandi, M.D.C.; Bouzat, C. A human-specific, truncated alpha7 nicotinic receptor subunit assembles with full-length alpha7 and forms functional receptors with different stoichiometries. J. Biol. Chem. 2018, 293, 10707–10717. [Google Scholar] [CrossRef] [PubMed]
- Maldifassi, M.C.; Martín-Sánchez, C.; Atienza, G.; Cedillo, J.L.; Arnalich, F.; Bordas, A.; Zafra, F.; Giménez, C.; Extremera, M.; Renart, J.; et al. Interaction of the α7-nicotinic subunit with its human-specific duplicated dupα7 isoform in mammalian cells: Relevance in human inflammatory responses. J. Biol. Chem. 2018, 293, 13874–13888. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Sine, S.M. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature 2005, 438, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Bouzat, C.; Gumilar, F.; Spitzmaul, G.; Wang, H.L.; Rayes, D.; Hansen, S.B.; Taylor, P.; Sine, S.M. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 2004, 430, 896–900. [Google Scholar] [CrossRef]
- Cameli, C.; Bacchelli, E.; De Paola, M.; Giucastro, G.; Cifiello, S.; Collo, G.; Cainazzo, M.M.; Pini, L.A.; Maestrini, E.; Zoli, M. Genetic variation in CHRNA7 and CHRFAM7A is associated with nicotine dependence and response to varenicline treatment. Eur. J. Hum. Genet. 2018, 26, 1824–1831. [Google Scholar] [CrossRef]
- Andersen, N.; Corradi, J.; Sine, S.M.; Bouzat, C. Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 20819–20824. [Google Scholar] [CrossRef]
- Liu, D.; de Souza, J.V.; Ahmad, A.; Bronowska, A.K. Structure, dynamics, and ligand recognition of human-specific CHRFAM7A (Dupα7) nicotinic receptor linked to neuropsychiatric disorders. Int. J. Mol. Sci. 2021, 22, 5466. [Google Scholar] [CrossRef]
- Chan, T.; Williams, E.; Cohen, O.; Eliceiri, B.P.; Baird, A.; Costantini, T.W. CHRFAM7A alters binding to the neuronal alpha-7 nicotinic acetylcholine receptor. Neurosci. Lett. 2019, 690, 126–131. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, H.; Huang, L.; Hou, X.; Zhou, R.; Dang, X. Global proteomic profiling of the uniquely human CHRFAM7A gene in transgenic mouse brain. Gene 2019, 714, 143996. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Rujescu, D.; Cichon, S.; Pietiläinen, O.P.; Ingason, A.; Steinberg, S.; Fossdal, R.; Sigurdsson, E.; Sigmundsson, T.; Buizer-Voskamp, J.E.; et al. Large recurrent microdeletions associated with schizophrenia. Nature 2008, 455, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.L.; O’Donovan, M.C.; Gurling, H.; Kirov, G.K.; Blackwood, D.H.R.; Corvin, A.; Craddock, N.J.; Gill, M.; Hultman, C.M.; Lichtenstein, P.; et al. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 2008, 455, 237–241. [Google Scholar] [CrossRef]
- Leonard, S.; Freedman, R. Genetics of chromosome 15q13-q14 in schizophrenia. Biol. Psychiatry 2006, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Elmslie, F.V.; Rees, M.; Williamson, M.P.; Kerr, M.; Kjeldsen, M.J.; Pang, K.A.; Sundqvist, A.; Friis, M.L.; Chadwick, D.; Richens, A.; et al. Genetic mapping of a major susceptibility locus for juvenile myoclonic epilepsy on chromosome 15q. Hum. Mol. Genet. 1997, 6, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, B.A.; Fiedler, B.; Himmelein, B.; Kämpfer, F.; Lässker, U.; Schwabe, G.; Spanier, I.; Tams, D.; Bretscher, C.; Moldenhauer, K.; et al. Centrotemporal spikes in families with rolandic epilepsy: Linkage to chromosome 15q14. Neurology 1998, 51, 1608–1612. [Google Scholar] [CrossRef]
- Joo, E.J.; Lee, K.Y.; Kim, H.S.; Kim, S.H.; Ahn, Y.M.; Kim, Y.S. Genetic association study of the alpha 7 nicotinic receptor (CHRNA7) with the development of schizophrenia and bipolar disorder in korean population. Psychiatry Investig. 2010, 7, 196–201. [Google Scholar] [CrossRef]
