New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds
Abstract
:1. Introduction
2. Novel Methods for the Green Oxidative Depolymerization of Lignin
2.1. Photochemical and Electrochemical Approaches
2.2. Mechanochemistry in Lignin Oxidation
2.3. Metal Catalysts in the Lignin Oxidation
2.4. Metal-Free Lignin Oxidative Depolymerization
2.5. Selenium Catalyzed Oxidation
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of Biofilms on the Conversion of Cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A Polymer of Caffeyl Alcohol in Plant Seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.; Pettersen, R.; Tshabalala, M. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 33–72. ISBN 9781439853801. [Google Scholar]
- Subbotina, E.; Rukkijakan, T.; Marquez-Medina, M.D.; Yu, X.; Johnsson, M.; Samec, J.S.M. Oxidative Cleavage of C–C Bonds in Lignin. Nat. Chem. 2021, 13, 1118–1125. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products through Kraft Lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Verma, O.P.; Manik, G.; Sethi, S.K. A Comprehensive Review of Renewable Energy Source on Energy Optimization of Black Liquor in MSE Using Steady and Dynamic State Modeling, Simulation and Control. Renew. Sustain. Energy Rev. 2019, 100, 90–109. [Google Scholar] [CrossRef]
- Kim, C.-H.; Lee, J.-Y.; Park, S.-H.; Moon, S.-O. Global Trends and Prospects of Black Liquor as Bioenergy. J. Korea Tech. Assoc. Pulp Pap. Ind. 2019, 51, 3–15. [Google Scholar] [CrossRef]
- Demirbaş, A. Pyrolysis and Steam Gasification Processes of Black Liquor. Energy Convers. Manag. 2002, 43, 877–884. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of Cellulose and Lignin Content on Pyrolysis and Combustion Characteristics for Several Types of Biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Kim, Y.; Lee, J.-H. Chemicals from Lignin: Recent Depolymerization Techniques and Upgrading Extended Pathways. Curr. Opin. Green Sustain. Chem. 2018, 14, 33–39. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, N.; Lapkin, A.A. Towards Circular Economy: Integration of Bio-Waste into Chemical Supply Chain. Curr. Opin. Chem. Eng. 2019, 26, 148–156. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Gu, S.; Luo, K.H. State-of-the-Art Catalytic Hydrogenolysis of Lignin for the Production of Aromatic Chemicals. Catal. Sci. Technol. 2018, 8, 6275–6296. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic Oxidation of Lignin to Valuable Biomass-Based Platform Chemicals: A Review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Vega-Aguilar, C.A.; Rodrigues, A.E. Added-Value Chemicals from Lignin Oxidation. Molecules 2021, 26, 4602. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Li, X.; Zhang, Y. Hydrogen Peroxide as an Oxidant in Biomass-to-Chemical Processes of Industrial Interest. Green Chem. 2019, 21, 5753–5780. [Google Scholar] [CrossRef]