- Lai, I.C.; Hong, C.J.; Tsai, S.J. Association study of nicotinic-receptor variants and major depressive disorder. J. Affect. Disord. 2001, 66, 79–82. [Google Scholar] [CrossRef]
- Stassen, H.H.; Bridler, R.; Hägele, S.; Hergersberg, M.; Mehmann, B.; Schinzel, A.; Weisbrod, M.; Scharfetter, C. Schizophrenia and smoking: Evidence for a common neurobiological basis? Am. J. Med. Genet. 2000, 96, 173–177. [Google Scholar] [CrossRef]
- Cooper, G.M.; Coe, B.P.; Girirajan, S.; Rosenfeld, J.A.; Vu, T.H.; Baker, C.; Williams, C.; Stalker, H.; Hamid, R.; Hannig, V.; et al. A copy number variation morbidity map of developmental delay. Nat. Genet. 2011, 43, 838–846. [Google Scholar] [CrossRef]
- Kaminsky, E.B.; Kaul, V.; Paschall, J.; Church, D.M.; Bunke, B.; Kunig, D.; Moreno-De-Luca, D.; Moreno-De-Luca, A.; Mulle, J.G.; Warren, S.T.; et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet. Med. 2011, 13, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.R.; Noor, A.; Vincent, J.B.; Lionel, A.C.; Feuk, L.; Skaug, J.; Shago, M.; Moessner, R.; Pinto, D.; Ren, Y.; et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008, 82, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Blouin, J.L.; Radhakrishna, U.; Gehrig, C.; Lasseter, V.K.; Wolyniec, P.; Nestadt, G.; Dombroski, B.; Kazazian, H.H.; Pulver, A.E.; et al. No evidence for linkage between schizophrenia and markers at chromosome 15q13-14. Am. J. Med. Genet. 1999, 88, 109–112. [Google Scholar] [CrossRef]
- Iwata, Y.; Nakajima, M.; Yamada, K.; Nakamura, K.; Sekine, Y.; Tsuchiya, K.J.; Sugihara, G.; Matsuzaki, H.; Suda, S.; Suzuki, K.; et al. Linkage disequilibrium analysis of the CHRNA7 gene and its partially duplicated region in schizophrenia. Neurosci. Res. 2007, 57, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Neves-Pereira, M.; Bassett, A.S.; Honer, W.G.; Lang, D.; King, N.A.; Kennedy, J.L. No evidence for linkage of the CHRNA7 gene region in Canadian schizophrenia families. Am. J. Med. Genet. 1998, 81, 361–363. [Google Scholar] [CrossRef]
- Sanders, A.R.; Duan, J.; Levinson, D.F.; Shi, J.; He, D.; Hou, C.; Burrell, G.J.; Rice, J.P.; Nertney, D.A.; Olincy, A.; et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: Implications for psychiatric genetics. Am. J. Psychiatry 2008, 165, 497–506. [Google Scholar] [CrossRef]
- Tsuang, D.W.; Skol, A.D.; Faraone, S.V.; Bingham, S.; Young, K.A.; Prabhudesai, S.; Haverstock, S.L.; Mena, F.; Menon, A.S.; Bisset, D.; et al. Examination of genetic linkage of chromosome 15 to schizophrenia in a large Veterans Affairs Cooperative Study sample. Am. J. Med. Genet. 2001, 105, 662–668. [Google Scholar] [CrossRef]
- Tsuang, M.T.; Stone, W.S.; Faraone, S.V. Schizophrenia: A review of genetic studies. Harv. Rev. Psychiatry 1999, 7, 185–207. [Google Scholar] [CrossRef]
- Freedman, R.; Coon, H.; Myles-Worsley, M.; Orr-Urtreger, A.; Olincy, A.; Davis, A.; Polymeropoulos, M.; Holik, J.; Hopkins, J.; Hoff, M.; et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. USA 1997, 94, 587–592. [Google Scholar] [CrossRef]
- Freedman, R.; Olincy, A.; Ross, R.G.; Waldo, M.C.; Stevens, K.E.; Adler, L.E.; Leonard, S. The genetics of sensory gating deficits in schizophrenia. Curr. Psychiatry Rep. 2003, 5, 155–161. [Google Scholar] [CrossRef]
- Leonard, S.; Adler, L.E.; Benhammou, K.; Berger, R.; Breese, C.R.; Drebing, C.; Gault, J.; Lee, M.J.; Logel, J.; Olincy, A.; et al. Smoking and mental illness. Pharmacol. Biochem. Behav. 2001, 70, 561–570. [Google Scholar] [CrossRef]
- Glassman, A.H. Cigarette smoking: Implications for psychiatric illness. Am. J. Psychiatry 1993, 150, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.H.; Franks, A.; Berger, R.; Palionyte, M.; Fingerlin, T.E.; Wagner, B.; Logel, J.; Olincy, A.; Ross, R.G.; Freedman, R.; et al. Multiple genes in the 15q13-q14 chromosomal region are associated with schizophrenia. Psychiatr. Genet. 2012, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Mexal, S.; Freedman, R. Smoking, genetics and schizophrenia: Evidence for self medication. J. Dual. Diagn. 2007, 3, 43–59. [Google Scholar] [CrossRef]
- Koike, K.; Hashimoto, K.; Takai, N.; Shimizu, E.; Komatsu, N.; Watanabe, H.; Nakazato, M.; Okamura, N.; Stevens, K.E.; Freedman, R.; et al. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr. Res. 2005, 76, 67–72. [Google Scholar] [CrossRef]
- Toyohara, J.; Hashimoto, K. α7 Nicotinic receptor agonists: Potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and alzheimer’s disease. Open Med. Chem. J. 2010, 4, 37–56. [Google Scholar] [CrossRef][Green Version]
- Mackowick, K.M.; Barr, M.S.; Wing, V.C.; Rabin, R.A.; Ouellet-Plamondon, C.; George, T.P. Neurocognitive endophenotypes in schizophrenia: Modulation by nicotinic receptor systems. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 52, 79–85. [Google Scholar] [CrossRef][Green Version]
- Parikh, V.; Kutlu, M.G.; Gould, T.J. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: Current trends and perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef]
- Severance, E.G.; Dickerson, F.B.; Stallings, C.R.; Origoni, A.E.; Sullens, A.; Monson, E.T.; Yolken, R.H. Differentiating nicotine- versus schizophrenia-associated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J. Neural Transm. 2009, 116, 213–220. [Google Scholar] [CrossRef]
- Kalmady, S.V.; Agrawal, R.; Venugopal, D.; Shivakumar, V.; Amaresha, A.C.; Agarwal, S.M.; Subbanna, M.; Rajasekaran, A.; Narayanaswamy, J.C.; Debnath, M.; et al. CHRFAM7A gene expression in schizophrenia: Clinical correlates and the effect of antipsychotic treatment. J. Neural Transm. 2018, 125, 741–748. [Google Scholar] [CrossRef]
- Bencherif, M.; Stachowiak, M.K.; Kucinski, A.J.; Lippiello, P.M. Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med. Hypotheses 2012, 78, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.; Hall, M.; Adler, L.E.; Leonard, S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry 1995, 38, 22–33. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Zhang, X.; Blennow, K.; Nordberg, A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 1999, 10, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Guillozet-Bongaarts, A.L.; Hyde, T.M.; Dalley, R.A.; Hawrylycz, M.J.; Henry, A.; Hof, P.R.; Hohmann, J.; Jones, A.R.; Kuan, C.L.; Royall, J.; et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry 2014, 19, 478–485. [Google Scholar] [CrossRef]
- Marutle, A.; Zhang, X.; Court, J.; Piggott, M.; Johnson, M.; Perry, R.; Perry, E.; Nordberg, A. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J. Chem. Neuroanat. 2001, 22, 115–126. [Google Scholar] [CrossRef]
- Court, J.; Spurden, D.; Lloyd, S.; McKeith, I.; Ballard, C.; Cairns, N.; Kerwin, R.; Perry, R.; Perry, E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: Alpha-bungarotoxin and nicotine binding in the thalamus. J. Neurochem. 1999, 73, 1590–1597. [Google Scholar] [CrossRef]
- Stephens, S.H.; Logel, J.; Barton, A.; Franks, A.; Schultz, J.; Short, M.; Dickenson, J.; James, B.; Fingerlin, T.E.; Wagner, B.; et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr. Res. 2009, 109, 102–112. [Google Scholar] [CrossRef]