- Mathieu, Y.; Vidal, J.D.; Arribas Martínez, L.; Abad Fernández, N.; Iborra, S.; Corma, A. Molecular Oxygen Lignin Depolymerization: An Insight into the Stability of Phenolic Monomers. ChemSusChem 2020, 13, 4743–4758. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of Green Chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Sharma-Shivappa, R. Heterogeneous Catalytic Oxidation of Lignin into Value-Added Chemicals. Biofuels 2012, 3, 155–166. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K. Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef] [Green Version]
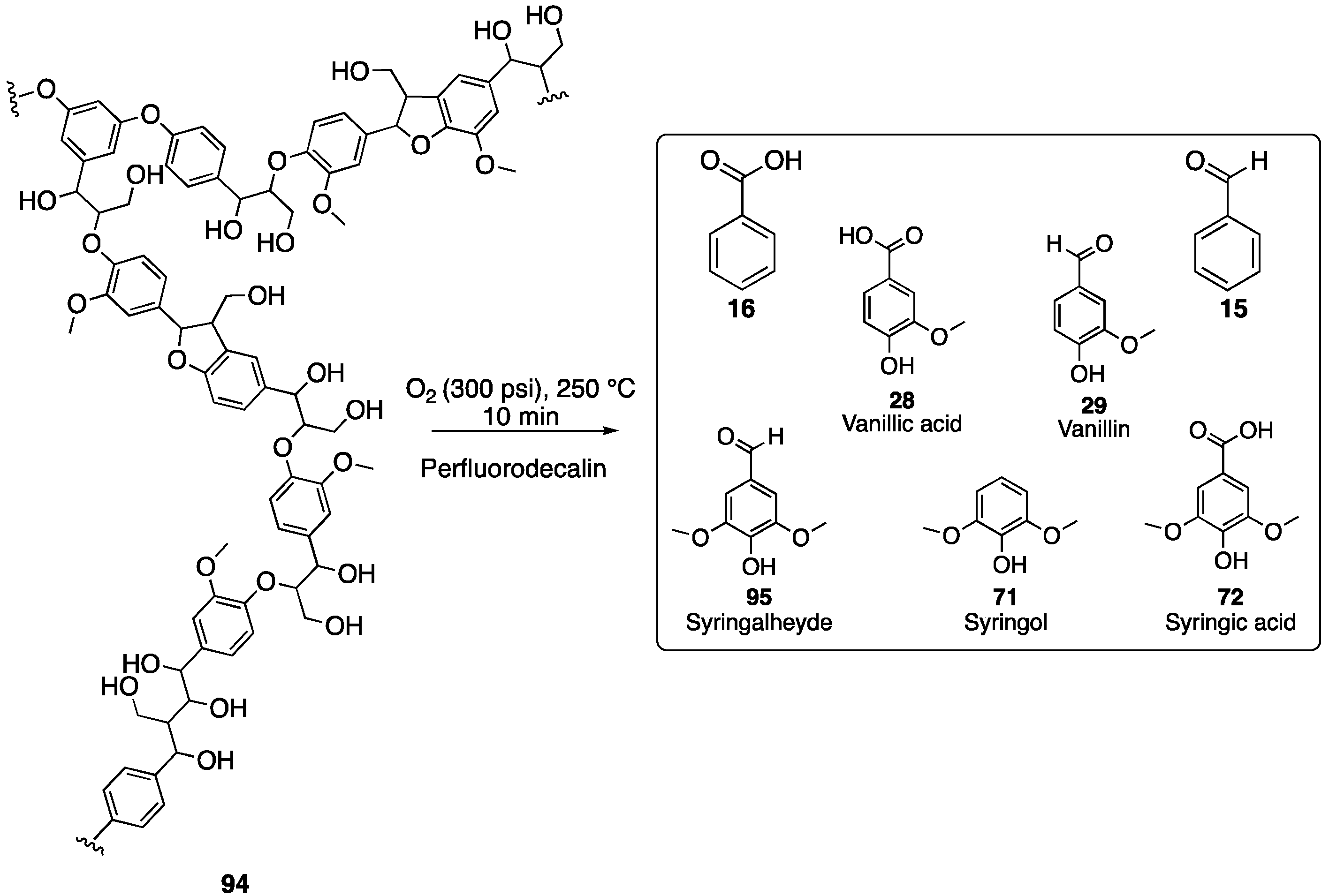
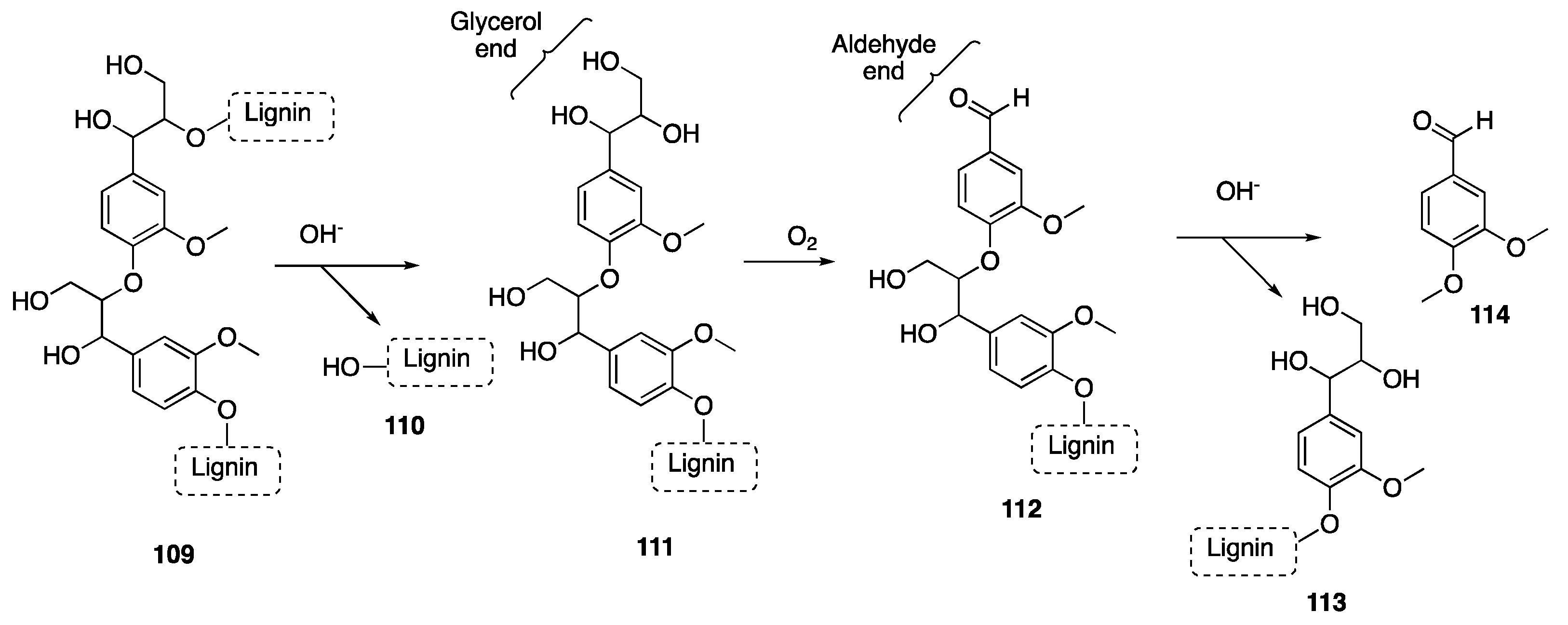
- Hafezisefat, P.; Lindstrom, J.K.; Brown, R.C.; Qi, L. Non-Catalytic Oxidative Depolymerization of Lignin in Perfluorodecalin to Produce Phenolic Monomers. Green Chem. 2020, 22, 6567–6578. [Google Scholar] [CrossRef]
- Khan, A.; Nair, V.; Colmenares, J.C.; Gläser, R. Lignin-Based Composite Materials for Photocatalysis and Photovoltaics. Top. Curr. Chem. 2018, 376, 20. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, S.; Thiruvengetam, P.; Karthick, K.; Sankar, S.S.; Karmakar, A.; Kundu, S. Green and Sustainable Route for Oxidative Depolymerization of Lignin: New Platform for Fine Chemicals and Fuels. Biotechnol. Prog. 2021, 37, e3111. [Google Scholar] [CrossRef]
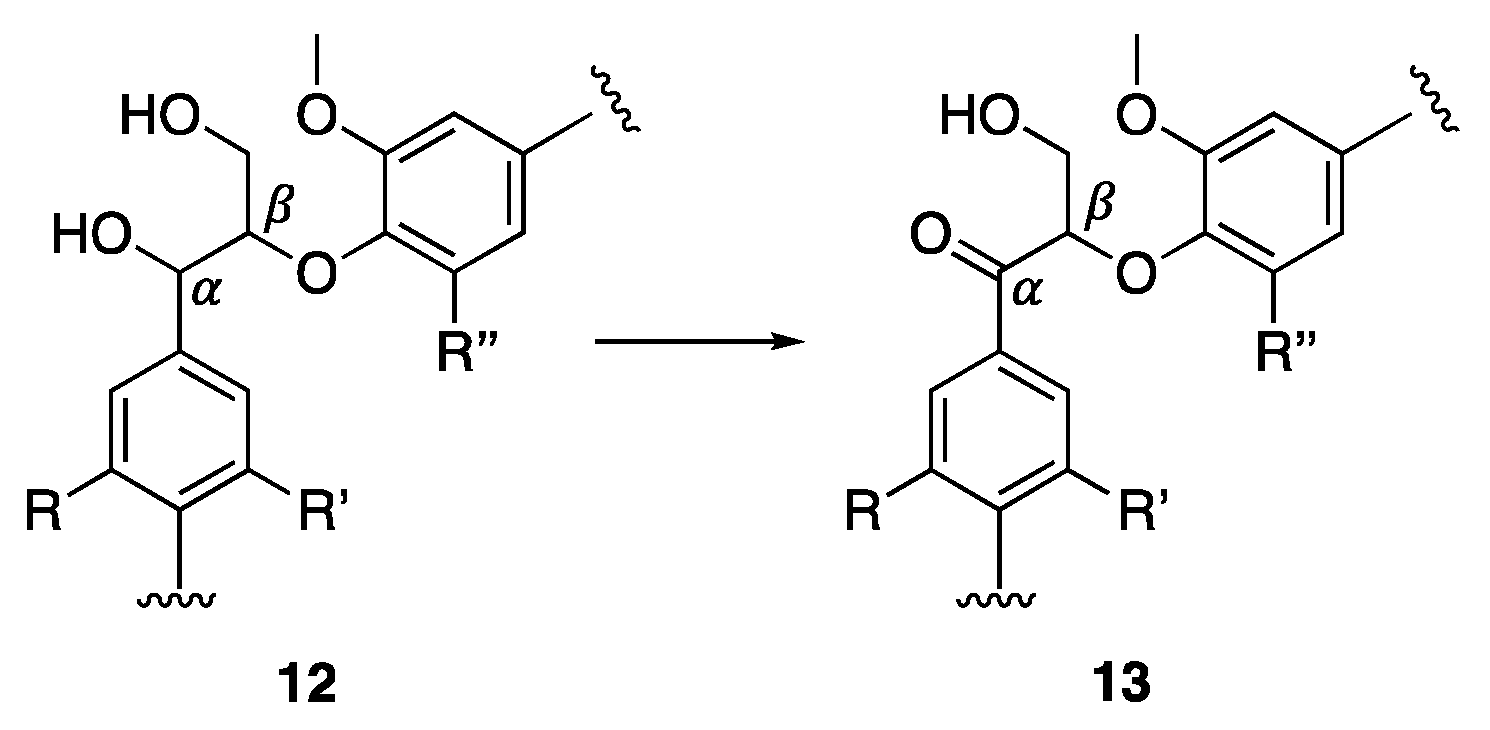
- Mottweiler, J.; Rinesch, T.; Besson, C.; Buendia, J.; Bolm, C. Iron-Catalysed Oxidative Cleavage of Lignin and β-O-4 Lignin Model Compounds with Peroxides in DMSO. Green Chem. 2015, 17, 5001–5008. [Google Scholar] [CrossRef] [Green Version]
- Crestini, C.; Pro, P.; Neri, V.; Saladino, R. Methyltrioxorhenium: A New Catalyst for the Activation of Hydrogen Peroxide to the Oxidation of Lignin and Lignin Model Compounds. Bioorg. Med. Chem. 2005, 13, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized Methyltrioxo Rhenium (MTO)/H2O2 Systems for the Oxidation of Lignin and Lignin Model Compounds. Bioorg. Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Du, X.; Liu, W.; Tricker, A.W.; Dai, H.; Deng, Y. Highly Efficient Lignin Depolymerization via Effective Inhibition of Condensation during Polyoxometalate-Mediated Oxidation. Energy Fuels 2019, 33, 6483–6490. [Google Scholar] [CrossRef]
- Voitl, T.; Rudolf von Rohr, P. Oxidation of Lignin Using Aqueous Polyoxometalates in the Presence of Alcohols. ChemSusChem 2008, 1, 763–769. [Google Scholar] [CrossRef]