- Bertelsen, B.; Oranje, B.; Melchior, L.; Fagerlund, B.; Werge, T.M.; Mikkelsen, J.D.; Tümer, Z.; Glenthøj, B.Y. Association study of CHRNA7 promoter variants with sensory and sensorimotor gating in schizophrenia patients and healthy controls: A danish case-control study. Neuromolecular. Med. 2015, 17, 423–430. [Google Scholar] [CrossRef]
- Gault, J.; Hopkins, J.; Berger, R.; Drebing, C.; Logel, J.; Walton, C.; Short, M.; Vianzon, R.; Olincy, A.; Ross, R.G.; et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 123B, 39–49. [Google Scholar] [CrossRef]
- Zhou, D.; Gochman, P.; Broadnax, D.D.; Rapoport, J.L.; Ahn, K. 15q13.3 duplication in two patients with childhood-onset schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 777–783. [Google Scholar] [CrossRef]
- Perl, O.; Strous, R.D.; Dranikov, A.; Chen, R.; Fuchs, S. Low levels of alpha7-nicotinic acetylcholine receptor mRNA on peripheral blood lymphocytes in schizophrenia and its association with illness severity. Neuropsychobiology 2006, 53, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Perl, O.; Ilani, T.; Strous, R.D.; Lapidus, R.; Fuchs, S. The alpha7 nicotinic acetylcholine receptor in schizophrenia: Decreased mRNA levels in peripheral blood lymphocytes. FASEB J. 2003, 17, 1948–1950. [Google Scholar] [CrossRef] [PubMed]
- Raux, G.; Bonnet-Brilhault, F.; Louchart, S.; Houy, E.; Gantier, R.; Levillain, D.; Allio, G.; Haouzir, S.; Petit, M.; Martinez, M.; et al. The -2 bp deletion in exon 6 of the ‘alpha 7-like’ nicotinic receptor subunit gene is a risk factor for the P50 sensory gating deficit. Mol. Psychiatry 2002, 7, 1006–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, C.J.; Lai, I.C.; Liou, L.L.; Tsai, S.J. Association study of the human partially duplicated alpha7 nicotinic acetylcholine receptor genetic variant with bipolar disorder. Neurosci. Lett. 2004, 355, 69–72. [Google Scholar] [CrossRef]
- Flomen, R.H.; Collier, D.A.; Osborne, S.; Munro, J.; Breen, G.; St Clair, D.; Makoff, A.J. Association study of CHRFAM7A copy number and 2 bp deletion polymorphisms with schizophrenia and bipolar affective disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 571–575. [Google Scholar] [CrossRef]
- Dempster, E.L.; Toulopoulou, T.; McDonald, C.; Bramon, E.; Walshe, M.; Wickham, H.; Sham, P.C.; Murray, R.M.; Collier, D.A. Episodic memory performance predicted by the 2bp deletion in exon 6 of the “alpha 7-like” nicotinic receptor subunit gene. Am. J. Psychiatry 2006, 163, 1832–1834. [Google Scholar] [CrossRef]
- Flomen, R.H.; Shaikh, M.; Walshe, M.; Schulze, K.; Hall, M.H.; Picchioni, M.; Rijsdijk, F.; Toulopoulou, T.; Kravariti, E.; Murray, R.M.; et al. Association between the 2-bp deletion polymorphism in the duplicated version of the alpha7 nicotinic receptor gene and P50 sensory gating. Eur. J. Hum. Genet. 2013, 21, 76–81. [Google Scholar] [CrossRef]
- Lai, I.C.; Hong, C.J.; Tsai, S.J. Association study of a nicotinic receptor variant with schizophrenic disorders. Neuropsychobiology 2001, 43, 15–18. [Google Scholar] [CrossRef]
- Petrovsky, N.; Schmechtig, A.; Flomen, R.H.; Kumari, V.; Collier, D.; Makoff, A.; Wagner, M.; Ettinger, U. CHRFAM7A copy number and 2-bp deletion polymorphisms and antisaccade performance. Int. J. Neuropsychopharmacol. 2009, 12, 267–273. [Google Scholar] [CrossRef]
- Rozycka, A.; Dorszewska, J.; Steinborn, B.; Lianeri, M.; Winczewska-Wiktor, A.; Sniezawska, A.; Wisniewska, K.; Jagodzinski, P.P. Association study of the 2-bp deletion polymorphism in exon 6 of the CHRFAM7A gene with idiopathic generalized epilepsy. DNA Cell Biol. 2013, 32, 640–647. [Google Scholar] [CrossRef]
- Helbig, I.; Mefford, H.C.; Sharp, A.J.; Guipponi, M.; Fichera, M.; Franke, A.; Muhle, H.; de Kovel, C.; Baker, C.; von Spiczak, S.; et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat. Genet. 2009, 41, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Mullen, S.A.; Carvill, G.L.; Bellows, S.; Bayly, M.A.; Trucks, H.; Lal, D.; Sander, T.; Berkovic, S.F.; Dibbens, L.M.; Scheffer, I.E.; et al. Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology 2013, 81, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Mefford, H.C.; Muhle, H.; Ostertag, P.; von Spiczak, S.; Buysse, K.; Baker, C.; Franke, A.; Malafosse, A.; Genton, P.; Thomas, P.; et al. Genome-wide copy number variation in epilepsy: Novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010, 6, e1000962. [Google Scholar] [CrossRef] [PubMed]
- Dibbens, L.M.; Mullen, S.; Helbig, I.; Mefford, H.C.; Bayly, M.A.; Bellows, S.; Leu, C.; Trucks, H.; Obermeier, T.; Wittig, M.; et al. Familial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: Precedent for disorders with complex inheritance. Hum. Mol. Genet. 2009, 18, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Muhle, H.; Mefford, H.C.; Obermeier, T.; von Spiczak, S.; Eichler, E.E.; Stephani, U.; Sander, T.; Helbig, I. Absence seizures with intellectual disability as a phenotype of the 15q13.3 microdeletion syndrome. Epilepsia 2011, 52, e194–e198. [Google Scholar] [CrossRef] [PubMed]
- Melchior, L.; Bertelsen, B.; Debes, N.M.; Groth, C.; Skov, L.; Mikkelsen, J.D.; Brøndum-Nielsen, K.; Tümer, Z. Microduplication of 15q13.3 and Xq21.31 in a family with Tourette syndrome and comorbidities. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162B, 825–831. [Google Scholar] [CrossRef]
- Hogart, A.; Leung, K.N.; Wang, N.J.; Wu, D.J.; Driscoll, J.; Vallero, R.O.; Schanen, N.C.; LaSalle, J.M. Chromosome 15q11-13 duplication syndrome brain reveals epiGenet.ic alterations in gene expression not predicted from copy number. J. Med. Genet. 2009, 46, 86–93. [Google Scholar] [CrossRef]
- Meguro-Horike, M.; Yasui, D.H.; Powell, W.; Schroeder, D.I.; Oshimura, M.; Lasalle, J.M.; Horike, S. Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum. Mol. Genet. 2011, 20, 3798–3810. [Google Scholar] [CrossRef]
- Good, K.V.; Vincent, J.B.; Ausió, J. MeCP2: The Genet.ic driver of rett syndrome epiGenet.ics. Front. Genet. 2021, 12, 620859. [Google Scholar] [CrossRef]
- Nagarajan, R.P.; Hogart, A.R.; Gwye, Y.; Martin, M.R.; LaSalle, J.M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. EpiGenetics 2006, 1, e1–e11. [Google Scholar] [CrossRef]
- Rozycka, A.; Dorszewska, J.; Steinborn, B.; Kempisty, B.; Lianeri, M.; Wisniewska, K.; Jagodzinski, P.P. A transcript coding for a partially duplicated form of α7 nicotinic acetylcholine receptor is absent from the CD4+ T-lymphocytes of patients with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). Folia Neuropathol. 2013, 51, 65–75. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Murray, K.; Lomax, A.E. Neuroimmune communication in health and disease. Physiol. Rev. 2018, 98, 2287–2316. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, C.C.; Alejandra, T.G.; Guadalupe, D.K.J.; Herminia, V.G.; Lenin, P.; Enrique, B.V.; Evandro, B.M.; Oscar, B.; Iván, G.M. Modulation of the extraneuronal cholinergic system on main innate response leukocytes. J. Neuroimmunol. 2019, 327, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Cedillo, J.L.; Arnalich, F.; Martín-Sánchez, C.; Quesada, A.; Rios, J.J.; Maldifassi, M.C.; Atienza, G.; Renart, J.; Fernández-Capitán, C.; García-Rio, F.; et al. Usefulness of α7 nicotinic receptor messenger RNA levels in peripheral blood mononuclear cells as a marker for cholinergic antiinflammatory pathway activity in septic patients: Results of a pilot study. J. Infect. Dis. 2015, 211, 146–155. [Google Scholar] [CrossRef]