- Badamali, S.K.; Luque, R.; Clark, J.H.; Breeden, S.W. Microwave Assisted Oxidation of a Lignin Model Phenolic Monomer Using Co(Salen)/SBA-15. Catal. Commun. 2009, 10, 1010–1013. [Google Scholar] [CrossRef]
- Badamali, S.K.; Luque, R.; Clark, J.H.; Breeden, S.W. Co(Salen)/SBA-15 Catalysed Oxidation of a β-O-4 Phenolic Dimer under Microwave Irradiation. Catal. Commun. 2011, 12, 993–995. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Prieto, P. Chapter 1. Microwave-Assisted Green Organic Synthesis. In Green Chemistry Series; Stefanidis, G., Stankiewicz, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–33. ISBN 9781782621409. [Google Scholar]
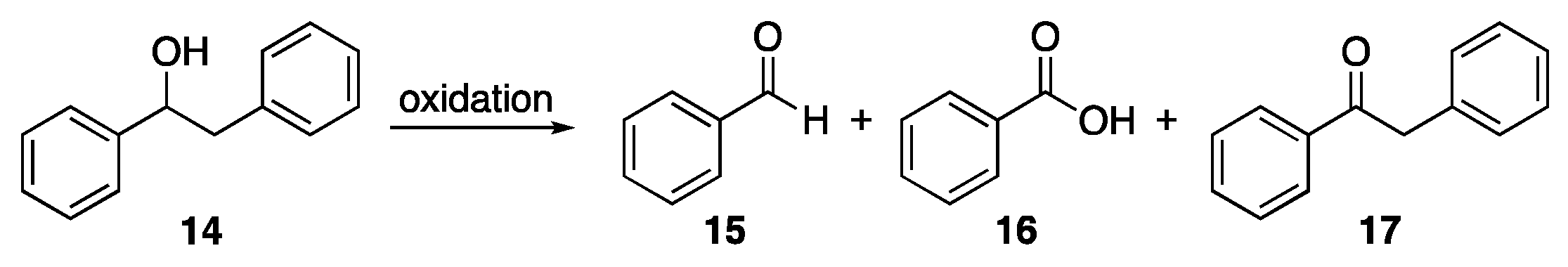
- Dai, J.; Patti, A.F.; Styles, G.N.; Nanayakkara, S.; Spiccia, L.; Arena, F.; Italiano, C.; Saito, K. Lignin Oxidation by MnO2 under the Irradiation of Blue Light. Green Chem. 2019, 21, 2005–2014. [Google Scholar] [CrossRef]
- Dai, J.; Patti, A.F.; Longé, L.; Garnier, G.; Saito, K. Oxidized Lignin Depolymerization Using Formate Ionic Liquid as Catalyst and Solvent. ChemCatChem 2017, 9, 2684–2690. [Google Scholar] [CrossRef]
- Wu, X.; Lin, J.; Zhang, H.; Xie, S.; Zhang, Q.; Sels, B.F.; Wang, Y. Z-Scheme Nanocomposite with High Redox Ability for Efficient Cleavage of Lignin C–C Bonds under Simulated Solar Light. Green Chem. 2021, 23, 10071–10078. [Google Scholar] [CrossRef]
- Yang, G.; Shi, J.; Sun, H.; Tong, X. The Product-Controllable Aerobic Oxidative Cleavage of Vicinal Diols Using Vanadium-Based Photocatalysts. React. Chem. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Han, S.; Kim, J.; Trang, N.V.T.; Kim, K.; Choi, E.-G.; Boonmongkolras, P.; Lee, Y.W.; Shin, B.; et al. Lignin-Fueled Photoelectrochemical Platform for Light-Driven Redox Biotransformation. Green Chem. 2020, 22, 5151–5160. [Google Scholar] [CrossRef]
- Lan, C.; Fan, H.; Shang, Y.; Shen, D.; Li, G. Electrochemically Catalyzed Conversion of Cornstalk Lignin to Aromatic C ompounds: An Integrated Process of Anodic Oxidation of a Pb/PbO2 Electrode and Hydrogenation of a Nickel Cathode in Sodium Hydroxide Solution. Sustain. Energy Fuels 2020, 4, 1828–1836. [Google Scholar] [CrossRef]
- Di Fidio, N.; Timmermans, J.W.; Antonetti, C.; Raspolli Galletti, A.M.; Gosselink, R.J.A.; Bisselink, R.J.M.; Slaghek, T.M. Electro-Oxidative Depolymerisation of Technical Lignin in Water Using Platinum, Nickel Oxide Hydroxide and Graphite Electrodes. New J. Chem. 2021, 45, 9647–9657. [Google Scholar] [CrossRef]