- Baird, A.; Coimbra, R.; Dang, X.; Eliceiri, B.P.; Costantini, T.W. Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 2016, 5, 66–71. [Google Scholar] [CrossRef]
- van Maanen, M.A.; Lebre, M.C.; van der Poll, T.; LaRosa, G.J.; Elbaum, D.; Vervoordeldonk, M.J.; Tak, P.P. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009, 60, 114–122. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Gao, L. CHRFAM7A Overexpression attenuates cerebral ischemia-reperfusion injury via inhibiting microglia pyroptosis mediated by the NLRP3/Caspase-1 pathway. Inflammation 2021, 44, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yu, S.; Chen, X.; Ye, R.; Zhu, W.; Liu, X. NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol. Disord. Drug Targets 2016, 15, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, W.; Zhang, Q.; Deng, C. Human-specific gene CHRFAM7A mediates M2 macrophage polarization via the Notch pathway to ameliorate hypertrophic scar formation. Biomed. Pharmacother. 2020, 131, 110611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, J.; Ren, H.; Meng, F.; Ma, R.; Xu, B. Human-specific CHRFAM7A protects against radiotherapy-induced lacrimal gland injury by inhibiting the p38/JNK signalling pathway and oxidative stress. Int. J. Clin. Exp. Pathol. 2017, 10, 9001–9011. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Changeux, J.P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for COVID-19 with preventive and therapeutic implications. C. R. Biol. 2020, 343, 33–39. [Google Scholar] [CrossRef]
- Farsalinos, K.; Angelopoulou, A.; Alexandris, N.; Poulas, K. COVID-19 and the nicotinic cholinergic system. Eur. Respir. J. 2020, 56, 2001589. [Google Scholar] [CrossRef]
- Gonzalez-Rubio, J.; Navarro-Lopez, C.; Lopez-Najera, E.; Lopez-Najera, A.; Jimenez-Diaz, L.; Navarro-Lopez, J.D.; Najera, A. Cytokine release syndrome (CRS) and nicotine in COVID-19 patients: Trying to calm the storm. Front. Immunol. 2020, 11, 1359. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with COVID-19? Bioelectron. Med. 2020, 6, 15. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55, 2001116. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Huang, W.; Kabbani, N.; Brannan, T.K.; Lin, M.K.; Theiss, M.M.; Hamilton, J.F.; Ecklund, J.M.; Conley, Y.P.; Vodovotz, Y.; Brienza, D.; et al. Association of a functional polymorphism in the CHRFAM7A gene with inflammatory response mediators and neuropathic pain after spinal cord injury. J. Neurotrauma 2019, 36, 3026–3033. [Google Scholar] [CrossRef]
- Kiguchi, N.; Sakaguchi, H.; Kadowaki, Y.; Saika, F.; Fukazawa, Y.; Matsuzaki, S.; Kishioka, S. Peripheral administration of interleukin-13 reverses inflammatory macrophage and tactile allodynia in mice with partial sciatic nerve ligation. J. Pharmacol. Sci. 2017, 133, 53–56. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef]
- Chan, T.W.; Langness, S.; Cohen, O.; Eliceiri, B.P.; Baird, A.; Costantini, T.W. CHRFAM7A reduces monocyte/macrophage migration and colony formation in vitro. Inflamm. Res. 2020, 69, 631–633. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.R.; Hahn, M.J. Regulation of inflammatory gene expression in macrophages by epithelial-stromal interaction 1 (Epsti1). Biochem. Biophys. Res. Commun. 2018, 496, 778–783. [Google Scholar] [CrossRef]