- Cui, T.; Ma, L.; Wang, S.; Ye, C.; Liang, X.; Zhang, Z.; Meng, G.; Zheng, L.; Hu, H.-S.; Zhang, J.; et al. Atomically Dispersed Pt–N3C1 Sites Enabling Efficient and Selective Electrocatalytic C–C Bond Cleavage in Lignin Models under Ambient Conditions. J. Am. Chem. Soc. 2021, 143, 9429–9439. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Alternative Energy Input: Mechanochemical, Microwave and Ultrasound-Assisted Organic Synthesis. Chem Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Dabral, S.; Wotruba, H.; Hernández, J.G.; Bolm, C. Mechanochemical Oxidation and Cleavage of Lignin β-O-4 Model Compounds and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 3242–3254. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, L.; Xu, W.; Dushkin, A.V.; Su, W. Mechanochemical Cleavage of Lignin Models and Lignin via Oxidation and a Subsequent Base-Catalyzed Strategy. Green Chem. 2020, 22, 3489–3494. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical Degradation of Lignin and Wood by Solvent-Free Grinding in a Reactive Medium. Green Chem 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Zeng, J.; Yoo, C.G.; Wang, F.; Pan, X.; Vermerris, W.; Tong, Z. Biomimetic Fenton-Catalyzed Lignin Depolymerization to High-Value Aromatics and Dicarboxylic Acids. ChemSusChem 2015, 8, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Arefieva, O.D.; Vasilyeva, M.S.; Zemnukhova, L.A.; Timochkina, A.S. Heterogeneous Photo-Fenton Oxidation of Lignin of Rice Husk Alkaline Hydrolysates Using Fe-Impregnated Silica Catalysts. Environ. Technol. 2021, 42, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Patankar, S.C.; Liu, L.-Y.; Ji, L.; Ayakar, S.; Yadav, V.; Renneckar, S. Isolation of Phenolic Monomers from Kraft Lignin Using a Magnetically Recyclable TEMPO Nanocatalyst. Green Chem. 2019, 21, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yan, L.; Zhao, X.; Wang, C.; Li, S.; Zhang, X.; Ma, L.; Zhang, Q. Mild Selective Oxidative Cleavage of Lignin C–C Bonds over a Copper Catalyst in Water. Green Chem. 2021, 23, 7030–7040. [Google Scholar] [CrossRef]
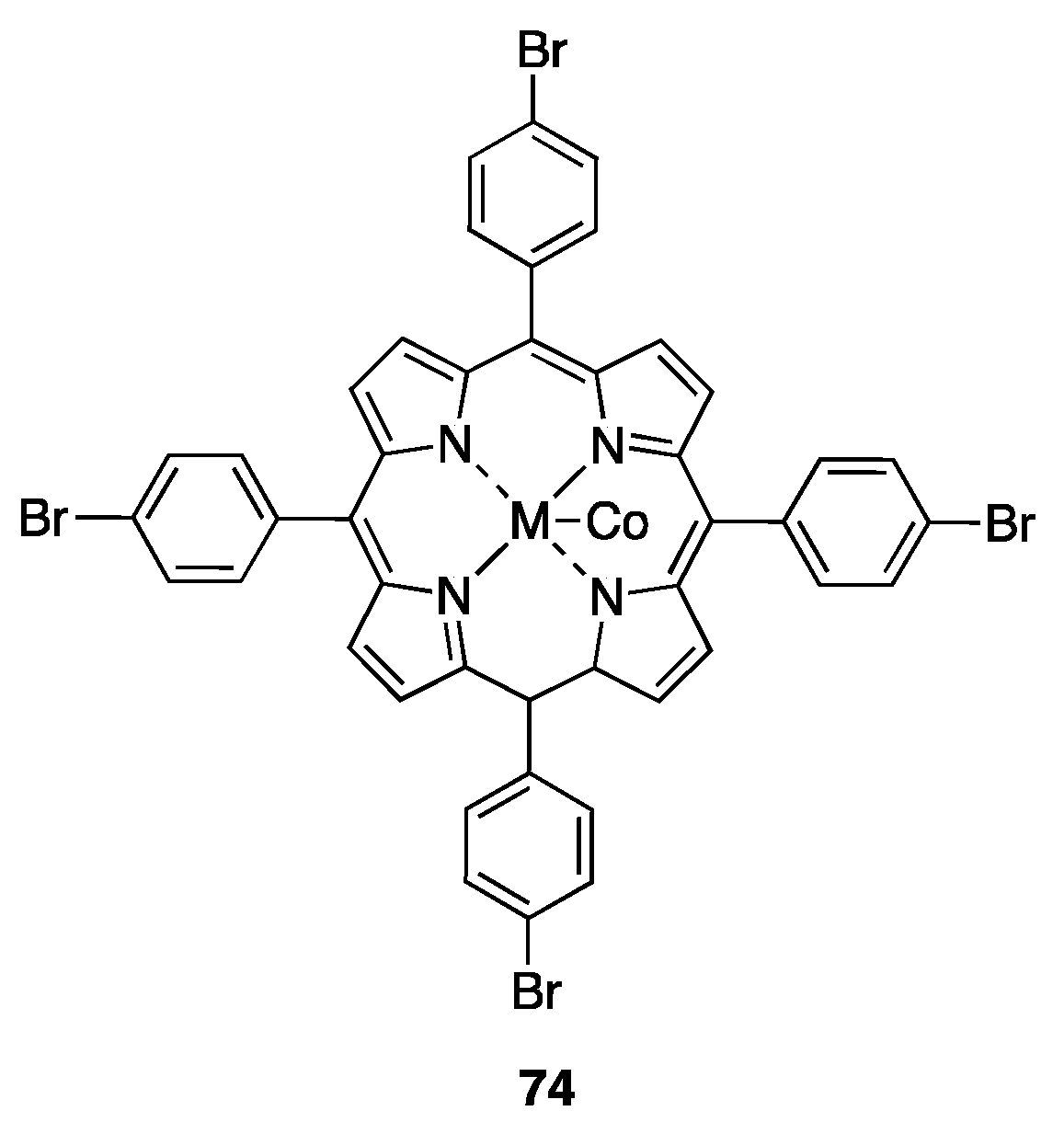
- Crestini, C.; Pastorini, A.; Tagliatesta, P. Metalloporphyrins Immobilized on Motmorillonite as Biomimetic Catalysts in the Oxidation of Lignin Model Compounds. J. Mol. Catal. Chem. 2004, 208, 195–202. [Google Scholar] [CrossRef]
- Artaud, I.; Ben-Aziza, K.; Mansuy, D. Iron Porphyrin-Catalyzed Oxidation of 1,2-Dimethoxyarenes: A Discussion of the Different Reactions Involved and the Competition between the Formation of Methoxyquinones or Muconic Dimethyl Esters. J. Org. Chem. 1993, 58, 3373–3380. [Google Scholar] [CrossRef]
- Xie, J.; Ma, G.; Ouyang, X.; Zhao, L.; Qiu, X. Metalloporphyrin as a Biomimetic Catalyst for the Catalytic Oxidative Degradation of Lignin to Produce Aromatic Monomers. Waste Biomass Valorization 2020, 11, 4481–4489. [Google Scholar] [CrossRef]
- Halma, M.; Lachenal, D.; Marlin, N.; Deronzier, A.; Brochier, M.C.; Zarubin, M. H2O2 Oxidation of Lignin Model Dimers Catalyzed by Copper(II)–phenanthroline. Ind. Crops Prod. 2015, 74, 514–522. [Google Scholar] [CrossRef]
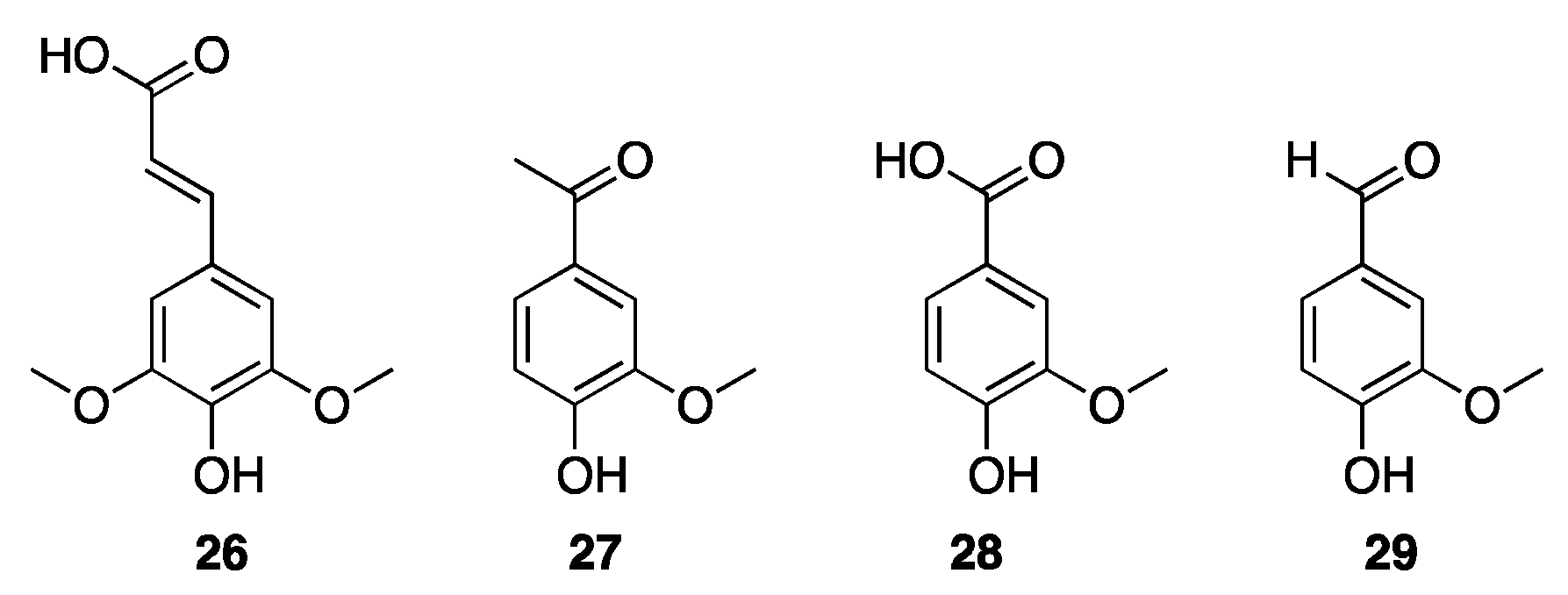
- Yu, Q.; Song, Z.; Chen, X.; Fan, J.; Clark, J.H.; Wang, Z.; Sun, Y.; Yuan, Z. A Methanol–Choline Chloride Based Deep Eutectic Solvent Enhances the Catalytic Oxidation of Lignin into Acetovanillone and Acetic Acid. Green Chem. 2020, 22, 6415–6423. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Q.; Pan, T.; Zuo, Y.; Fu, Y.; Guo, Q.-X. Depolymerization of Lignin by Catalytic Oxidation with Aqueous Polyoxometalates. Appl. Catal. Gen. 2013, 467, 504–508. [Google Scholar] [CrossRef]
- Du, X.; Tricker, A.W.; Yang, W.; Katahira, R.; Liu, W.; Kwok, T.T.; Gogoi, P.; Deng, Y. Oxidative Catalytic Fractionation and Depolymerization of Lignin in a One-Pot Single-Catalyst System. ACS Sustain. Chem. Eng. 2021, 9, 7719–7727. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Li, C.; Zhang, B.; Xiao, J.; Wang, A.; Zhang, T.; et al. Mild Redox-Neutral Depolymerization of Lignin with a Binuclear Rh Complex in Water. ACS Catal. 2019, 9, 4441–4447. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; He, J.; Zhang, Y. Highly Effective C–C Bond Cleavage of Lignin Model Compounds. Green Chem. 2017, 19, 3135–3141. [Google Scholar] [CrossRef]
- Hosoya, T.; Yamamoto, K.; Miyafuji, H.; Yamada, T. Selective Production of Bio-Based Aromatics by Aerobic Oxidation of Native Soft Wood Lignin in Tetrabutylammonium Hydroxide. RSC Adv. 2020, 10, 19199–19210. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hosoya, T.; Yoshioka, K.; Miyafuji, H.; Ohno, H.; Yamada, T. Tetrabutylammonium Hydroxide 30-Hydrate as Novel Reaction Medium for Lignin Conversion. ACS Sustain. Chem. Eng. 2017, 5, 10111–10115. [Google Scholar] [CrossRef]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-Catalyzed Oxacyclization of Alkenoic Acids and Alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319924045. [Google Scholar]
- Sancineto, L.; Tidei, C.; Bagnoli, L.; Marini, F.; Lenardão, E.; Santi, C. Selenium Catalyzed Oxidation of Aldehydes: Green Synthesis of Carboxylic Acids and Esters. Molecules 2015, 20, 10496–10510. [Google Scholar] [CrossRef]
- Cao, H.; Qian, R.; Yu, L. Selenium-Catalyzed Oxidation of Alkenes: Insight into the Mechanisms and Developing Trend. Catal. Sci. Technol. 2020, 10, 3113–3121. [Google Scholar] [CrossRef]
- Rathore, V.; Upadhyay, A.; Kumar, S. An Organodiselenide with Dual Mimic Function of Sulfhydryl Oxidases and Glutathione Peroxidases: Aerial Oxidation of Organothiols to Organodisulfides. Org. Lett. 2018, 20, 6274–6278. [Google Scholar] [CrossRef]