- York, M.R.; Nagai, T.; Mangini, A.J.; Lemaire, R.; van Seventer, J.M.; Lafyatis, R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007, 56, 1010–1020. [Google Scholar] [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the channel: Metabotropic signaling by nicotinic receptors. Trends. Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Wang, H.X. Smoking and Parkinson’s and Alzheimer’s disease: Review of the epidemiological studies. Behav. Brain Res. 2000, 113, 117–120. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, P.H.; Ahn, Y.W.; Choi, Y.J.; Lee, G.; Lee, D.Y.; Chung, E.S.; Jin, B.K. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur. J. Neurosci. 2007, 26, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, X.; Hui, Y.; Zhu, C.; Wu, J.; Taylor, D.H.; Ji, J.; Fan, W.; Huang, Z.; Hu, J. Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: Implications for Parkinson’s disease. Neuropharmacology 2015, 91, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; León, R.; Lopez, M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Gillevet, T.C.; Kabbani, N. A G protein-coupled alpha7 nicotinic receptor regulates signaling and TNF-alpha release in microglia. FEBS Open Biol. 2017, 7, 1350–1361. [Google Scholar] [CrossRef]
- De Simone, R.; Ajmone-Cat, M.A.; Carnevale, D.; Minghetti, L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J. Neuroinflamm. 2005, 2, 4. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Kalkman, H.O.; Feuerbach, D. Modulatory effects of α7 nAChRs on the immune system and its relevance for CNS disorders. Cell Mol. Life Sci. 2016, 73, 2511–2530. [Google Scholar] [CrossRef]
- Taylor, P. Development of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease. Neurology 1998, 51, S30–S35, discussion S65–S67. [Google Scholar] [CrossRef]
- Neri, M.; Bonassi, S.; Russo, P. Genet.ic variations in CHRNA7 or CHRFAM7 and susceptibility to dementia. Curr. Drug Targets 2012, 13, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Carson, R.; Craig, D.; McGuinness, B.; Johnston, J.A.; O’Neill, F.A.; Passmore, A.P.; Ritchie, C.W. Alpha7 nicotinic acetylcholine receptor gene and reduced risk of Alzheimer’s disease. J. Med. Genet. 2008, 45, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Dziewczapolski, G.; Glogowski, C.M.; Masliah, E.; Heinemann, S.F. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2009, 29, 8805–8815. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Bordia, T.; Zhang, D.; Perez, X.A. Nicotine and nicotinic receptor drugs: Potential for Parkinson’s disease and drug-induced movement disorders. Int. Rev. Neurobiol. 2015, 124, 247–271. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Green, K.N.; Liang, K.; Tran, L.; Chen, Y.; Leslie, F.M.; LaFerla, F.M. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 3046–3051. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fà, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Yang, K.H.; Petroianu, G. On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 618–630. [Google Scholar] [CrossRef]
- Lasala, M.; Fabiani, C.; Corradi, J.; Antollini, S.; Bouzat, C. Molecular modulation of human α7 Nicotinic receptor by amyloid-β peptides. Front. Cell Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lee, D.H.; Davis, C.B.; Shank, R.P. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J. Neurochem. 2000, 75, 1155–1161. [Google Scholar] [CrossRef]
- Dineley, K.T.; Westerman, M.; Bui, D.; Bell, K.; Ashe, K.H.; Sweatt, J.D. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J. Neurosci. 2001, 21, 4125–4133. [Google Scholar] [CrossRef]
- Parri, H.R.; Hernandez, C.M.; Dineley, K.T. Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer’s disease. Biochem. Pharmacol. 2011, 82, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Nagele, R.G.; D’Andrea, M.R.; Anderson, W.J.; Wang, H.Y. Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience 2002, 110, 199–211. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, W.; Benedetti, N.J.; Lee, D.H. Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J. Biol. Chem. 2003, 278, 31547–31553. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Shen, L.; Kim, S.; Inlow, M.; West, J.D.; Faber, K.M.; Foroud, T.; Mayeux, R.; Saykin, A.J.; Initiative, A.S.D.N.; et al. Analysis of copy number variation in Alzheimer’s disease: The NIALOAD/ NCRAD family study. Curr. Alzheimer Res. 2012, 9, 801–814. [Google Scholar] [CrossRef]
- Swaminathan, S.; Huentelman, M.J.; Corneveaux, J.J.; Myers, A.J.; Faber, K.M.; Foroud, T.; Mayeux, R.; Shen, L.; Kim, S.; Turk, M.; et al. Analysis of copy number variation in Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. PLoS ONE 2012, 7, e50640. [Google Scholar] [CrossRef]
- Swaminathan, S.; Kim, S.; Shen, L.; Risacher, S.L.; Foroud, T.; Pankratz, N.; Potkin, S.G.; Huentelman, M.J.; Craig, D.W.; Weiner, M.W.; et al. Genomic copy number analysis in alzheimer’s disease and mild cognitive impairment: An ADNI study. Int. J. Alzheimers Dis. 2011, 2011, 729478. [Google Scholar] [CrossRef]
- Szigeti, K.; Kellermayer, B.; Lentini, J.M.; Trummer, B.; Lal, D.; Doody, R.S.; Yan, L.; Liu, S.; Ma, C.; Consortium, T.A.R.a.C. Ordered subset analysis of copy number variation association with age at onset of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 1063–1071. [Google Scholar] [CrossRef]
- Liou, Y.J.; Lai, I.C.; Hong, C.J.; Liu, H.C.; Liu, T.Y.; Tsai, S.J. Association analysis of the partially duplicated alpha7 nicotinic acetylcholine receptor Genet.ic variant and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2001, 12, 301–304. [Google Scholar] [CrossRef]
- Fehér, A.; Juhász, A.; Rimanóczy, A.; Csibri, E.; Kálmán, J.; Janka, Z. Association between a Genet.ic variant of the alpha-7 nicotinic acetylcholine receptor subunit and four types of dementia. Dement. Geriatr. Cogn. Disord. 2009, 28, 56–62. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Hansen, H.H.; Timmerman, D.B.; Mikkelsen, J.D. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: From animal models to human pathophysiology. Curr. Pharm. Des. 2010, 16, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Ihnatovych, I.; Nayak, T.K.; Ouf, A.; Sule, N.; Birkaya, B.; Chaves, L.; Auerbach, A.; Szigeti, K. iPSC model of CHRFAM7A effect on α7 nicotinic acetylcholine receptor function in the human context. Transl. Psychiatry 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Ihnatovych, I.; Birkaya, B.; Notari, E.; Szigeti, K. iPSC-derived microglia for modeling human-specific DAMP and PAMP responses in the context of alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 9668. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Capó-Vélez, C.M.; García-Beltrán, W.F.; Ramos, F.M.; Vázquez-Rosa, E.; Ríos, R.; Mercado, J.R.; Meléndez, R.I.; Lasalde-Dominicci, J.A. Up-regulation of the neuronal nicotinic receptor α7 by HIV glycoprotein 120: Potential implications for HIV-associated neurocognitive disorder. J. Biol. Chem. 2012, 287, 3079–3086. [Google Scholar] [CrossRef]
- Cedillo, J.L.; Bordas, A.; Arnalich, F.; Esteban-Rodríguez, I.; Martín-Sánchez, C.; Extremera, M.; Atienza, G.; Rios, J.J.; Arribas, R.L.; Montiel, C. Anti-tumoral activity of the human-specific duplicated form of α7-nicotinic receptor subunit in tobacco-induced lung cancer progression. Lung Cancer 2019, 128, 134–144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).