- Tan, K.H.; Xu, W.; Stefka, S.; Demco, D.E.; Kharandiuk, T.; Ivasiv, V.; Nebesnyi, R.; Petrovskii, V.S.; Potemkin, I.I.; Pich, A. Selenium-Modified Microgels as Bio-Inspired Oxidation Catalysts. Angew. Chem. Int. Ed. 2019, 58, 9791–9796. [Google Scholar] [CrossRef]
- Santos, W.C.C.; Dias, K.A.; Santos, L.P.; Kisukuri, C.M.; Rodrigues, T.S.; Geonmonond, R.S.; Camargo, P.H.C.; Andrade, L.H. Evaluating Gold and Selenium Chemistry for Selective Transformations of Lignin Model Compounds. Adv. Synth. Catal. 2018, 360, 1376–1383. [Google Scholar] [CrossRef]

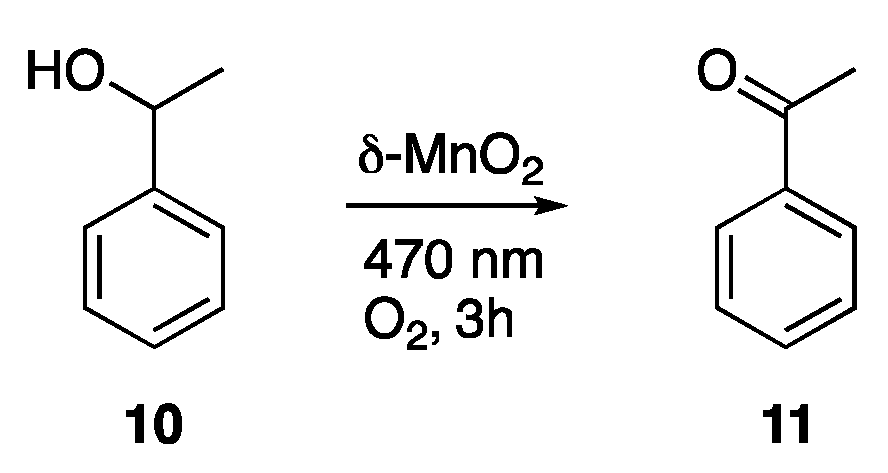



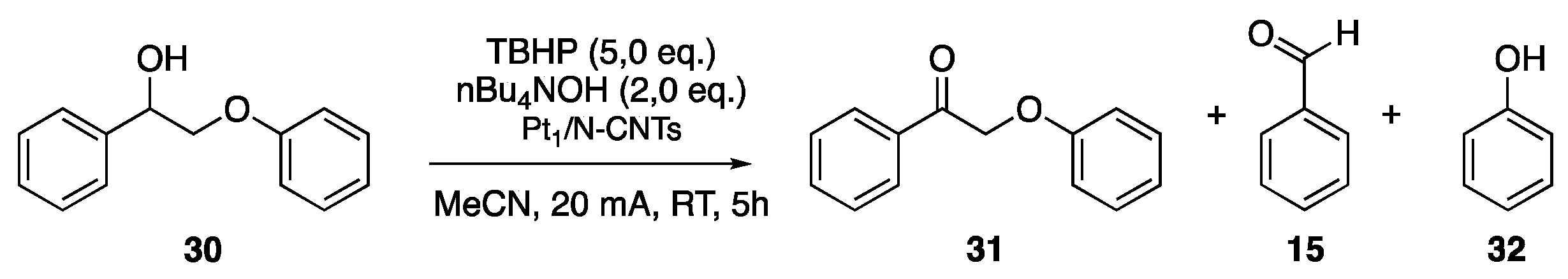
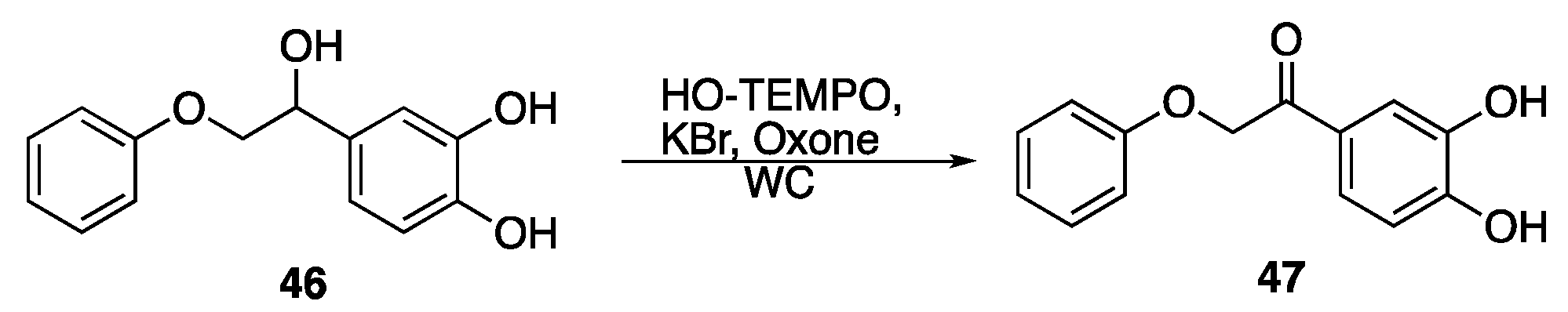


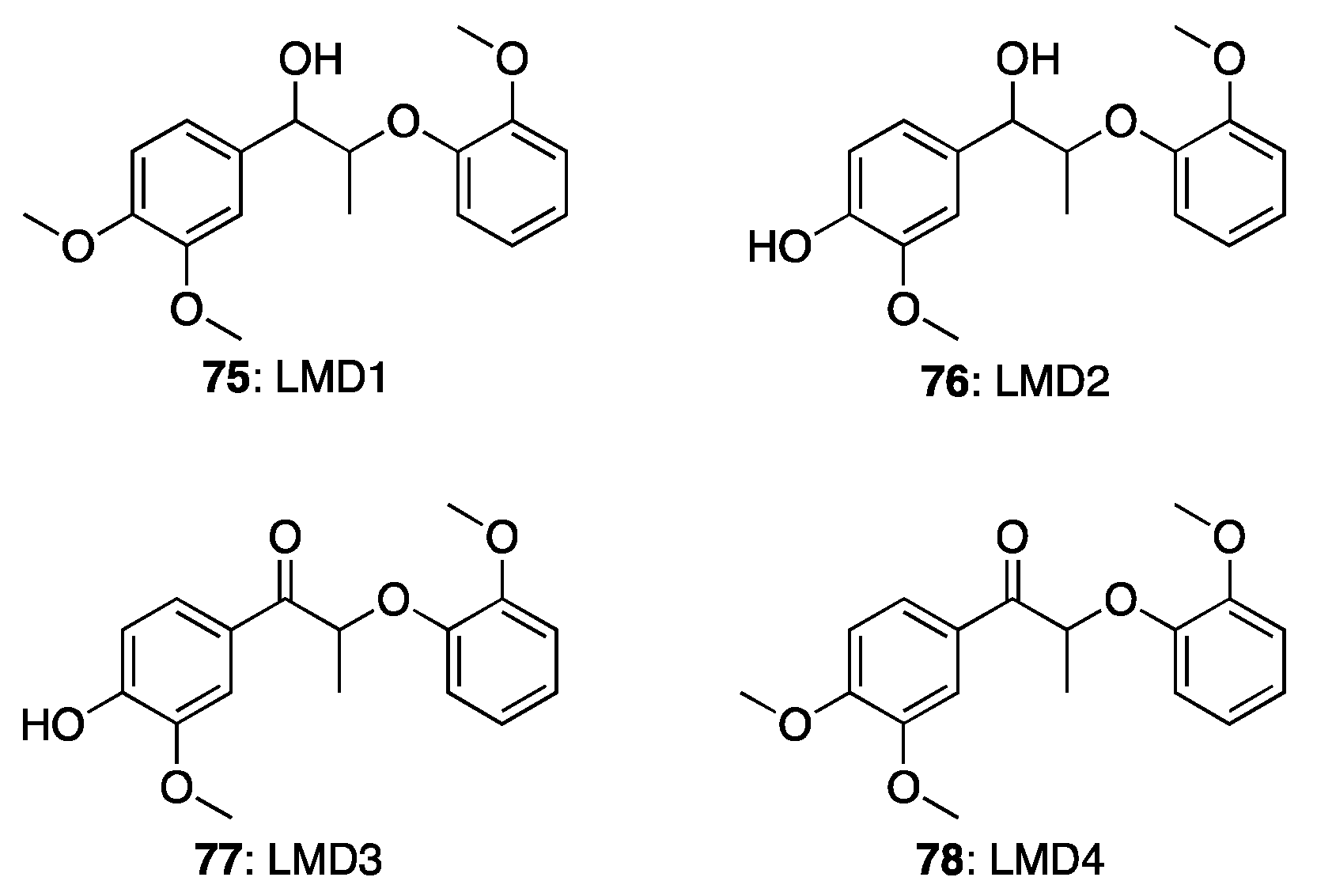

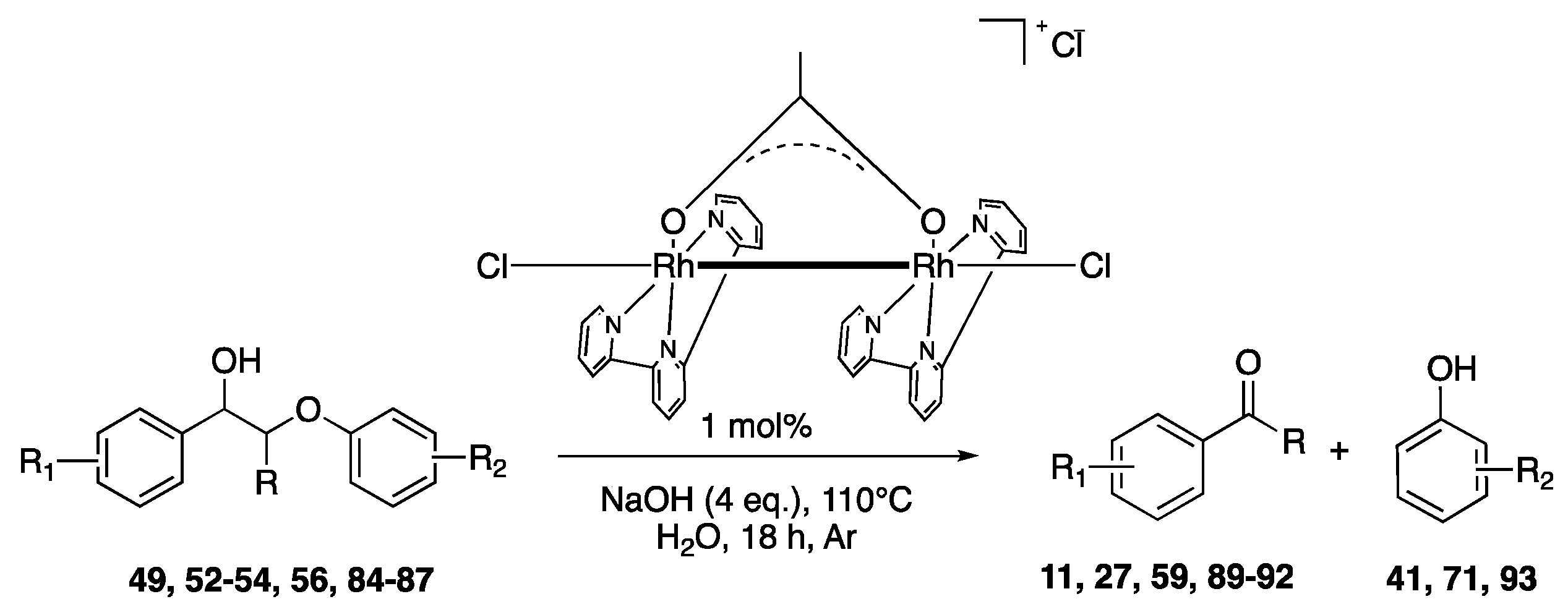
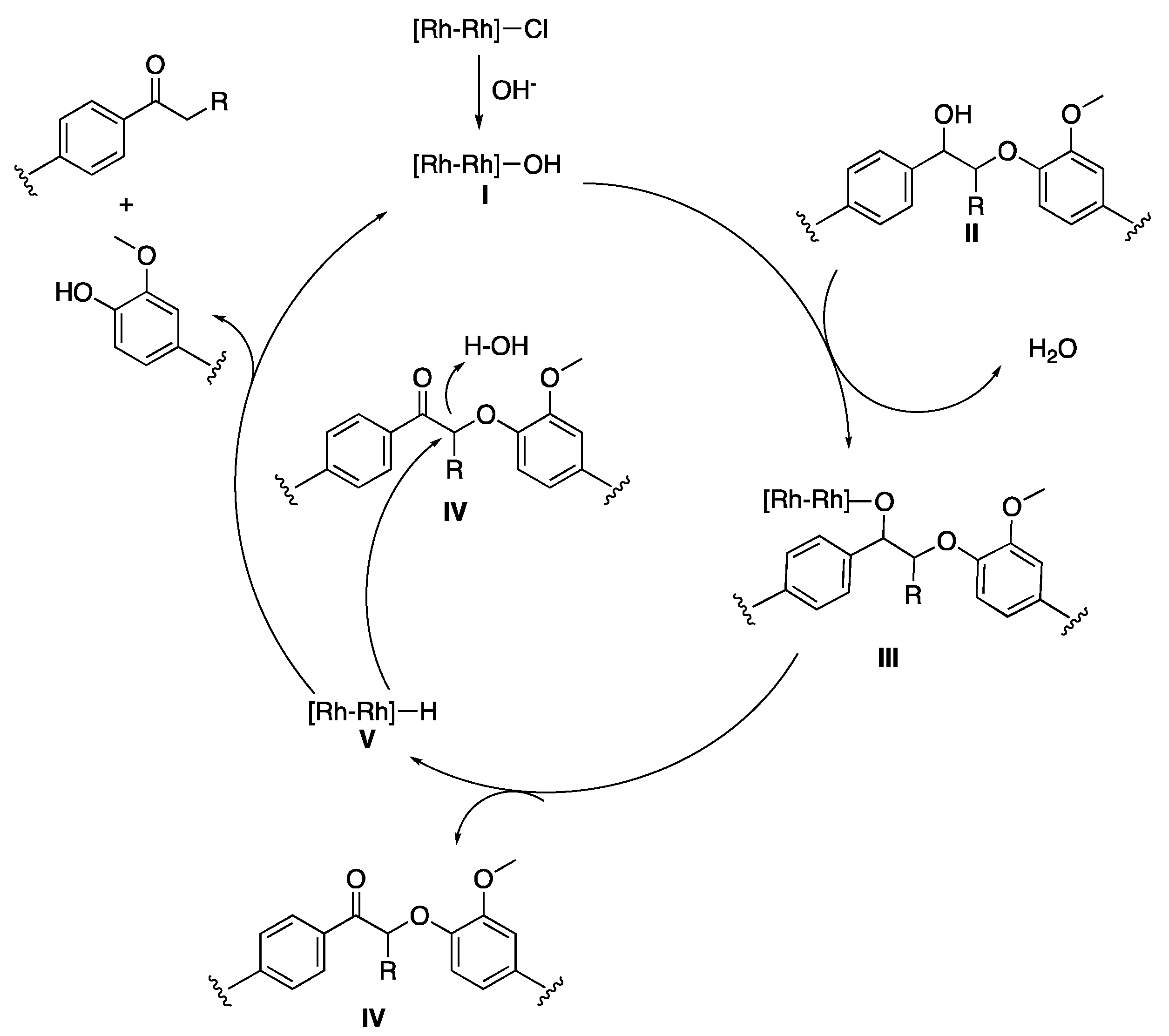

| Lignin Model Compounds | Conversion % | Products (Yield %) | |
|---|---|---|---|
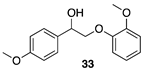 | >99 |  | 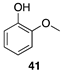 |
| (52%) | (31%) | ||
 | >99 |  |  |
| (86%) | (65%) | ||
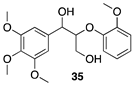 | >99 | 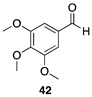 | 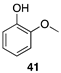 |
| (46%) | (23%) | ||
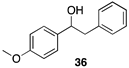 | >99 | 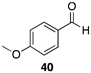 | 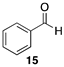 |
| (36%) | (34%) | ||
 | >99 | 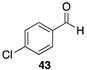 | |
| (78%) | |||
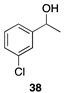 | 79 | 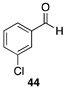 | |
| (59%) | |||
 | >99 | 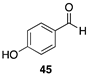 | |
| (36%) | |||
| First Step Product (Yield %) | Second Step Product (Yield %) | |
|---|---|---|
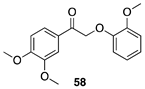 | 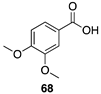 |  |
| (94%) | (80%) | (95%) |
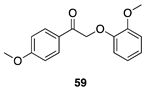 | 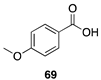 | 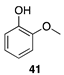 |
| (95%) | (92%) | (99%) |
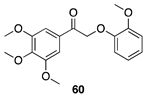 |  | 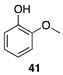 |
| (78%) | (88%) | (98%) |
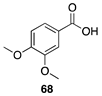
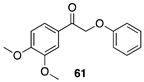 | 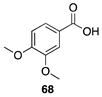 |  |
| (91%) | (87%) | (96%) |
 |  | 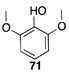 |
| (92%) | (84%) | (88%) |
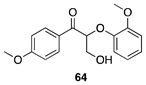
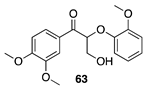 |  | 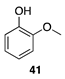 |
| (91%) | (70%) | (92%) |
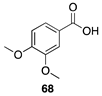
 |  | 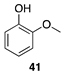 |
| (80%) | (73%) | (90%) |
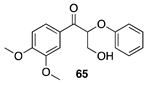 | 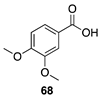 |  |
| (82%) | (83%) | (96%) |
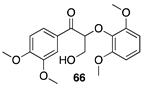 | 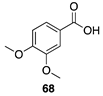 | 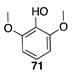 |
| (86%) | (68%) | (87%) |
 | 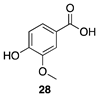 |  |
| (88%) | (58%) | (92%) |
| Substrates | Conditions | Products (Yields %) | |
|---|---|---|---|
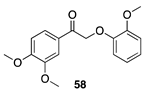 | 30 °C-10 h | 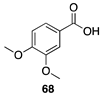 | 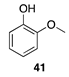 |
| (76.38%) | (82.59%) | ||
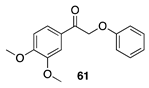 | 30 °C-15 h | 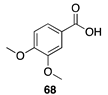 | 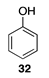 |
| (80.58%) | (78.23%) | ||
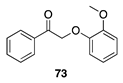 | 30 °C-4 h | 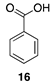 | 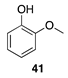 |
| (85.56%) | (91.28%) | ||
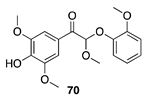 | 50 °C-8 h | 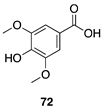 | 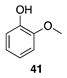 |
| (76.06%) | (83.57%) | ||
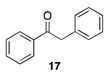 | 50 °C-10 h |  | 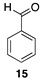 |
| (82.53%) | (76.65%) | ||
| Substrates | Products (Yields %) | ||
|---|---|---|---|
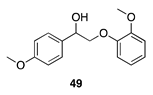 | 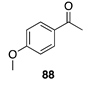 | 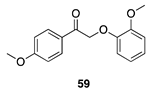 | 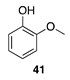 |
| (86%) | (8%) | (89%) | |
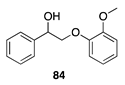 |  |  | |
| (61%) | (86%) | ||
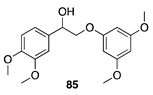 | 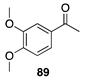 | 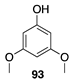 | |
| (85%) | (90%) | ||
 | 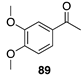 | 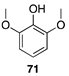 | |
| (88%) | (92%) | ||
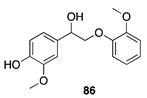 | 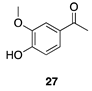 | 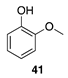 | |
| (82%) | (86%) | ||
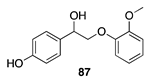 |  | 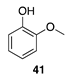 | |
| (76%) | (90%) | ||
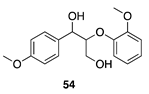 | 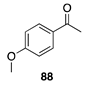 | 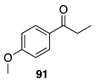 |  |
| (24%) | (34%) | (62%) | |
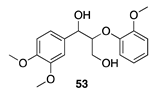 |  | 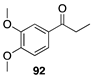 |  |
| (26%) | (33%) | (61%) | |
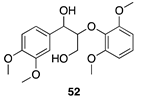 |  | 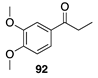 | 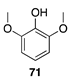 |
| (29%) | (32%) | (64%) | |
 | ||||||
|---|---|---|---|---|---|---|
| Substrate | Conversion (%) | Products 97–102 (Yield%) | Products 103–108 (Yield%) | |||
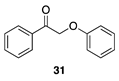 | 100 A | 92 B |  | 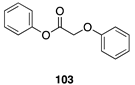 | ||
| (99%) A | (92%) B | (0%) A | (0%) B | |||
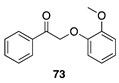 | 100 A | 98 B | 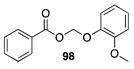 | 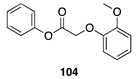 | ||
| (92%) A | (94%) B | (0%) A | (0%) B | |||
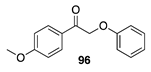 | 100 A | >99 B | 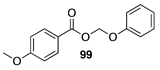 | 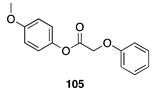 | ||
| (6%) A | (24%) B | (90%) A | (66%) B | |||
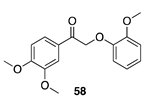 | 100 A | >99 B | 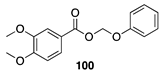 | 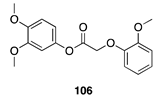 | ||
| (3%) A | (9%) B | (86%) A | (82%) B | |||
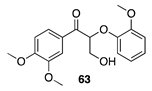 | >99 A | >99 B | 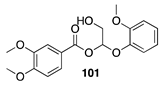 | 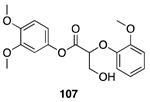 | ||
| (26%) A | (37%) B | (66%) A | (44%) B | |||
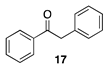 | >99 A | 30 B | 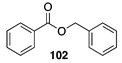 | 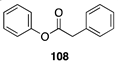 | ||
| (78%) A | (20%) B | (7%) A | (2%) B | |||
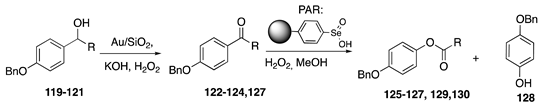 | |||
|---|---|---|---|
| Substrate | First Step | Second Step | |
| Product (Yield%) | Product (Yield%) | Product (Yield%) | |
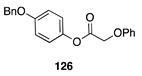
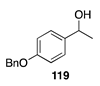 |  | 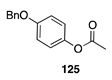 |  |
| (86%) | (13%) | (58%) | |
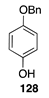
 | 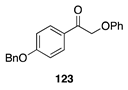 |  | 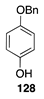 |
| (51%) | (40%) | (46%) | |
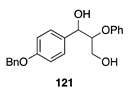 | 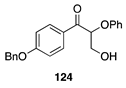 |  | |
| (74%) | (90%) | ||
 | 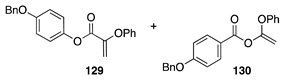 | ||
| (25%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scimmi, C.; Sancineto, L.; Drabowicz, J.; Santi, C. New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds. Int. J. Mol. Sci. 2022, 23, 4378. https://doi.org/10.3390/ijms23084378
Scimmi C, Sancineto L, Drabowicz J, Santi C. New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds. International Journal of Molecular Sciences. 2022; 23(8):4378. https://doi.org/10.3390/ijms23084378
Chicago/Turabian StyleScimmi, Cecilia, Luca Sancineto, Jozef Drabowicz, and Claudio Santi. 2022. "New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds" International Journal of Molecular Sciences 23, no. 8: 4378. https://doi.org/10.3390/ijms23084378
APA StyleScimmi, C., Sancineto, L., Drabowicz, J., & Santi, C. (2022). New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds. International Journal of Molecular Sciences, 23(8), 4378. https://doi.org/10.3390/ijms23084378








